Abstract
Reactions of benzoquinone with amines can potentially lead to the formation of coupled merocyanine or merocyanine/polymethine systems. In this study, several diamino-substituted 1,4-benzoquinones were synthesized. The crystal structures for three derivatives bearing 2-hydroxyethylamino or 2-(2-hydroxyethoxy)ethyl)amino substituents were determined using single-crystal X-ray crystallographic analysis. A characteristic feature of all molecular structures is the presence of an extensive network of intermolecular interactions, significantly stabilized by hydrogen bonding. Additionally, changes in the optical behavior of the synthesized compounds were monitored by UV-Vis spectroscopy in the presence of Cu2+ and Zn2+ ions, followed by the addition of primary, secondary or biogenic (butane-1,4-diamine) amines.
1. Introduction
Quinones are small molecules found widespread in nature and known due to involvement into numerous biological processes such as photosynthesis [1] and respiratory chains [2] as well as their derivatives have a deep impact on the composition and function of bacterial ecosystems [3]. Moreover, quinone motifs are found in the structure of some co-factors, e.g., Pyrroloquinoline Quinone (PQQ) [4] and 2,4,5-trihydroxyphenylalanine quinone (TPQ). TPQ serves as a part of the copper-containing amine oxidases (CuAOs) where both (copper ion and quinone) are involved in a catalytic cycle [5]. Quinones have shown promise in antimalarial drug design [6]. Interaction between quinones and amino acids can lead to enzymatic browning of foods, the formation of humic substances, and the discoloration of various plants during processing [7]. Also, benzoquinones with replaceable halide or pseudohalide substituents were tested for optical chirality sensing of chiral amines, amino alcohols, and amino acids [8]. Additionally, naturally occurring quinone derivatives can potentially replace synthetic azodyes due to the latter’s known toxicity [9].
During the last decade, quinone derivatives have been intensively studied for application as electroactive materials in advanced electrochemical energy storage devices [10]. Quinones have been investigated for the creation of all-organic aqueous redox systems [11] and to serve as both cathodes [12] and anodes [13] in Li, Na or Zn [14] ion batteries.
It is known that derivatives of 2,5-dihydroxy-1,4-benzoquinone or 2,5-diamino-1,4-benzoquinone are extensively studied as ligands for metal complexes and MOFs [15] which have been investigated for spin–spin coupling, redox isomerism and sensors based on supramolecular assemblies [16].
2,5-Diaminosubstituted 1,4-benzoquinones represent a class of coupled polymethines that possess a unique electronic structure. The simplest derivative-2,5-diamino-3,6-dichloro-1,4-benzoquinone-consist of two nearly independent merocyanine fragments connected by two long single bonds (approximately 1.52 Å). Each merocyanine fragment contains 6π electrons delocalized over the fragment [17]. Such coupled polymethines show a bathochromic shift in their UV-Vis spectra in comparison to the absorption of a single polymethine [18]. Incorporation of substituents that do not affect the merocyanines fragments but can form different types intra- and intermolecular interactions can be beneficial.
In this paper, we report on the results received from the study of 1,4-benzoquinones bearing 2-hydroxyethylamino or 2-(2-hydroxyethoxy)ethyl)amino groups along to halide or pseudohalide (CN) substituents. Molecular structures of quinones 3–5 have been determined by single-crystal X-ray diffraction to investigate the effects of substituents on the formed intra- and intermolecular interactions. Furthermore, considering the potential synergistic effects of quinones and metal ions, we investigated the optical behavior of the synthesized compounds 3–6 in the presence of various amines (sec-butylamine, diethylamine and butane-1,4-diamine) and Cu2+ or Zn2+ ions. Butane-1,4-diamine is a representative of biogenic amines, which are compounds formed during the food spoilage process [19].
2. Materials and Methods
2.1. Materials and Instrumentation
Reagents and solvents were purified by standard means or used without further purification. Melting points were measured on KSPII Melting Point Analyzer (A.Krüss Optronic, Hamburg, Germany). 1H and 13C NMR spectra were recorded on a Bruker Avance Neo 500 spectrometer (Bruker BioSpin AG BBIO, Rheinstetten, Germany) at 500 Hz in DMSO-d6 solutions. Chemical shifts were expressed in parts per million (δ, ppm) relative to solvent signal (DMSO-d6: 2.50 ppm for 1H and 39.52 ppm for 13C) [20]. Elemental CHN analysis was carried on Euro Vector EA 3000 analyzer (EuroVector, Milan, Italy). IR spectra were recorded on a Spectrum 100 FTIR spectrometer (PerkinElmer instruments, Waltham, MA, USA). The UV-Vis absorption spectra were acquired with Lambda 35 35 UV/Vis spectrometer (PerkinElmer instruments, Shelton, CT, USA) using 1 cm length quartz cuvettes with a concentration c = 5 × 10−5 M of compounds 3–6 in DMF/MeOH (3/2) solutions. Cu2+ and Zn2+ ions were added as chloride salts (10 eq.) and selected amines were added in excess. Low resolution mass spectra were acquired on a Waters EMD 1000MS mass detector (Waters, Milford, MA, USA) (ESI + mode, voltage 30 V) with Xterra MS C18 5 μm 2.1 100 mm column and gradient eluent mode using 0.1% HCOOH in deionized water and MeCN or MeOH.
2.2. Synthesis of Compounds 3–6
2.2.1. Synthesis Method for Compounds 3, 4 and 6
Chloranil (1a) (0.5 g, 2.03 mmol) or bromanil (1c) (0.86 g, 2.03 mmol) was dissolved in toluene (50 mL) and aminoethanol (2a, 8.13 mmol) or 2-(2-aminoethoxy)ethanol (2b, 8.13 mmol) was added to a stirring solution dropwise at room temperature. The solution was stirred for 8 h. Formed precipitate was filtered and washed with toluene and n-hexane. The precipitate can be recrystallized from MeOH or MeCN.
2.2.2. 2,5-Dichloro-3,6-bis((2-hydroxyethyl)amino)cyclohexa-2,5-diene-1,4-dione (3)
Yield: 0.21 g (35%), dark red solid. M.P.: 187–188 °C (toluene). MS: C10H12Cl2N2O4 requires [M + H]+ 297.02; found [M + H]+ 297.2. 1H NMR (500 MHz, DMSO-d6): δ 7.93 (br.s, 2H, exchange with D2O, NH), 4.97 (s, 2H, exchange with D2O, OH), 3.84 (m, 4H, CH2), 3.58 (m, 4H, CH2). 13C{1H} NMR could not be acquired due to poor solubility of a compound. IR (KBr pellet, cm−1): 3401, 3314, 3179, 2968, 2926, 1655, 1583, 1503, 1430, 1325, 1082. Anal. Calcd. for C10H12Cl2N2O4 + H2O (a water molecule is presented in a crystal structure of the compound): C, 38.36; H, 4.51; N, 8.95; found C, 38.75; H, 4.51; N, 9.08.
2.2.3. 2,5-Dichloro-3,6-bis((2-(2-hydroxyethoxy)ethyl)amino)cyclohexa-2,5-diene-1,4-dione (4)
Yield: 0.37 g (48%), dark red solid. M.P.: 137–138 °C (4-I, MeOH). MS: C14H20Cl2N2O6 requires [M + H]+ 383.07; found [M + H]+ 383.2. 1H NMR (500 MHz, DMSO-d6): δ 7.91 (br.s, 2H, exchange with D2O, NH), 4.62 (t, J = 5.2 Hz, 2H, exchange with D2O, OH), 3.93 (t, J = 5.2 Hz, 4H, CH2), 3.62 (t, J = 5.7 Hz, 4H, CH2), 3.50 (dd, J = 9.9, 4.9 Hz, 4H, CH2), 3.45 (m, 4H, CH2). 13C{1H} NMR could not be acquired due to poor solubility of a compound. IR (KBr pellet, cm−1): 3468, 3246, 2960, 2925, 2882, 1659, 1606, 1508, 1479. Anal. Calcd. for C14H20Cl2N2O6: C, 43.88; H, 5.26; N, 7.31; found C, 43.99; H, 5.33; N, 7.45.
2.2.4. 4-Chloro-2,5-bis((2-hydroxyethyl)amino)-3,6-dioxocyclohexa-1,4-dienecarbonitrile (5)
DDQ (1b) (0.5 g, 2.20 mmol) was dissolved in THF (50 mL) and aminoethanol (2a, 0.53 mL, 8.81 mmol) was added to a stirring solution dropwise at room temperature. Solution was stirred for 8 h. Formed precipitate was filtered and recrystallized from MeCN. Yield: 0.25 g (40%), red solid. M.P.: 223–225 °C. MS: C11H12ClN3O4 requires [M − H]− 284.05; found [M − H]− 284.2. 1H NMR (500 MHz, DMSO-d6): δ 9.12 (br.s, 1H, exchange with D2O, NH), 8.07 (br.s, 1H, exchange with D2O, NH), 5.05 (t, J = 5.2 Hz, 1H, exchange with D2O, OH), 4.99 (s, 1H, exchange with D2O, OH), 3.86 (m, 2H, CH2), 3.82 (t, J = 4.5 Hz, 2H, CH2), 3.66 (q, J = 5.2 Hz, 2H, CH2), 3.59 (d, J = 5.1 Hz, 2H, CH2). 13C{1H} NMR (126 MHz, DMSO-d6): δ 175.6 (C=O), 170.22 (C=O), 152.37 (C-NH), 146.24 (C-NH), 131.01 (C-Cl), 116.3 (CN), 109.2 (C-CN), 60.6 (CH2), 59.5(CH2), 46.6(CH2), 46.3(CH2). IR (KBr pellet, cm−1): 3370, 3268, 3219, 2958, 2892, 2212, 1662, 1558, 1504, 1450. Anal. Calcd. for C11H12ClN3O4: C, 46.25; H, 4.23; N, 14.71; found: C, 46.40; H, 4.31; N, 14.73
2.2.5. 2,5-Dibromo-3,6-bis((2-(2-hydroxyethoxy)ethyl)amino)cyclohexa-2,5-diene-1,4-dione (6)
Yield: 0.13 g (24%), dark red solid. M.P.: 136–137 °C (MeOH). MS: C14H20Br2N2O6 requires [M + H]+ 473.13; found [M + H]+ 473.3. 1H NMR (500 MHz, DMSO-d6): δ 7.98 (br.s, 2H, exchange with D2O, NH), 4.64 (s, 2H, exchange with D2O, OH), 3.98 (t, J = 5.4 Hz, 4H, CH2), 3.63 (t, J = 5.4 Hz, 4H, CH2), 3.49 (s, 4H, CH2), 3.46 (m, 4H, CH2). 13C{1H} NMR could not be acquired due to poor solubility of a compound. IR (KBr pellet, cm−1): 3449, 3257, 3008, 2949, 2919, 2880, 2815, 1651, 1611, 1505, 1478, 1317, 1108, 1077, 1062.
2.3. X-Ray Crystallography Analysis
Crystals of compound 3 were obtained from a reaction mixture solution of toluene and were washed several times with toluene and n-hexane. Single crystals of compounds 3 were investigated on a Bruker Nonius KappaCCD diffractometer. The structures of compounds have been deposited to the Cambridge Crystallographic Data Centre (CCDC), with deposition number CCDC 2247575.
Crystals of compound 4 were obtained by slow solvent evaporation from a hot saturated solution of the compound in MeOH (crystalline forms 4-I) or acetonitrile (MeCN) (crystalline form 4-II). Experimental Diffraction data of 4-I and 4-II were collected at 150 K on a Rigaku, XtaLAB Synergy, Dualflex, HyPix diffractometer using Cu Kα radiation (λ = 1.54184 Å). The crystal structure was solved by direct methods and refined by full-matrix least squares. For further details, see crystallographic data for 4-I and 4-II deposited at the Cambridge Crystallographic Data Centre as Supplementary Publication Numbers CCDC 2487932 (for 4-I) and CCDC 2487933 (for 4-II). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK.
Crystals of compound 5 were obtained by slow solvent evaporation from a hot saturated solution of the compound in acetonitrile (MeCN). Diffraction data were collected at 150.0 (1) K on a Rigaku, XtaLAB Synergy, Dualflex, HyPix diffractometer diffractometer. The structures were solved with the help of the Superflip [21,22,23] structure solution program using Charge Flipping and refined with the olex2.refine [24] refinement package using Levenberg-Marquardt minimisation. For further details, see crystallographic data for compound 5 deposited at the Cambridge Crystallographic Data Centre as Supplementary Publication Numbers CCDC 2455226.
For crystal packing visualization program Mercury [25] was used.
3. Results and Discussion
3.1. Synthesis
Synthesis of substituted 1,4-benzoquinones 3–6 starts from commercially available chloranil (1a), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (1b) or bromanil (1c). Reaction with aminoethanol (2a) or 2-(2-aminoethoxy)ethanol (2b) proceeds at room temperature during a few hours with precipitation of the final product. It should be noted that only disubstituted products were isolated that can be explained by a greater stability of the formed coupled polymethine. It is known [8] that synthesis of monosubstituted product requires more complex approach.
3.2. X-Ray Crystallography
Crystals of compounds 3–5 suitable for X-ray analysis were obtained either directly from the reaction mixture (compound 3) or by recrystallization followed by solvent evaporation from a saturated solution of the respective compound in MeOH or MeCN (compounds 4 and 5). The crystal structure of compound 3 contains a water molecule. For compound 4, two different crystals were obtained for X-ray crystallography analysis—one form from MeOH (4-I) and the other from MeCN (4-II). Table 1 lists the main crystallographic data for compounds 3, 4-I, 4-II and 5.

Table 1.
Crystal data and structure refinement for crystals of compounds 3–5.
Compound 3 (Figure 1) was isolated from the reaction of chloranil (1a) with aminoethanol (2a) (Scheme 1). Compound 3 can be described as quadrupole meropolymethine [17] that consists of two polymethine fragments coupled by two long C-C single bonds with electronic delocalization between polarized amino and carbonyl groups. In 1H NMR spectrum of compound 3 NH group appeared at 7.93 ppm but signals of both methylene groups appeared at 3.58 (CH2-OH) and 3.84 (HN-CH2) ppm and were assigned from 2D 1H-1H-COSY spectrum (Figure S2).
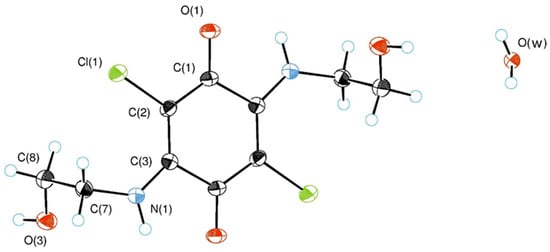
Figure 1.
ORTEP diagram of the asymmetric unit for compound 3 showing thermal ellipsoids at the 50% probability level.
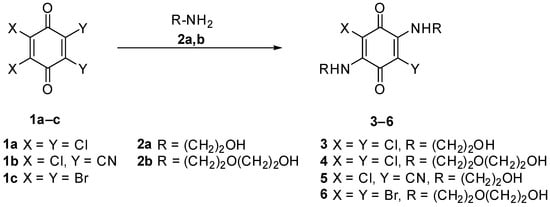
Scheme 1.
Synthesis of the compounds 3–6.
In the crystal structure of compound 3 formed H-bond network was assisted by water molecules (Figure 2). The crystalline structure has the following elements: twofold symmetry axes and symmetry centers. Molecules of compound 3 are located at the symmetry centers, while the water molecules are located at the twofold axes of symmetry. Each hydrogen atom of a water molecule forms H-bonds with carbonyl group. At the same time, each oxygen atom of water molecule (as an acceptor) forms two hydrogen bonds with OH groups of substituents (R = (CH2)2OH). Additionally, H-bonds were found between sidechains leading to the formation of centrosymmetric dimers.
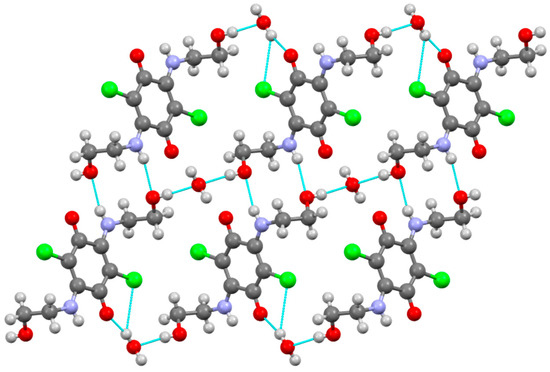
Figure 2.
Molecular packing in the crystal of compound 3. H-bonds are dotted lines colored blue. C, H, Cl, N, and O atoms are represented by grey, light grey, green, violet, and red ellipsoids, respectively. Hydrogen bonds are shown as blue dashed lines.
Compound 4 gives different crystalline forms when crystallized from different solvents. We obtained crystalline modifications of 4-I and 4-II. In accordance with single-crystal X-ray diffraction data the title compound gives monoclinic centrosymmetric molecular crystals. Figure 3A shows a perspective view of molecule 4-I with thermal ellipsoids and the atom-numbering scheme followed in the text.
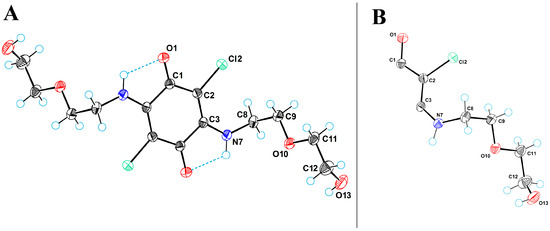
Figure 3.
(A) ORTEP diagram for molecule 4-I. (B) The asymmetric unit in crystal structures of 4-I and 4-II. C, Cl, N, and O atoms are represented by grey, green, blue, and red ellipsoids, respectively. The hydrogens are represented by circles of arbitrary radii. Hydrogen bonds are shown as blue dashed lines.
Molecules of compound 4 are stabilized by intramolecular hydrogen bonds of the NH···O type between the amino group and the carbonyl oxygen atom. The conformations of molecules in 4-I and 4-II differ very slightly. Table 2 gives the values of torsion angles characterizing the conformation of molecules in compound 4.

Table 2.
Values of selected torsion angles in 4-I and 4-II (in °).
In both crystal structures, the gravity center of the molecule of compound 4 is on the inversion center, which results in the symmetry equivalency of its components, so the asymmetric unit in 4-I and 4-II contains only half a molecule (Figure 3B). Based on the coupling principles used in organic dyes, this half of the molecule corresponds to one of the two polymethine units of the quinone derivative [17]. The merocyanine unit is determined in O1-C1-C2-C3-N7 fragment as C-C bonds are equivalized (d(C1-C2) = 1.43 Å, d(C2-C3) = 1.38 Å), C1=O bond is elongated (1.23 Å) and C3-N7 bond is shortened (1.33 Å).
Both crystal structures are stabilized by intermolecular hydrogen bonds of NH···O and OH···O types. Table 3 gives the main parameters of hydrogen bonds in 4-I and 4-II.

Table 3.
Parameters of hydrogen bonds in 4-I and 4-II.
In the crystal structure of 4-I, that was crystallized from MeOH, the amino group forms a bifurcated hydrogen bond: N7-H7···O1 and N7-H7···O13; the first bond is intramolecular, and the second is intermolecular. In addition, the crystal structure is stabilized by the intermolecular hydrogen bond O13-H13···O10. Due to these intermolecular bonds, the molecule is linked to its first neighbor via a two-fold symmetry axis; a second 2-(2-hydroxyethoxy)ethylamino group allows the molecule to bind to an additional neighbor, thus forming molecular chains along the crystallographic direction [1 0 1]. Figure 4 shows a projection of the crystal structure of 4-I along the monoclinic axis.
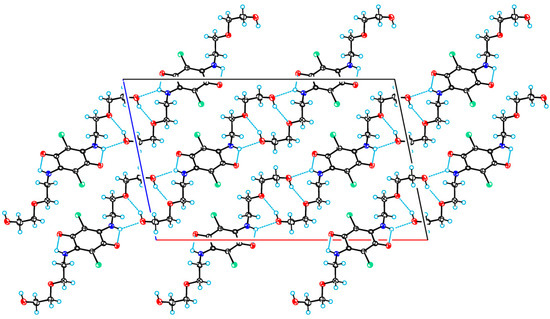
Figure 4.
A projection of the crystal structure of 4-I along the monoclinic axis. C, Cl, N, and O atoms are represented by grey, green, blue, and red ellipsoids, respectively. The hydrogens are represented by circles of arbitrary radii. Hydrogen bonds are shown as blue dashed lines.
The molecular chains (see Figure 5) are formed along the hydrogen-bonded motifs, where quinone rings are located almost perpendicular to each other (interplanar angle is 87.96°).
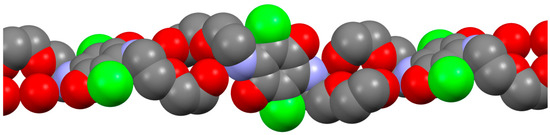
Figure 5.
Molecular chains of crystallographic form 4-I. Color map: C, grey; Cl, green; N, violet; O, red. For the sake of clarity, all hydrogen atoms were omitted.
In the crystal structure of 4-II (crystallized from MeCN) there are similar hydrogen bonds, by means of which the molecule is united with neighboring ones. The resulting centrosymmetric molecular chains are extended along the crystallographic direction of [1 0 0] (see Figure 6). The crystal structure of 4-II denser, and therefore it can be expected that at lower temperatures this modification should predominantly crystallize.
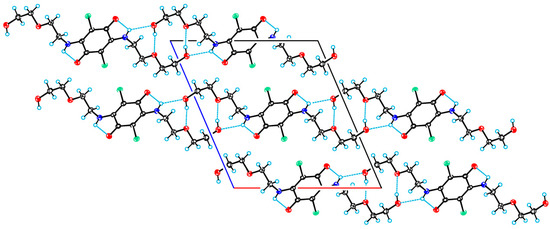
Figure 6.
A projection of the crystal structure of 4-II viewed along the monoclinic axis. C, Cl, N, and O atoms are represented by grey, green, violet, and red ellipsoids, respectively. The hydrogens are represented by circles of arbitrary radii. Hydrogen bonds are shown as blue dashed lines.
The molecular chains (Figure 7) of crystalline from 4-II are formed along the hydrogen-bonded motifs, where quinone rings are located parallel to each other.
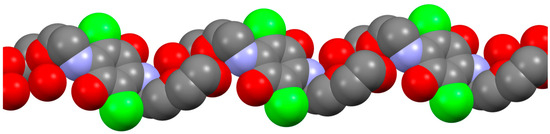
Figure 7.
Molecular chains of crystallographic form 4-II. Color map: C, grey; Cl, green; N, violet; O, red. For the sake of clarity, all hydrogen atoms were omitted.
Figure 8 shows a perspective view of the content of the asymmetric unit for compound 5. The asymmetric unit consists of four molecules, one of which (molecule D) is disordered.
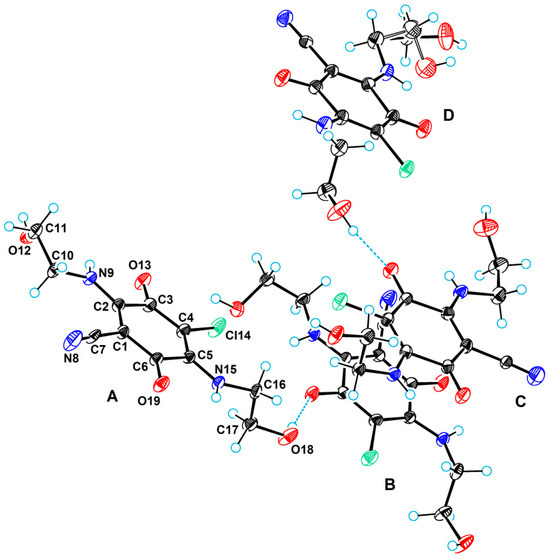
Figure 8.
A perspective view of the independent molecules 5 in the asymmetric unit with labels of the molecules (A, B, C, and D) and atomic numbering scheme. C, Cl, N, and O atoms are represented by grey, green, blue, and red ellipsoids, respectively. The hydrogens are represented by circles of arbitrary radii.
The large number of crystallographically independent molecules in the asymmetric unit is a consequence of the fact that the compound belongs to a conformationally rich class due to the presence of (2-hydroxyethyl)amino groups. All four of these molecules differ in conformation. Table 4 shows the values of selected torsion angles characterizing the molecular conformation.

Table 4.
Values of selected torsion angles in molecules 5 (in °).
Despite sharing the same short sidechain with compound 3, compound 5 does not form centrosymmetric dimers. Instead, crystal structure of compound 5 is characterized by layers formed through the inclusion of sidechains in an extensive system of intermolecular hydrogen bonds of OH···O, OH···Cl and NH···N types (Figure 9).
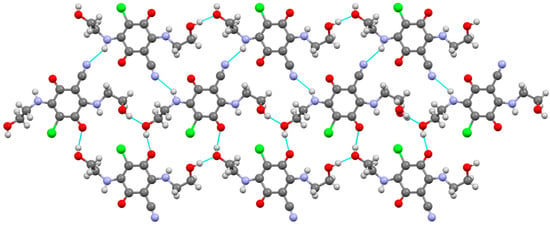
Figure 9.
Molecular packing in the crystal of compound 5. C, H, Cl, N, and O atoms are represented by grey, light grey, green, violet, and red ellipsoids, respectively. H-bonds are dotted lines colored blue.
Table 5 lists the main geometrical parameters of these bonds. By means of these hydrogen bonds, double molecular layers (Figure S11) are formed in the crystal structure, perpendicular to the monoclinic axis. All molecular layers in the crystal are related by symmetry operations, which are generated by the screw axis of symmetry 21.

Table 5.
Hydrogen-bond geometry for 5.
To compare the intermolecular interactions in crystal structures, CrystalExplorer [26] software (version 21.5) was utilized and normalized contact distances (dnorm), which are calculated relative to van der Waals radii, are mapped onto the Hirshfeld surfaces (Figures S12–S18). The quantitative analysis utilizes fingerprint plots that are created as two-dimensional histograms by collecting data points from every surface spot, using the internal distance (di) and external distance (de) as coordinates [27].
Two-dimensional fingerprint plots in crystal of compounds 3–5 are shown in Figure 10. In all cases the fingerprint plots exhibit two pronounced spikes, which indicate strong O···H bonding. An additional spike, corresponding to H···H interactions, is observed in the case of compound 4-I and all molecules of compound 5.
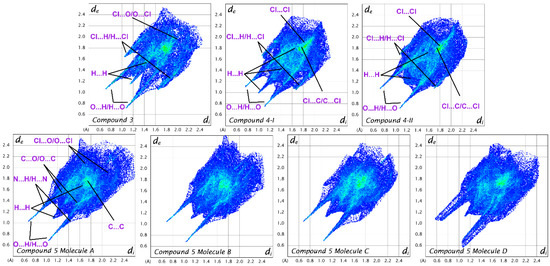
Figure 10.
Two-dimensional fingerprint plots in the crystals of compounds 3–5. In the case of compound 5, the interactions are shown only for molecule (A), as the interactions involving the other molecules (B–D) are similar.
Figure 11 represents the contribution (in %) of each contact type to the crystal packing of compounds 3–5. Overall, H···H and strong O···H interactions collectively constitute more than 50% of all intermolecular contacts. There is higher proportion of H···H interactions in compounds 4-I and 4-II which can be attributed to the long sidechains and additional methylene groups.
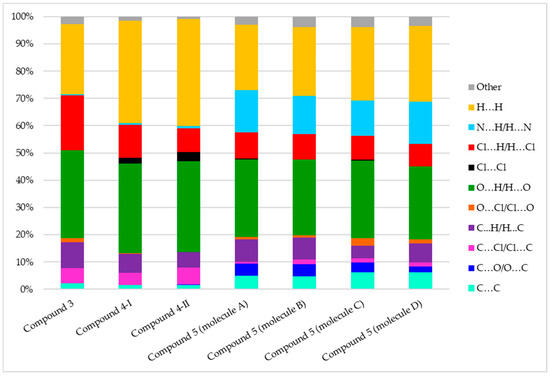
Figure 11.
Population of close contacts of compounds 3–5 in the crystal packing.
Compound 5 exhibits fewer O···H contacts (26.7–28.5%) compared to compounds 3 and 4 (32.3–33.4%). Conversely, strong N···H contacts were found in all four molecules of compound 5 (12.9–15.5%) due to the presence of the CN substituent; however, N···H contacts in compounds 3 and 4 were negligible (less than 1%). Interestingly, compound 3 showed Cl···H interactions (20%), a percentage higher than that observed for the other compounds, although Cl-Cl interaction (around 3%) was found in the crystal packing of compounds 4-I and 4-II.
Approximately 5% of C···C contacts and minimal C···O interactions were detected for all molecules of compound 5. These contacts are associated with π-π stacking interactions, which result from the layered crystal structure. For example, the distance between the quinone rings of molecules B and C is 3.262 Å.
Compounds 3–5 are assessed by comparing their crystal data and supramolecular architecture with six related, symmetrically diaminosubstituted 1,4-benzoquinones (7a–f) (Figure 12): 2,5-bis(2-propylamino)-1,4-benzoquinone (7a) [28], 2,5-bis(cyclohexylamino)-1,4-benzoquinone (7b) [28], 2,5-bis(3-methylanilino)-1,4-benzoquinone (7c) [28], (S,S)-2,5-difluoro-3,6-bis((1-phenylethyl)amino)-1,4-benzoquinone (7d) [8], (S,S)-2-chloro-5-cyano-3,6-bis((1-phenylethyl)amino)-1,4-benzoquinone (7e) [8], and 2,5-diamino-3,6-dichloro-1,4-benzoquinone (7f) [29].
Figure 12.
Structure of compounds 7a–f with numbering of quinone core positions. The structures are deposited at CCDC under deposition numbers 607637 (7a), 610155 (7b), 610156 (7c), 2077251 (7d), 2077251 (7e), 1108900 (7f).
Compounds 3–5 feature a 2-hydroxyethylamino or 2-(2-hydroxyethoxy)ethylamino substituents, while compounds 7a–e contain simple alkyl or aryl amino groups. Both series of compounds (3–5 and 7a–f) exist in the solid state as coupled polymethines. This is evidenced by the equalization of bonds within the O=C1-C2=C3-N fragment. The two single carbon-carbon bonds (C3-C4 and C1-C6) connecting the polymethine fragments exhibit similar lengths in both series of compounds: 1.52(8)–1.53(9) Å for compounds 3–5 and 1.50(9)–1.52(3) Å for 7a–f. Analysis of the torsion angles in the O=C1-C6-N and O=C4-C3-N fragments of compounds 3–5 (0.07–6.71°) and 7a–f (0.42–3.68°) revealed slight deviations from planarity for the benzoquinone core in both series. These structural similarities confirm that the core electronic structure is maintained across both sets of compounds, regardless of the substituents’ structure.
The dominant structural motif for compounds 7a–f is the formation of dimeric supramolecular synthons, , achieved via intermolecular N-H…O=C hydrogen bonding between the amino and carbonyl groups of two molecules. Despite the similar core molecular structures, the intermolecular interactions and resulting supramolecular architecture of compounds 3–5 are different. This distinction arises due to the presence of the 2-hydroxyethylamino or 2-(2-hydroxyethoxy)ethylamino groups, which contain hydrogen bond donor and acceptor sites. Consequently, intermolecular interactions among the side chains of compounds 3–5 led to the formation of molecular chains.
3.3. UV-Vis Studies
We first investigated the UV-Vis spectra of compounds 3–6 in polar solvent (DMF), revealing a weak absorption band in the region of 450–650 nm (Table 6). The addition of various amines (sec-butylamine, diethylamine, and butane-1,4-diamine) had practically no effect on the absorption of compounds 3–6 in this region (Figures S19–S22). Next, we tested the effect on the absorption band produced by the addition of Cu2+ or Zn2+ ions (added as their chloride salts) to the solutions of compounds 3–6. Despite the presence of flexible side chains in the structure of quinones 3–6 with potential metal coordination sites, no pronounced effect on absorption was observed. This can be explained by the stability of the coupled polymethine structure that is responsible for the absorption in the 450–600 nm region. Finally, metal ion and amine were added to the solution of compound of interest (3–6).

Table 6.
Data from UV-Vis spectra of compounds 3–6 in DMF/MeOH solutions with or without additives.
Analysis of the obtained data revealed a universal increase in absorbance intensity across the 450–600 nm region upon the addition of M2+ (Cu2+ or Zn2+) ions and amine to the solution of compounds 3–6 (Figure 13). For compounds 4 and 6 (possessing long side chains (R = (CH2)2O(CH2)2OH)), the addition of Cu2+ ions and butane-1,4-diamine resulted in a bathochromic shift in the UV-Vis spectra relative to the initial quinone. The addition of Zn2+ ions and amines induced a pronounced increase in absorption intensity as well as a hypsochromic shift relative to compound 4 solution. At the same time, absorption intensity of quinone 6 was low upon addition of Zn2+ ions and amines. Quinones 3 and 5 with short sidechains (R = (CH2)2OH) differ in the halide/pseudohalide at the 2-position (Cl or CN). In the case of quinone 3, hypsochromic shift (62 nm) was observed upon addition of Zn2+/sec-butylamine, and bathochromic shift (22 nm) after the addition of Cu2+/butane-1,4-diamine. The addition of Zn2+ and butane-1,4-diamine to the solution of compound 5 resulted in a hypsochromic shift of 22 nm.
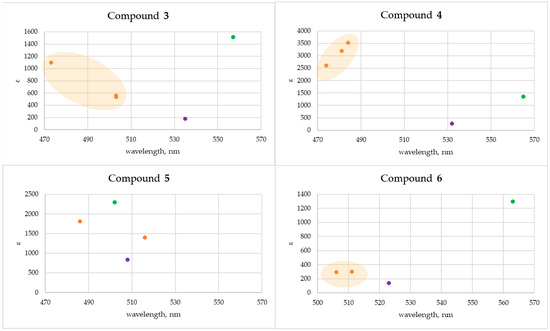
Figure 13.
Graphical plots of UV-Vis spectral data for interaction between compounds 3–6 and Zn2+ ions (orange color) or Cu2+ ions (green color) in a solution. The initial compound is designated by purple.
It should be noted that fast precipitation (within a few minutes) occurs upon addition of Cu2+ ions and amine to solutions of all compounds, whereas addition of Zn2+ ions and amines leads to precipitate formation over a few hours.
4. Conclusions
Single crystal X-ray diffraction data for three 1,4-benzoquinone derivatives 3–5 with flexible side chains were obtained. Compounds exist as quadrupole meropolymethines and consist of two fragments with equalized bonds, which are connected by two long single bonds. Additionally, the solid-state structure is characterized by an extensive network of intra- and intermolecular interactions. Compounds 3–6 were tested on optical response towards amines including representative of biogenic amines (butane-1,4-diamine). Analysis of UV-Vis data revealed that the optical response can be obtained from a combination of the amine and the metal ion, which is a promising result for further investigations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15110986/s1, Figures S1–S8: NMR spectroscopy data; Figures S9–S11: additional X-ray crystallography data; Figures S12–S18: Hirshfeld surfaces for compounds 3–5; Figures S19–S22: additional UV-Vis spectroscopy data.
Author Contributions
Conceptualization, N.B.; formal analysis, A.G. and S.B.; investigation, S.B., A.G. and S.Z.; writing—original draft preparation, S.B. and N.B.; writing—review and editing, N.B. and A.G.; visualization, A.G. and S.B.; supervision, N.B.; project administration, N.B.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by research and development grant No RTU-PA-2024/1-0028 under the EU Recovery and Resilience Facility funded project No. 5.2.1.1.i.0/2/24/I/CFLA/003 “Implementation of consolidation and management changes at Riga Technical University, Liepaja University, Rezekne Academy of Technology, Latvian Maritime Academy and Liepaja Maritime College for the progress towards excellence in higher education, science, and innovation”.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| NMR | Nuclear Magnetic Resonance |
| MeCN | acetonitrile |
| MeOH | methanol |
| MOF | Metal–organic framework |
| THF | Tetrahydrofuran |
| UV-Vis | Ultraviolet–visible spectroscopy |
References
- Mao, R.; Guo, J.; Bie, L.; Liu, L.; Gao, J. Tunneling Mechanisms of Quinones in Photosynthetic Reaction Center–Light Harvesting 1 Supercomplexes. Small Sci. 2024, 4, 2400188. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, J.; Kaszuba, K.; Minhas, G.S.; Baradaran, R.; Tambalo, M.; Gallagher, D.T.; Sazanov, L.A. Key role of quinone in the mechanism of respiratory complex I. Nat. Commun. 2020, 11, 4135. [Google Scholar] [CrossRef]
- Franza, T.; Gaudu, P. Quinones: More than electron shuttles. Res. Microbiol. 2022, 173, 103953. [Google Scholar] [CrossRef]
- Yan, T.; Nisar, M.F.; Hu, X.; Chang, J.; Wang, Y.; Wu, Y.; Liu, Z.; Cai, Y.; Jia, J.; Xiao, Y.; et al. Pyrroloquinoline Quinone (PQQ): Its impact on human health and potential benefits. Curr. Res. Food Sci. 2024, 9, 100889. [Google Scholar] [CrossRef]
- Brazeau, B.J.; Johnson, B.J.; Wilmot, C.M. Copper-containing amine oxidases. Biogenesis and catalysis; a structural perspective. Arch. Biochem. Biophys. 2004, 428, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. European Journal of Medicinal Chemistry Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Formen, J.S.S.K.; Wolf, C. Chiroptical Switching and Quantitative Chirality Sensing with (Pseudo)halogenated Quinones. Angew. Chem. Int. Ed. 2021, 60, 27031–27038. [Google Scholar] [CrossRef] [PubMed]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Waste Biomass Valorization 2021, 12, 6339–6374. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Hasan, F.; Mahanta, V.; Abdelazeez, A.A.A. Quinones for Aqueous Organic Redox Flow Battery: A Prospective on Redox Potential, Solubility, and Stability. Adv. Mater. Interfaces 2023, 10, 2300268. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, L.; Shen, J.; Gu, T.; Yang, Y.; Ji, S.; Zhu, M.; Liu, J. Raising the Redox Potential in a Quinone-Based Positive Organic Cathode via Space Charge Modulation. ACS Energy Lett. 2025, 10, 1107–1116. [Google Scholar] [CrossRef]
- Gou, Y.; Liu, N.; Yu, P.; Zhang, J.; Peng, J.; Han, J.; Huang, Y.; Fang, C. Activating Redox Chemistry of Quinones for High Energy Density Aqueous Sodium-Ion Batteries. J. Am. Chem. Soc. 2025, 147, 4993–5003. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, H.-Y.; Lin, L.; Yang, X.; Wu, W.; Sun, X. A high capacity small molecule quinone cathode for rechargeable aqueous zinc-organic batteries. Nat. Commun. 2021, 12, 4424. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.V.; Zhang, L.; Zhang, Q.; Liu, T.L. A strategic high yield synthesis of 2,5-dihydroxy-1,4-benzoquinone Based MOFs. Inorg. Chem. 2019, 58, 10756–10760. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Schweinfurth, D.; Deibel, N.; Weisser, F. Functional metal complexes based on bridging “imino”-quinonoid ligands. Coord. Chem. Rev. 2015, 293–294, 250–262. [Google Scholar] [CrossRef]
- Dähne, S.; Leupold, D. Coupling Principles in Organic Dyes. Angew. Chem. Int. Ed. Engl. 1966, 5, 984–993. [Google Scholar] [CrossRef]
- Mourot, B.; Jacquemin, D.; Siri, O.; Pascal, S. Coupled Polymethine Dyes: Six Decades of Discoveries. Chem. Rec. 2024, 24, e202400183. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Wolfe, K.M.; Harding, C.R.; Welch, G.C. Biogenic amine sensors using organic π-conjugated materials as active sensing components and their commercialization potential. J. Mater. Chem. C 2023, 11, 9749–9767. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; van der Lee, A. Symmetry determination following structure solution in P 1. J. Appl. Crystallogr. 2008, 41, 975–984. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A computer program for topological analysis of discrete electron densities. J. Appl. Crystallogr. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Spackman, M.A.; Mckinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Bayen, S.; Barooah, N.; Sarma, R.J.; Sen, T.K.; Karmakar, A.; Baruah, J.B. Synthesis, structure and electrochemical properties of 2,5-bis(alkyl/arylamino)1,4-benzoquinones and 2-arylamino-1,4-naphthoquinones. Dye. Pigment. 2007, 75, 770–775. [Google Scholar] [CrossRef]
- Kulpe, S. Chinofarbstoffe. I. Die Molekül- und Kristallstruktur des 3,6-Dichlor-2,5-diamino-1,4-benzochinons. Acta Cryst. 1969, B25, 1411–1417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).