Influence of the Etching Material Deposition Rate and Annealing Time on Nanohole Morphology Etched into InP/In0.52Al0.48As Layers via Local Droplet Epitaxy
Abstract
1. Introduction
2. Experimental Details
3. Results
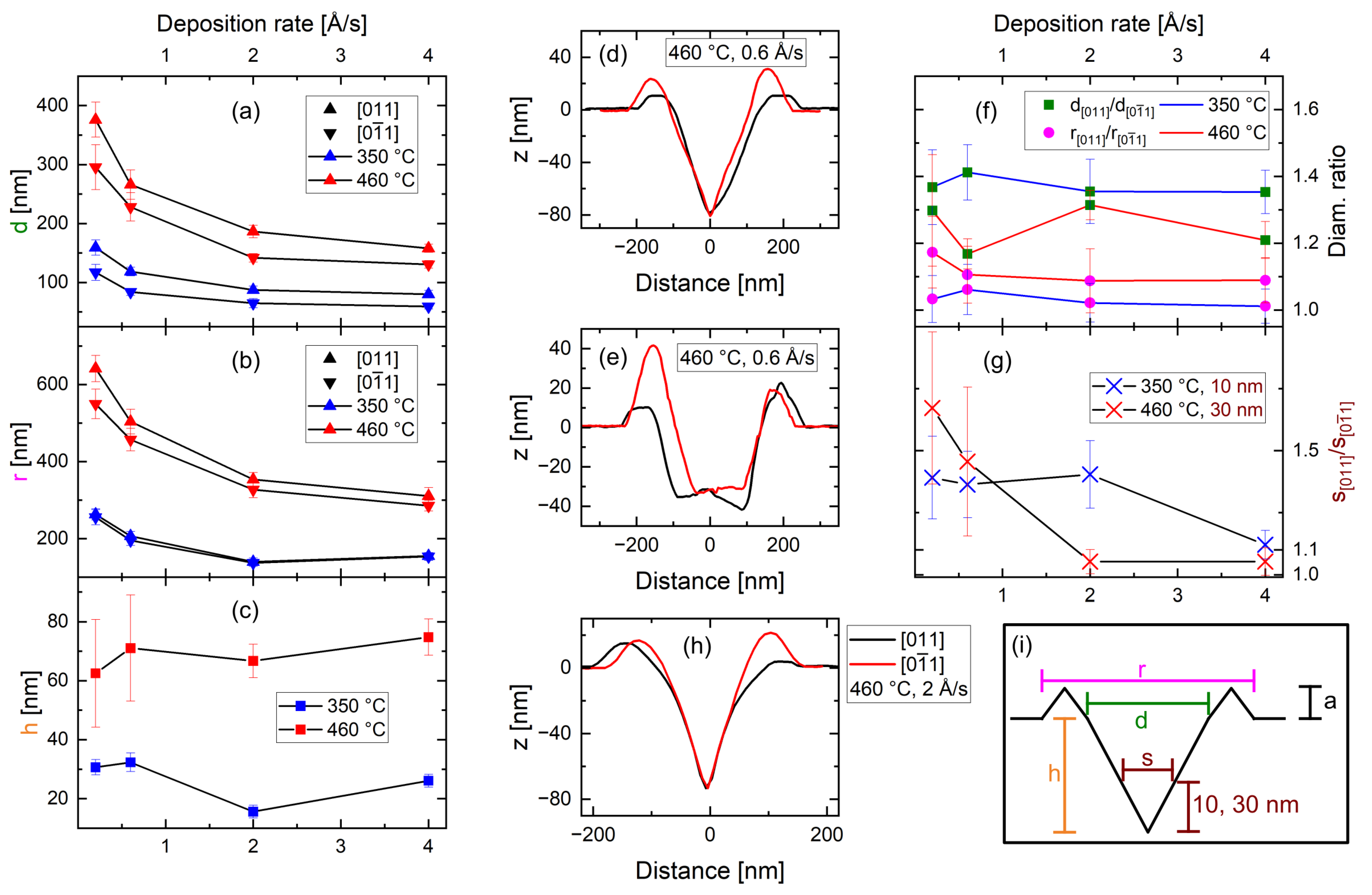
3.1. Etching Material Deposition Rate
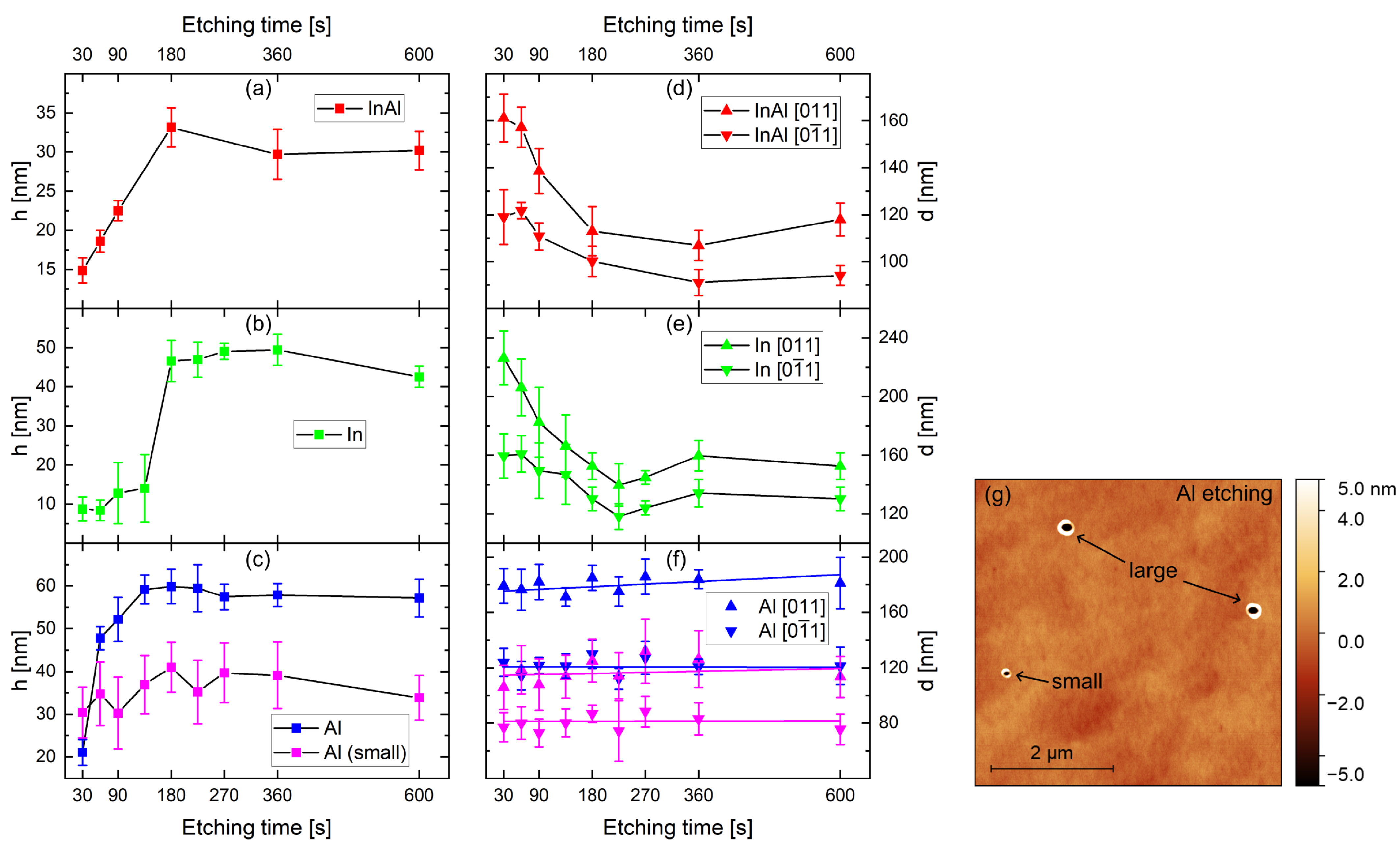
3.2. Etching Time
4. Discussion
4.1. Phase I—Drilling and Excavation
4.2. Phase II—Refilling
4.3. Al Droplets
4.4. In and InAl Droplets
5. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDE | Local droplet etching |
| QD | Quantum dot |
| MBE | Molecular beam epitaxy |
| AFM | Atomic force microscopy |
| HRXRD | High-resolution X-ray diffractometry |
| SEM | Scanning electron microscopy |
| Ga | Gallium |
| As | Arsenic |
| In | Indium |
| Al | Aluminum |
| P | Phosphor |
References
- Jiang, L.; Taylor, J.M.; Nemoto, K.; Munro, W.J.; Van Meter, R.; Lukin, M.D. Quantum repeater with encoding. Phys. Rev. A 2009, 79, 032325. [Google Scholar] [CrossRef]
- Senellart, P.; Solomon, G.; White, A. High-performance semiconductor quantum-dot single-photon sources. Nat. Nanotechnol. 2017, 12, 1026–1039. [Google Scholar] [CrossRef]
- Basso Basset, F.; Valeri, M.; Roccia, E.; Muredda, V.; Poderini, D.; Neuwirth, J.; Spagnolo, N.; Rota, M.B.; Carvacho, G.; Sciarrino, F.; et al. Quantum key distribution with entangled photons generated on demand by a quantum dot. Sci. Adv. 2021, 7, eabe6379. [Google Scholar] [CrossRef]
- Hopfmann, C.; Nie, W.; Sharma, N.L.; Weigelt, C.; Ding, F.; Schmidt, O.G. Maximally entangled and gigahertz-clocked on-demand photon pair source. Phys. Rev. B 2021, 103, 075413. [Google Scholar] [CrossRef]
- da Silva, S.F.C.; Undeutsch, G.; Lehner, B.; Manna, S.; Krieger, T.M.; Reindl, M.; Schimpf, C.; Trotta, R.; Rastelli, A. GaAs quantum dots grown by droplet etching epitaxy as quantum light sources. Appl. Phys. Lett. 2021, 119, 120502. [Google Scholar] [CrossRef]
- Huber, D.; Reindl, M.; Huo, Y.; Huang, H.; Wildmann, J.S.; Schmidt, O.G.; Rastelli, A.; Trotta, R. Highly indistinguishable and strongly entangled photons from symmetric GaAs quantum dots. Nat. Commun. 2017, 8, 15506. [Google Scholar] [CrossRef]
- Keil, R.; Zopf, M.; Chen, Y.; Höfer, B.; Zhang, J.; Ding, F.; Schmidt, O.G. Solid-state ensemble of highly entangled photon sources at rubidium atomic transitions. Nat. Commun. 2017, 8, 15501. [Google Scholar] [CrossRef] [PubMed]
- Hilska, J.; Chellu, A.; Hakkarainen, T. Nanohole Etching in AlGaSb with Gallium Droplets. Cryst. Growth Des. 2021, 21, 1917–1923. [Google Scholar] [CrossRef]
- Deutsch, D.; Buchholz, C.; Zolatanosha, V.; Jöns, K.D.; Reuter, D. Telecom C-band photon emission from (In, Ga)As quantum dots generated by filling nanoholes in In0.52Al0.48As layers. AIP Adv. 2023, 13, 055009. [Google Scholar] [CrossRef]
- Deutsch, D.; Zolatanosha, V.; Reuter, D. Local droplet etching with In, Al and InAl in In0.52Al0.48As layers for generation of quantum dots emitting in the optical C-band. J. Cryst. Growth 2025, 668, 128247. [Google Scholar] [CrossRef]
- K-Space Associates, Inc. Available online: https://k-space.com/product/bandit/ (accessed on 10 September 2025).
- Galiev, G.B.; Vasiliev, A.L.; Imamov, R.M.; Klimov, E.A.; Maltsev, P.P.; Pushkarev, S.S.; Presniakov, M.Y.; Trunkin, I.N. Structural and electrical properties of InAlAs/InGaAs/InAlAs HEMT heterostructures on InP substrates with InAs inserts in quantum well. Crystallogr. Rep. 2014, 59, 900–907. [Google Scholar] [CrossRef]
- Heyn, C.; Sonnenberg, D.; Hansen, W. Local Droplet Etching: Self-assembled Nanoholes for Quantum Dots and Nanopillars. In Nanodroplets; Springer: New York, NY, USA, 2013; pp. 363–384. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Auler, N.; Deutsch, D.; Reuter, D. Statistical Analysis of the Spatial Distribution of InAl Droplet-Etched Nanoholes in In0.52Al0.48As Layers. Crystals 2025, 15, 770. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Ma, C.; Wang, Y.; Brechtken, B.; Haug, R.J.; Rugeramigabo, E.P.; Zopf, M.; Ding, F. Local droplet etching on InAlAs/InP surfaces with InAl droplets. AIP Adv. 2022, 12, 055302. [Google Scholar] [CrossRef]
- Vonk, V.; Slobodskyy, T.; Keller, T.F.; Richard, M.I.; Fernández, S.; Schulli, T.; Heyn, C.; Hansen, W.; Stierle, A. Faceting of local droplet-etched nanoholes in AlGaAs. Phys. Rev. Mater. 2018, 2, 106001. [Google Scholar] [CrossRef]
- Huo, Y.H.; Witek, B.J.; Kumar, S.; Cardenas, J.R.; Zhang, J.X.; Akopian, N.; Singh, R.; Zallo, E.; Grifone, R.; Kriegner, D.; et al. A light-hole exciton in a quantum dot. Nat. Phys. 2013, 10, 46–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Grünewald, L.; Cao, X.; Abdelbarey, D.; Zheng, X.; Rugeramigabo, E.P.; Verbeeck, J.; Zopf, M.; Ding, F. Unveiling the 3D Morphology of Epitaxial GaAs/AlGaAs Quantum Dots. Nano Lett. 2024, 24, 10106–10113. [Google Scholar] [CrossRef]
- Fuster, D.; González, Y.; González, L. Fundamental role of arsenic flux in nanohole formation by Ga droplet etching on GaAs(001). Nanoscale Res. Lett. 2014, 9, 309. [Google Scholar] [CrossRef]
- Heyn, C. Kinetic model of local droplet etching. Phys. Rev. B 2011, 83, 165302. [Google Scholar] [CrossRef]
- Heyn, C.; Bartsch, T.; Sanguinetti, S.; Jesson, D.; Hansen, W. Dynamics of mass transport during nanohole drilling by local droplet etching. Nanoscale Res. Lett. 2015, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Wang, Z.M.; Liang, B.; Lee, J.; Kim, E.S.; Salamo, G.J. Origin of nanohole formation by etching based on droplet epitaxy. Nanoscale 2014, 6, 2675. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deutsch, D.; Reuter, D. Influence of the Etching Material Deposition Rate and Annealing Time on Nanohole Morphology Etched into InP/In0.52Al0.48As Layers via Local Droplet Epitaxy. Crystals 2025, 15, 913. https://doi.org/10.3390/cryst15110913
Deutsch D, Reuter D. Influence of the Etching Material Deposition Rate and Annealing Time on Nanohole Morphology Etched into InP/In0.52Al0.48As Layers via Local Droplet Epitaxy. Crystals. 2025; 15(11):913. https://doi.org/10.3390/cryst15110913
Chicago/Turabian StyleDeutsch, Dennis, and Dirk Reuter. 2025. "Influence of the Etching Material Deposition Rate and Annealing Time on Nanohole Morphology Etched into InP/In0.52Al0.48As Layers via Local Droplet Epitaxy" Crystals 15, no. 11: 913. https://doi.org/10.3390/cryst15110913
APA StyleDeutsch, D., & Reuter, D. (2025). Influence of the Etching Material Deposition Rate and Annealing Time on Nanohole Morphology Etched into InP/In0.52Al0.48As Layers via Local Droplet Epitaxy. Crystals, 15(11), 913. https://doi.org/10.3390/cryst15110913





