Molecular Dynamics Study on the Molar Ratio-Dependent Interaction Regulation Mechanisms in CL-20/FOX-7 Energetic Cocrystal Explosives
Abstract
1. Introduction
2. Computational Methods
3. Results
3.1. Cohesive Energy Density Comparison
3.2. Mechanical Properties
3.3. Detonation Performance
3.4. Performance Comparison on Alternative Crystal Facets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolton, O.; Matzger, A. Improved compatibility and smart material functionality realized in an energetic cocrystal. Angew. Chem. Int. Ed. 2011, 38, 8960–8963. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Li, F. Thermal behavior and decomposition mechanism of ammonium perchlorate and ammonium nitrate in the presence of nanometer triamino guanidine nitrate. ACS Omega 2019, 4, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Luo, P.; Xiao, L.; Xin, Y.; Zhang, G.; Hu, Y.; Yang, J.; Gao, H.; Zhao, F.; Jiang, W.; et al. New insights in nano-copper chromite catalyzing ultrafine AP: Evaluation of dispersity and mixing uniformity. Def. Technol. 2023, 32, 120–133. [Google Scholar] [CrossRef]
- Song, X.; Gao, X.; Kou, Y.; Yu, Z.; Wang, Y.; An, C.; Li, F. Thermolysis and sensitivities of solid propellants using characterized nano oxidizers involving energy performance evaluation. FirePhysChem 2022, 2, 323–333. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, Y.; Zhang, J.; Guo, K. Song Iron/aluminum nanocomposites prepared by one-step reduction method and their effects on thermal decomposition of AP and AN. Def. Technol. 2023, 22, 74–87. [Google Scholar] [CrossRef]
- Yu, Z.; Song, X.; Kou, Y.; Wang, Y.C. An Characterization and testing for lowest eutectic mixture of TNBA/DNTF. Propellants Explos. Pyrotech. 2023, 48, 1–13. [Google Scholar] [CrossRef]
- Song, X.; Kou, Y.; Wang, Y.; Cheng, Z.; Li, F. Preparation and properties of lowest eutectic mixture MTNP/TNAZ. J. Energ. Mater. 2022, 40, 119–135. [Google Scholar] [CrossRef]
- Kou, Y.; Song, X.; Guo, K.; Cheng, Z.; Wang, Y. Characterization, thermolysis, and energetic properties of an MTNP/PETN eutectic prepared via the solvent/anti-solvent method. Propellants Explos. Pyrotech. 2020, 46, 299–308. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.; Pagoria, P.; Matzger, A. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20: HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Mao, J.; Wang, B.; Chen, Y.; Fu, J.; Tian, X.; Ye, B. Molecular dynamics simulation of CL-20/DNDAP cocrystalbased PBXs. J. Mol. Model. 2023, 29, 199. [Google Scholar] [CrossRef]
- Pang, W.; Wang, K.; Zhang, W.; LTD, L.; Fan, X.; Li, J. CL-20-based cocrystal energetic materials: Simulation, preparation and performance. Molecules 2020, 25, 4311. [Google Scholar] [CrossRef]
- Millar, D.; Maynard-Casely, H.; Allan, D.; Cumming, A.; Lennie, A.; Mackay, A.; Oswald, I.; Tang, C.; Pulham, C. Crystal engineering of energetic materials: Co-crystals of CL-20. Crys. Eng. Commun. 2012, 10, 3742–3749. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Huang, H.; Zhou, X.; Li, J.; Nie, F. Preparation and performance of a HNIW/TNT cocrystal explosive. Propellants Explos. Pyrotech. 2013, 4, 495–501. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Wang, X.; Xu, J.; Liu, Y.; Liu, X.; Huang, H.; Sun, J. Crystal structure and explosive performance of a new CL-20/caprolactam cocrystal. J. Mol. Struct. 2013, 1048, 267–273. [Google Scholar] [CrossRef]
- Agrawal, J. Recent trends in high-energy materials. Prog. Energy Combust. Sci. 1998, 24, 1–30. [Google Scholar] [CrossRef]
- Shu, Y.J.; Wu, Z.K.; Liu, N.; Ding, X.Y.; Wu, M.J.; Wang, K.; Lu, Y.Y. Crystal control and cocrystal formation: Important route of modification research of energetic materials. Chin. J. Explos. Propellants 2015, 38, 1–9. [Google Scholar]
- Landenberger, K.B.; Matzger, A.J. Cocrystal engineering of a prototype energetic material: Supramolecular chemistry of 2,4,6-trinitrotoluene. Cryst. Growth Des. 2010, 10, 5341–5345. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Song, Z.; Song, D.; An, C.-W.; Wang, J.Y.; Zhang, J.-L. Preparation of CL-20/DNT cocrystal explosive and study on its performance. Chin. J. Explos. Propellants 2016, 39, 23–27. [Google Scholar]
- Patil, V.B.; Yan, Q.-L.; Trzciński, W.A.; Bělina, P.; Shánělová, J.; Musil, T.; Zeman, S. Co-agglomerated crystals of cyclic nitramines with sterically crowded molecules. CrystEngComm 2022, 24, 7771–7785. [Google Scholar] [CrossRef]
- Patil, V.B.; Bělina, P.; Trzcinski, W.A.; Zeman, S. Co-agglomerated crystals of cyclic nitramines with the nitrogen rich 3, 6-bis(1H-1, 2, 3, 4-tetrazol-5-ylamino)-1,2,4,5-tetrazine (BTATz). Chem. Eng. J. 2024, 483, 149029. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhou, X.; Zhang, C.; Huang, H.; Li, J.; Nie, F. Characterization and properties of a novel energetic-energetic cocrystal explosive composed of HNIW and BTF. Cryst. Growth Des. 2012, 12, 5155–5158. [Google Scholar] [CrossRef]
- Patil, V.B.; Yan, Q.-L.; Trzciński, W.A.; Zeman, S. Co-agglomerated crystals of 2,2′,4,4′,6,6′-hexanitro-stilbene/-azobenzene with attractive nitramines. Chem. Eng. J. 2023, 457, 141200. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, L.; Wu, P.; Liu, Y.; Nie, F.; Huang, F. Huang Fabrication of RDX, HMX and CL-20 based microcapsules via in situ polymerization of melamine-formaldehyde resins with reduced sensitivity. Chem. Eng. J. 2015, 268, 60–66. [Google Scholar] [CrossRef]
- Nair, U.; Sivabalan, R.; Gore, G.; Geetha, M.; Asthana, S.; Singh, H. Hexanitrohexaazai-sowurtzitane(CL-20) and CL-20-based formulations. Combust. Explos. Shock Waves 2005, 41, 121–132. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Mao, X.; Li, Y. Research progress of FOX-7. New Technol. New Process 2023, 7–12. [Google Scholar]
- Liu, Q.; Xiao, J.; Zhang, J.; Zhou, F.; He, Z.; Xiao, H. Molecular dynamics simulation on CL-20/TNT cocrystal explosive. Chem. J. Chin. Univ. 2016, 37, 559–566. [Google Scholar]
- Wen, G. CL-20 Crystallization Study and Molecular Dynamics Simulation of CL-20/TATB Eutectic. Master’s Thesis, North University of China, Taiyuan, China, 2014. [Google Scholar]
- Wild, R.; Teipel, U. Characterization and explosive properties of FOX-7. In Proceedings of the 35th International Annua Conference of ICT, Karlsruhe, Germany, 29 June–2 July 2004. [Google Scholar]
- Fu, Q.; Shu, Y.; Huang, Y. Thermal Decomposition Mechanism of FOX-7. J. Solid Rocket Technol. 2010, 33, 77–80. [Google Scholar]
- Wu, Z.; Shu, Y.; Liu, N.; Ding, X.-Y.; Wu, M.-J.; Wang, K.; Wang, B.; Lu, Y.-Y. Molecular dynamics simulation of CL-20/FOX-7 eutectic. Chin. J. Explos. Propellants 2016, 39, 37–42. [Google Scholar]
- Hang, G.-Y.; Yu, W.-L.; Wang, T.; Wang, J.-T.; Li, Z. Molecular dynamics calculation on structures, stabilities, mechanical properties, and energy density of CL-20/FOX-7 cocrystal explosives. J. Mol. Model. 2017, 23, 362. [Google Scholar] [CrossRef]
- ACCELRYS Inc. Materials Studio20. 1[CP/CD]; Accelrys Inc.: San Diego, CA, USA, 2020. [Google Scholar]
- Sun, H. COMPASS: An ab initio force-field optimized for con densed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Andersen, H. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2374–2383. [Google Scholar] [CrossRef]
- Tosi, M. Cohesion of ionic solids in the Born model. Solid State Phys. 1964, 16, 1–120. [Google Scholar]
- Ewald, P. Evaluation of optical and electrostatic lattice potentials. Ann. Phys. 1921, 64, 253–287. [Google Scholar] [CrossRef]
- Sun, T. Molecular Dynamics Simulation Study on the Structure and Properties of CL-20 Cocrystal and Their Composite Materials. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2015. [Google Scholar]
- Ren, G. Preparation of Two Eutectic Explosives and Their Molecular Dynamics Simulation. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2020. [Google Scholar]
- Kamlet, M.; Jacobs, S. Chemistry of detonations. I. A simple method for calculating detonation properties of CHNO explosives. J. Chem. Phys. 1968, 48, 23–35. [Google Scholar] [CrossRef]
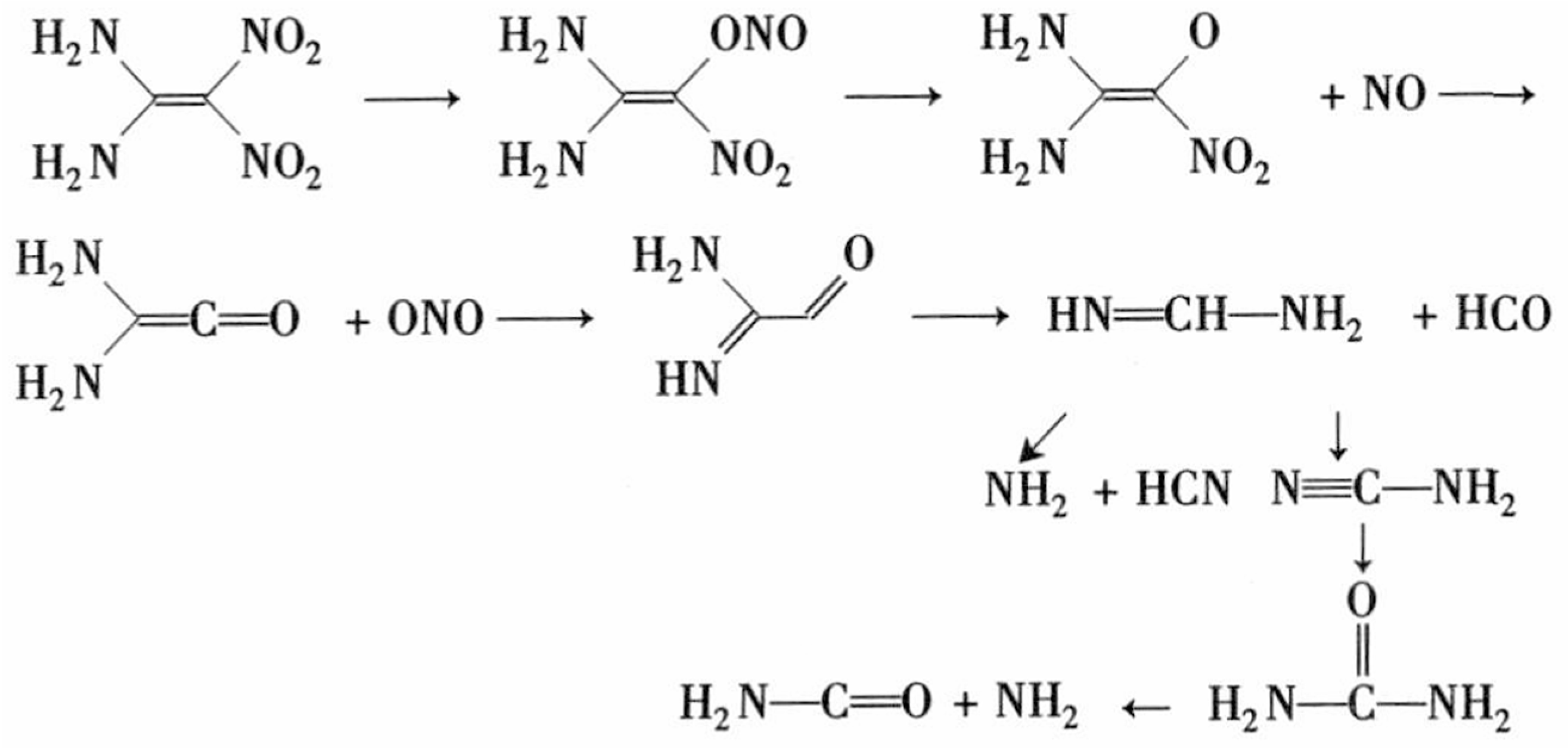

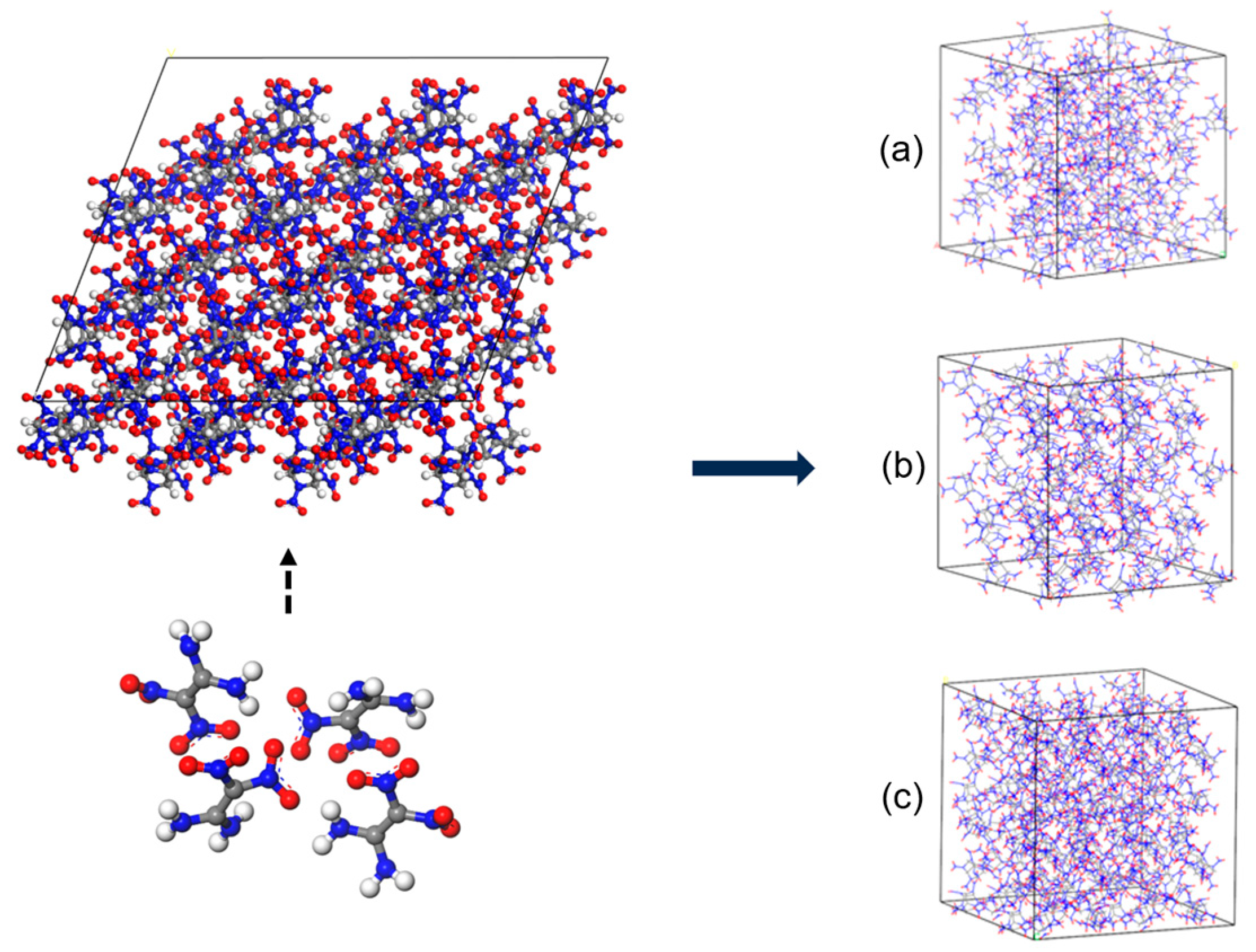
| Molar Ratio | Mass Percent (%) | Supercell Model | Total Number of Atoms |
|---|---|---|---|
| (CL-20:FOX-7) | |||
| 1:00 | 100 | 3 × 3 × 2 | 2592 |
| 8:01 | 72 | 3 × 3 × 2 | 2416 |
| 5:01 | 48 | 3 × 2 × 2 | 1552 |
| 3:01 | 64 | 4 × 2 × 2 | 1952 |
| 0:01 | 0 | 4 × 3 × 2 | 1344 |
| CED/kJ·cm−3 | van der Waals /kJ·cm−3 | Electrostatic/kJ·cm−3 | |
|---|---|---|---|
| CL-20 | 0.654 | 0.32 | 0.323 |
| FOX-7 | 1.208 | 0.537 | 0.657 |
| CL-20/FOX-7 (3:1) | 0.741 | 0.34 | 0.389 |
| CL-20/FOX-7 (5:1) | 0.773 | 0.329 | 0.432 |
| CL-20/FOX-7 (8:1) | 0.671 | 0.306 | 0.354 |
| E/GPa | K/GPa | G/GPa | C12–C44/GPa | γ | |
|---|---|---|---|---|---|
| CL-20 | 16.5 | 10.5 | 5.5 | 1.2 | 0.25 |
| FOX-7 | 5.616 | 5.082 | 2.134 | 2.3082 | 0.31 |
| CL-20/FOX-7 (3:1) | 8.562 | 6.264 | 3.365 | 0.6185 | 0.272 |
| CL-20/FOX-7 (5:1) | 7.405 | 5.024 | 2.952 | 1.6364 | 0.324 |
| CL-20/FOX-7 (8:1) | 6.14 | 4.612 | 2.402 | 0.1604 | 0.206 |
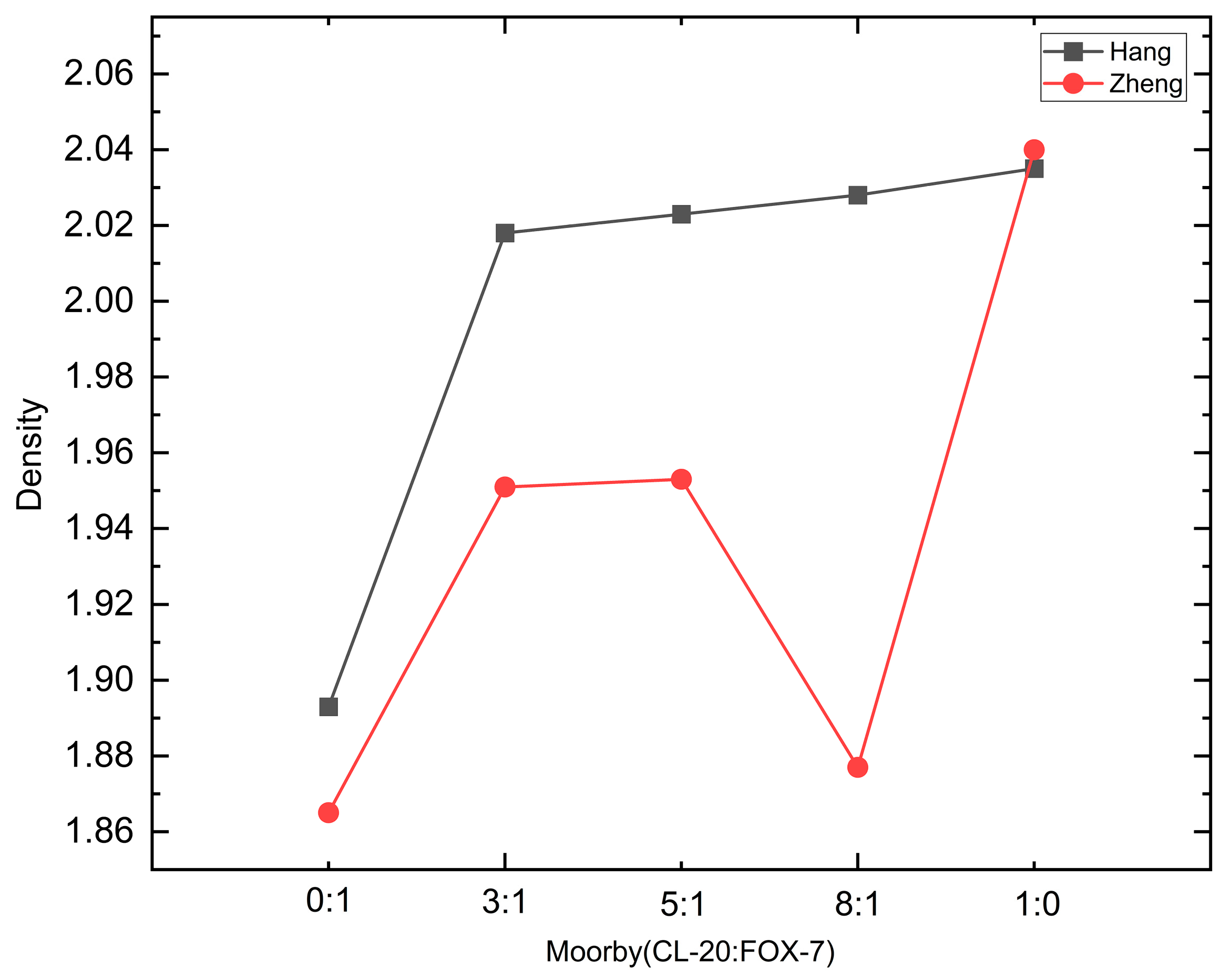
| Density/g·cm−3 | CL-20/% | Detonation/m·s−1 | Burst Pressure/GPa | |
|---|---|---|---|---|
| CL-20 | 2.04 | 100 | 9717 | 45.3 |
| FOX-7 | 1.865 | 0 | 8724 | 34.5 |
| CL-20/FOX-7 (3:1) | 1.951 | 89.75 | 9386.4 | 41.1 |
| CL-20/FOX-7 (5:1) | 1.953 | 92.1 | 9437.7 | 41.6 |
| CL-20/FOX-7 (8:1) | 1.877 | 95.34 | 9208.4 | 38.7 |
| Density/g·cm−3 | CED/kJ·cm−3 | van der Waals | Electrostatic | |
|---|---|---|---|---|
| CL-20 | 2.04 | 0.654 | 0.32 | 0.323 |
| FOX-7 | 1.865 | 1.208 | 0.537 | 0.657 |
| CL-20/FOX-7 (3:1) | 1.832 | 0.694 | 0.293 | 0.391 |
| CL-20/FOX-7 (5:1) | 1.867 | 0.654 | 0.293 | 0.391 |
| CL-20/FOX-7 (8:1) | 1.77 | 0.653 | 0.274 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Wang, Y.; Wang, T.; Li, S.; Luo, Y.; Liu, X.; Quan, K.; Zhang, S. Molecular Dynamics Study on the Molar Ratio-Dependent Interaction Regulation Mechanisms in CL-20/FOX-7 Energetic Cocrystal Explosives. Crystals 2025, 15, 912. https://doi.org/10.3390/cryst15110912
Zheng R, Wang Y, Wang T, Li S, Luo Y, Liu X, Quan K, Zhang S. Molecular Dynamics Study on the Molar Ratio-Dependent Interaction Regulation Mechanisms in CL-20/FOX-7 Energetic Cocrystal Explosives. Crystals. 2025; 15(11):912. https://doi.org/10.3390/cryst15110912
Chicago/Turabian StyleZheng, Ruikang, Yuling Wang, Tao Wang, Shuchang Li, Yibo Luo, Xingyu Liu, Kaizeng Quan, and Shusheng Zhang. 2025. "Molecular Dynamics Study on the Molar Ratio-Dependent Interaction Regulation Mechanisms in CL-20/FOX-7 Energetic Cocrystal Explosives" Crystals 15, no. 11: 912. https://doi.org/10.3390/cryst15110912
APA StyleZheng, R., Wang, Y., Wang, T., Li, S., Luo, Y., Liu, X., Quan, K., & Zhang, S. (2025). Molecular Dynamics Study on the Molar Ratio-Dependent Interaction Regulation Mechanisms in CL-20/FOX-7 Energetic Cocrystal Explosives. Crystals, 15(11), 912. https://doi.org/10.3390/cryst15110912







