Abstract
This study investigates the influence of Y2O3 content and sintering temperature on the microstructure, phase composition, and physico-mechanical properties of zirconia-based ceramics with Al2O3 addition. It was shown that varying the stabilizer content within 3.0–6.0 wt.% Y2O3 leads to observable changes in the phase composition and grain size, as well as affecting the density and microhardness of the material. The sintering conditions ensuring the required density and homogeneous microstructure at moderate temperatures were determined. This research is aimed at optimizing synthesis and heat treatment parameters to produce a dense material with minimal porosity and stable mechanical characteristics. The samples were shaped by uniaxial pressing and sintered in the temperature range of 1530–1570 °C. The structure and phase composition were analyzed using X-ray diffraction and scanning electron microscopy, while the physico-mechanical properties were evaluated by measuring apparent density, porosity, and microhardness. The sintering temperature of 1550 °C was identified as optimal for achieving the highest performance characteristics while maintaining structural homogeneity. The addition of Al2O3 contributes to a reduction in the sintering temperature without compromising the material’s properties.
1. Introduction
Despite extensive research on ZrO2–Y2O3 systems, quantitative data on the synergistic effect of Y2O3 content in the range of 3.0–6.0 wt.% and sintering temperature in the interval of 1530–1570 °C on the microstructure and properties of compositions containing a fixed small addition of Al2O3 (0.5 wt.%), synthesized by the chemical co-precipitation method, remain incomplete [1,2,3,4,5]. In particular, the threshold Y2O3 content required for the complete suppression of microcracking and the optimal sintering temperature regime that ensures maximum density without abnormal grain growth in this system still need to be clarified. In particular, the complex influence of Y2O3 content, sintering temperature, and their interrelationship on microcrack formation requires systematic investigation. A key objective is to identify the parameters at which a synergistic effect is achieved, including complete stabilization of the tetragonal phase, the formation of a fine-grained microstructure, and the relaxation of residual stresses, which collectively lead to the suppression of microcrack formation. The aim of this work is to establish these quantitative relationships in order to optimize the set of physical and mechanical properties of YSZ–Al2O3 ceramics.
Ceramics based on zirconium dioxide (ZrO2) are a promising material for various technical applications due to their high mechanical strength, resistance to thermal loads and corrosion resistance [6,7,8]. In pure form, ZrO2 exists in several crystalline modifications, but at high temperatures a phase transformation is observed, which is accompanied by significant volume expansion, which leads to the destruction of the material [9,10]. To stabilize the structure, oxides of rare earth metals are introduced, in particular Y2O3, which allows obtaining a tetragonal or cubic phase with improved performance characteristics. Additional introduction of aluminum oxide (Al2O3) helps to reduce the sintering temperature and improve the mechanical properties of the material [10,11,12].
One of the most important areas of zirconium ceramics research is phase composition control, which directly affects mechanical properties such as density, hardness and wear resistance. Modern research is aimed at reducing the sintering temperature of zirconium ceramics, since high temperatures lead to grain growth, which negatively affects its mechanical properties. One way to solve this problem is to introduce aluminum oxide (Al2O3), which prevents grain growth and improves the hardness and wear resistance of the material [10,13].
The mechanical properties of zirconium ceramics are highly dependent on the method of powder synthesis. Chemical coprecipitation enables the uniform distribution of stabilizing additives while allowing precise control over the granulometric composition of the powders, leading to improved homogeneity and enhanced mechanical performance [14,15]. The use of hydrothermal synthesis and sol–gel technology contributes significantly to refining the microstructure of the material [16,17,18].
Another important aspect is the influence of various alloying additives, such as rare earth oxides (CeO2, La2O3), on the structure and properties of zirconia ceramics. The addition of CeO2 enhances phase stability by promoting the retention of the tetragonal phase, which improves transformation toughening and increases the material’s ductility, thereby enhancing its fracture resistance and mechanical reliability [19]. The introduction of HfO2 enhances thermal stability and prevents microstructural degradation during prolonged exposure to high temperatures, making it particularly beneficial for applications requiring long-term thermal endurance [20].
Numerous studies show that the effect of sintering temperature on the final properties of zirconium ceramics requires careful selection of heat treatment modes. A temperature of 1500–1600 °C is optimal for obtaining a dense structure with high mechanical strength [21]. However, reducing the sintering temperature by introducing active additives remains an important task for reducing energy costs in the production of ceramic materials [22].
In addition to the well-known factors influencing the properties of zirconia-based ceramics stabilized with yttria—such as composition and sintering temperature—modern research pays significant attention to the fundamental strengthening mechanisms and the improvement of powder synthesis methods. The key phenomenon determining the high strength and fracture toughness of YSZ ceramics is transformation toughening, in which the martensitic phase transformation from the tetragonal to the monoclinic phase in the crack propagation zone absorbs energy and inhibits crack growth [23]. The efficiency of this mechanism directly depends on the stability of the tetragonal phase, its fraction in the material, and its ability to undergo a controlled transformation under stress, which makes phase composition control critically important [24].
The choice of powder synthesis method predetermines such key parameters as dispersity, homogeneity of component distribution, and sinterability. Comparative studies have shown that the co-precipitation method provides better reproducibility and control over particle size compared to the sol–gel technique and is more scalable than hydrothermal synthesis [7].
To achieve maximum density and optimal microstructure during sintering, precise control over the kinetics of densification and grain growth is essential. Optimization of thermal regimes, including the selection of optimal heating rates and isothermal holding times, makes it possible to decouple pore elimination from grain growth processes, which is a central task in the development of high-density fine-grained ceramics [8].
The obtained data are of great importance for the development of new zirconia-based ceramic compositions. In addition to traditional applications in mechanical engineering, medicine, and electronics, optimized YSZ-based materials show strong potential for use as radiation-resistant coatings in nuclear energy [25] and as matrices for luminescent materials in optoelectronics [26].
Despite the extensive research on zirconia-based systems stabilized with yttrium and aluminum oxides, the relationship between stabilizer content, densification kinetics, and microstructural evolution during chemical co-precipitation followed by sintering in the moderate temperature range (1530–1570 °C) remains insufficiently understood. The present study aims to clarify these interdependencies and identify the parameters that ensure the formation of a fine-grained, homogeneous, and defect-stable structure of zirconia ceramics with reduced energy consumption.
The scientific novelty of the research lies in the comprehensive analysis of phase stabilization processes and microstructural evolution during combined doping with Y2O3 and Al2O3, performed on materials synthesized via a unified chemical co-precipitation method under controlled pH and temperature conditions. This approach eliminated the influence of dispersion variability and enabled investigation of the effects of composition and thermal treatment alone. For the first time, quantitative relationships were obtained between Y2O3 content, grain size, and defect structure. It was demonstrated that the average grain size decreases (from 1.77 µm to 1.30 µm) and microcracks disappear as the Y2O3 content increases to 6.0 wt.%.
Additionally, it was found that sintering optimization at 1550 °C ensures the achievement of theoretical density while maintaining a fine-grained structure and reducing the thermal expansion coefficient (7.0–7.9 × 10−6 °C−1). The combined effects of Y3+-vacancy stabilization and Al2O3 grain-boundary pinning enable targeted control over grain growth kinetics and interfacial defect structure.
Thus, this study refines the understanding of structural stabilization mechanisms and sintering kinetics in ZrO2–Y2O3–Al2O3 systems, providing both fundamental insights into the behavior of partially stabilized solid solutions and practical guidance for the development of energy-efficient technologies for producing high-density ceramics with controlled thermomechanical characteristics.
The goal of this study is to investigate the effect of Y2O3 content (3–6 wt.%) and sintering temperature (1530–1570 °C) on the structure and properties of zirconium dioxide ceramics with the addition of 0.5 wt.% Al2O3, as well as to determine the optimal sintering parameters that provide high density, uniform microstructure, and minimized porosity of the material.
2. Materials and Methods
Initial powders were zirconium nitrate (Zr(NO3)4·H2O, CAS No. 13746-89-9, Wonaixi New Material Technology Co., Ltd., Leshan City, China), yttrium nitrate (Y(NO3)3·6H2O, CAS No. 13494-98-9, Xi’an Function Material Group Co., Ltd., Xi’an City, China) and aluminum nitrate (Al(NO3)3·9H2O, CAS No. 7784-27-2, Xi’an Function Material Group Co., Ltd., Xi’an City, China). Ammonia solution (NH4OH) was used as a precipitant.
The powder synthesis was carried out in a Kori BSF-5L reactor (Kori Instrument, Xingyang city, China) with a volume of 5 L. The precipitation temperature was 60 ± 2 °C, the pH of the solution was maintained in the range of 8.5–9.0 using an ammonia solution (25%). To ensure uniform precipitation, the rate of addition of the precipitating agent was 2 mL/min. The precipitation time was 4 h. To prevent particle agglomeration, the entire process took place with continuous stirring at a speed of 500 rpm. The resulting precipitate was decanted and dried in a muffle furnace (SNOL 400/12-VP, VNIIETO, Istra, Russia) at 110 °C for 12 h. After drying, annealing was performed at a temperature of 600 °C for 2 h to remove impurities and stabilize the phase composition. The process is schematically shown in Figure 1.
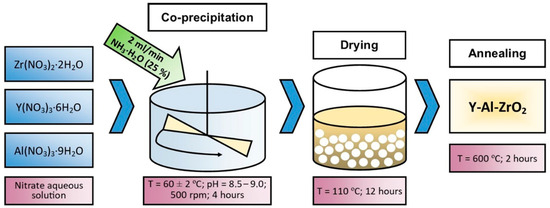
Figure 1.
Schematic diagram of the process for producing zirconium dioxide stabilized with yttrium oxide.
In order to study the properties of ceramics based on yttria-stabilized zirconium dioxide of composition 3–6 YSZ + 0.5 Al2O3, a series of samples were prepared by the chemical coprecipitation method (Table 1).

Table 1.
Chemical composition of synthesized ceramic samples in wt.%.
The synthesized powders were used to prepare a press mass with the addition of an organic binder based on a 10% solution of polyvinyl alcohol mixed with a plasticizer (glycerin), in a ratio of 7:3. The amount of organic binder introduced according to the formation indicators of press powder granules was 10 wt.%. The introduction of the binder and its subsequent granulation were carried out on a VLM 5 roller mixer (Versatile Equipments, Kolhapur, India).
The blanks were formed using the method of uniaxial semi-dry pressing on an ES0501 hydraulic press (EQFS, Reutov, Russia), the pressing force was 1000–1200 kg/cm2, holding at maximum pressure was 30 sec. The formed products had the following dimensions: diameter 14.0 mm, thickness 3.0–5.0 mm.
The burning of the organic binder was carried out in an oxidizing environment at 1000 °C using a chamber electric furnace SNOL 400/12-VP (VNIIETO, Istra, Russia), and the accuracy of maintaining the temperature was ±5 °C. The molded products were packed in alumina grade GK-1 (Rusal, Moscow, Russia) using a corundum capsule.
The final sintering of the samples was carried out in a SNOL 12/16 chamber furnace (VNIIETO, Istra, Russia) with a lanthanum chromite heater at a temperature of 1530–1570 °C; the maximum heating temperature of the furnace was 1700 °C. The heat treatment modes of the samples are shown in Figure 2.
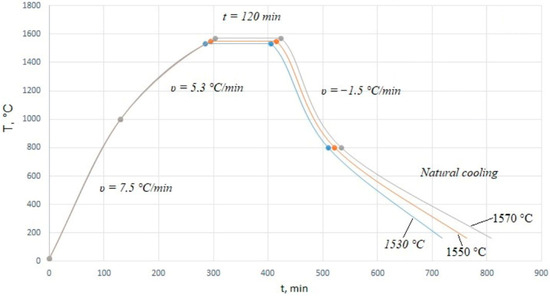
Figure 2.
Heat treatment modes.
Measurements of the mass of the powders used, binder components and other materials during the experiment and analysis were carried out on an AUM 210-E electronic analytical scale (Azzota Scientific, Claymont, DE, USA) with an accuracy of 0.0001 g. Drying of the powders was carried out in a ShS-80-01-SPU drying cabinet (Smolensk SDTB of PCS, Smolensk, Russia), with an accuracy of maintaining the temperature of ±1 °C.
The microhardness of the synthesized ceramics was determined by the Vickers method using a DuraScan-20 microhardness tester (EMCO TEST, Kuchl, Austria), test load was 2 kg. Holding at maximum load was 5 s. The obtained values were converted from kgf/mm2 to GPa. Microhardness measurements were carried out with averaging of results for at least 5 points on each sample.
The flexural strength was evaluated using the three-point bending test in accordance with ISO 6872:2015 [27]. After grinding and polishing, the sintered specimens had the form of bars with dimensions of approximately 25 × 4 × 2 mm (length × width × thickness). The tests were carried out with a support span of 20 mm and a crosshead speed of 0.5 mm/min. For each composition and sintering regime, at least five specimens were tested.
The crystal structure of powders and sintered samples was studied using an X PertPRO diffractometer (Malvern Panalytical Empyrean, Almelo, Netherland) using X-ray diffraction with monochromated copper radiation with a scanning step of 0.03° (Kα1 [Å] 1.5406). The measurement angle was 20–100°, the X-ray tube voltage was 40 kV, the current was 30 mA, the measurement time at each step was 10 s and an aluminum rectangular multi-purpose sample holder (PW1172/01, Malvern Panalytical Empyrean, Almelo, Netherland) was used. Quantitative analysis of the phase composition and structure of the samples was carried out using additional analysis software HighScore Plus 5.1 and Powder Cell 2.4.
The study of the values of apparent density, porosity and water absorption was carried out according to ASTM C20-00 [28].
The size of the initial powders and the powder mixture was estimated by laser diffraction on a Bettersizer S3 particle size and shape analyzer (Bettersize Instruments Ltd., Dandong, China). The size of the determined particles: 0.01–3500 μm; particle shape: 2–3500 μm.
The microstructure of the samples was analyzed using a Phenom™ XL G2 Desktop scanning electron microscope (ThermoFisher Scientific, Waltham, MA, USA). Maximum magnification ×100,000; resolution 14 nm; accelerating voltage 5, 10, 15 kV, backscattered electron detector, EMF attachment. Samples for microstructure analysis after grinding and polishing were prepared in boiling isopropyl alcohol, followed by washing in an ultrasonic bath UZM-25 (Medprompribor, Moscow, Russia) and washing in a chromium solution, followed by drying at 200 °C.
The thermal expansion coefficient of the ceramic samples was measured by linear dilatometry using a DIL0809PC horizontal dilatometer (Hunan Zhenhua Analysis Instrument, Xiangtan City, China). The temperature range of the device was −30 to +1600 °C. The samples studied had a cylindrical shape with a length of about 25 mm and a diameter of 5 mm. The end surfaces were carefully ground to ensure measurement accuracy. Measurements were carried out in the temperature range from 1530 to 1570 °C with a uniform heating rate of 20 °C/min. The change in the length of the sample was recorded in real time, which made it possible to determine the relative elongation as a function of temperature. The average thermal expansion coefficient (α) was calculated using Equation (1):
where ΔL is the absolute change in the length of the sample, L0 is the initial length, ΔT is the temperature range.
α = ΔL/L0·ΔT,
The obtained α values are presented as averages for five measurements for each composition; the measurement error did not exceed 5%. All experimental data are presented as mean values and corresponding standard deviations. The measurement error was estimated based on the results of repeated measurements and is indicated as the standard deviation (±SD). To estimate the statistical significance of differences between the studied groups, Student’s criterion was used. The significance level was considered reliable at p < 0.05. The repeatability of measurements was estimated using the coefficient of variation, calculated as the ratio of the standard deviation to the mean value, expressed as a percentage. The obtained coefficient of variation values did not exceed 10%, which indicates high reproducibility of the experimental data.
3. Results and Discussion
The sintering temperature range of 1530–1570 °C was selected not for a detailed study of sintering kinetics, but to determine the “optimal technological densification parameters” that ensure high density while maintaining a fine-grained structure. As is well known, increasing the temperature above 1570–1600 °C in ZrO2–Y2O3–Al2O3 systems leads to accelerated grain growth, a decrease in the tetragonal phase fraction, and degradation of mechanical properties.
In this study, the limited temperature interval made it possible to focus on the “energy-efficient sintering region”, which provides a balance between density, phase stability, and structural uniformity of the material.
Thus, the presented temperature series was not intended for a full analysis of diffusion kinetics but rather represents the “technologically relevant portion of the process”, within which the optimal sintering parameters and their influence on the microstructure and properties of the ceramics were determined.
All initial powder compositions have identical particle size, surface morphology and particle distribution. As an example, Figure 3 shows a micrograph of particles of sample 4Y.
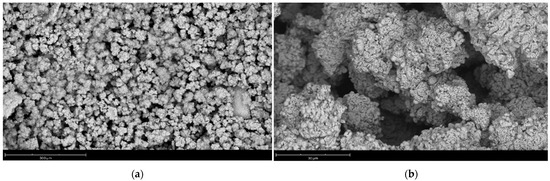
Figure 3.
Morphology of synthesized particles of zirconium oxide powder stabilized with yttrium oxide using the example of sample 4Y: (a) ×510 magnification; (b) ×4700 magnification.
The results of measuring the granulometric composition of the synthesized zirconium dioxide powders stabilized with 3.0, 4.0, 5.0, and 6.0 wt.% Y2O3 are shown in Figure 4.
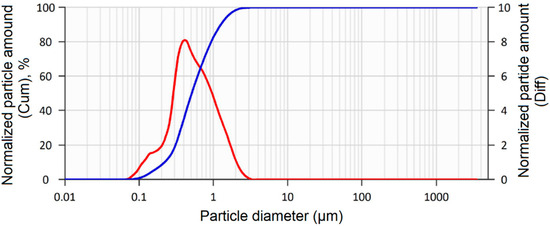
Figure 4.
Averaged curve of granulometric composition of synthesized powders. D10 = 0.219 μm; D50 = 0.508 μm; D90 = 1.249 μm. Blue line—cumulative; red line—differential.
The particle size distribution of the synthesized powders with different Y2O3 content is represented by a linear dependence, ascending from the nanoscale range of ~80–120 nm, then the peak of more than 80% falls on the particle size of ~600 nm. The particle size distribution curves are identical regardless of the yttrium oxide content, indicating the stability and repeatability of the chemical coprecipitation modes.
The particle size distribution for the studied compositions of the samples sintered at 1550 °C is shown in Figure 5, where an increase in the number of fine particles up to 0.8 μm in size with an increase in the yttrium oxide content is clearly visible. Thus, with an yttrium oxide content of 3.0 wt.%, the number of such particles is slightly less than 400 (Figure 5a); at 4.0 wt.%, the number of particles is about 450 (Figure 5b); at 5.0 and 6.0 wt.%, the number of particles is 480–520, respectively (Figure 5c,d). Thus, yttrium oxide Y2O3 plays an important role in stabilizing zirconium oxide ZrO2 and controlling its grain growth. Addition of yttrium oxide to zirconium oxide results in the formation of a solid solution in which yttrium ions Y3+ replace zirconium ions Zr4+ in the crystal lattice.
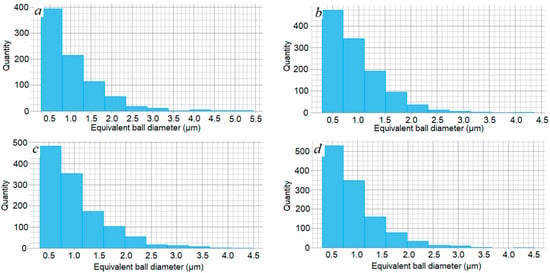
Figure 5.
Particle size distribution: (a) 3Y (d50 = 1.532 μm); (b) 4Y (d50 = 1.352 μm); (c) 5Y (d50 = 1.218 μm); (d) 6Y (d50 = 1.152 μm). Sintering temperature T = 1550 °C.
The contribution of yttria to the formation of the microstructure and phase composition should be viewed as a sequential, diffusion-controlled process. At the initial stage of sintering, when grain boundaries are highly mobile, Y3+ ions predominantly segregate to these boundaries due to the higher rate of grain-boundary diffusion compared to bulk diffusion. This segregation is not contradictory to the mechanism of forming a bulk solid solution; rather, it represents its initial, kinetically favored stage. The space-charge layers formed at the boundaries, depleted in oxygen vacancies, create a diffusion barrier (the “solute drag” effect), which mechanically impedes boundary motion. This barrier is exactly what is responsible for inhibiting grain growth and for the development of a fine-grained structure.
As heating proceeds and the material is held at the sintering temperature, slow bulk diffusion of Y3+ occurs from the boundaries into the grain interiors, completing the formation of a homogeneous substitutional solid solution (Y3+ → Zr4+) throughout the material. This stage provides bulk stabilization of the tetragonal phase. Thus, the observed stabilization of the microstructure is the result of synergy: boundary segregation controls the kinetics of grain growth, while bulk substitution determines phase stability. Together, these processes lead to the formation of a fine-grained structure, which enhances the mechanical properties of the material.
The results of the graph analysis show that the number of small particles 0.5–1.0 µm in size increases with the increase in the yttrium oxide content, from 390 to 530 pcs in the analyzed area.
The different yttrium oxide content determines the color of the ceramics, from light brown to white (Figure 6). Most likely, the main factors determining the change in shade are phase transformations, grain size, diffusion of stabilizing additives and the level of porosity. At 1530 °C, the ceramics have a lighter color due to the fine-grained structure and high porosity, while at 1570 °C a change in shade towards gray or light brown can be observed due to grain growth, increased density and diffusion processes.

Figure 6.
Change in ceramic color depending on the yttrium oxide content. Sintering temperature T = 1570 °C.
The sintering process in the range of 1530–1570 °C is accompanied by active compaction of the ceramics. At the initial stage (1530 °C), the pores begin to close due to the fusion of powder particles, but residual porosity is still preserved. With a further increase in temperature to 1570 °C, the pores are almost eliminated, and the density of the material approaches the theoretical value (6.08–6.10 g/cm3). With an increase in temperature to 1570 °C, grain growth accelerates due to increased diffusion processes. The introduction of aluminum oxide (Al2O3) allows this process to be controlled, preventing abnormal growth of individual grains. The uniform microstructure obtained by adding Al2O3 improves mechanical properties such as mechanical strength and hardness.
Since the main goal of the study is to obtain partially or completely tetragonally stabilized zirconia ceramics with an optimal balance between mechanical properties and density, the range of 3–6 wt.% Y2O3 was chosen based on the literature and industrial practice data on the most used yttrium concentrations for stabilizing ZrO2. At less than 3 wt.% Y2O3, it is impossible to achieve effective suppression of the monoclinic phase, and at more than 6 wt.%, the probability of forming a predominantly cubic phase increases, which leads to slightly different properties, including lower hardness. As for the sintering temperature, the range of 1530–1570 °C is typical for high-quality Y2O3-doped zirconia products, since at lower temperatures, there is a large proportion of residual porosity and under-compacted structure, while at significantly higher temperatures (above 1600 °C), there is a risk of abnormal grain growth and a reduction in mechanical properties. Thus, it is within the selected limits that the best combination of density, microstructural homogeneity and mechanical properties is achieved.
Figure 7 shows SEM images of the microstructure of ceramics sintered at 1530 °C with an yttrium oxide content of 3.0–6.0 wt.% and summary information based on computer calculation of grain sizes.
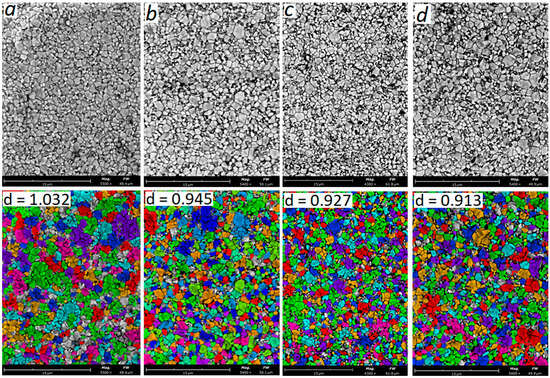
Figure 7.
SEM analysis of particle size and shape of samples: (a) 3Y; (b) 4Y; (c) 5Y; (d) 6Y. T = 1530 °C.
According to the results of the SEM image analysis of the microstructure of ceramics with 3.0 wt.% Y2O3, the number of particles is 818 pcs, and the average particle coverage is 68.7%; the average grain size estimated as the value of the equivalent circle diameter is 1.032 μm (Figure 7a). With an increase in the yttrium oxide content at 1530 °C, the average grain size slightly decreases from 1.032 μm with Y2O3 of 3.0 wt.% to 0.913 μm with Y2O3 of 6.0 wt.%.
Figure 8 shows SEM images of the microstructure of ceramics sintered at 1550 °C with an yttrium oxide content of 3.0–6.0 wt.% and summary information based on computer calculation of grain sizes.
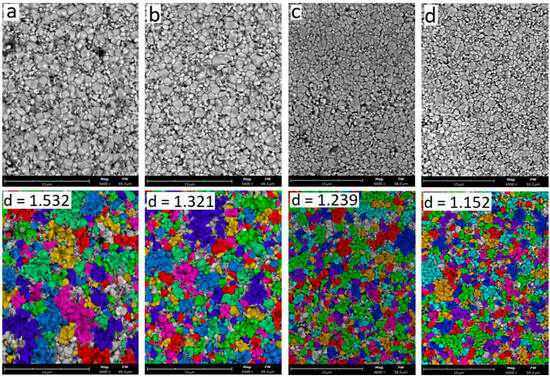
Figure 8.
SEM analysis of particle size and shape of samples: (a) 3Y; (b) 4Y; (c) 5Y; (d) 6Y. T = 1550 °C.
According to the results of the SEM image analysis of the microstructure of ceramics with 3.0 wt.% Y2O3 (Figure 8a), the average grain size estimated as the value of the equivalent circle diameter is 1.532 μm. With an increase in the yttrium oxide content at 1550 °C, the average grain size decreases insignificantly from 1.532 μm with Y2O3 of 3.0 wt.% to 1.152 μm with Y2O3 of 6.0 wt.%. The number of small particles of 0.5–1.0 μm in size, like Figure 7, increases with an increase in the yttrium oxide content.
Figure 9 shows SEM images of the microstructure of ceramics sintered at 1570 °C with an yttrium oxide content of 3.0–6.0 wt.% and summary information based on computer calculation of grain sizes.
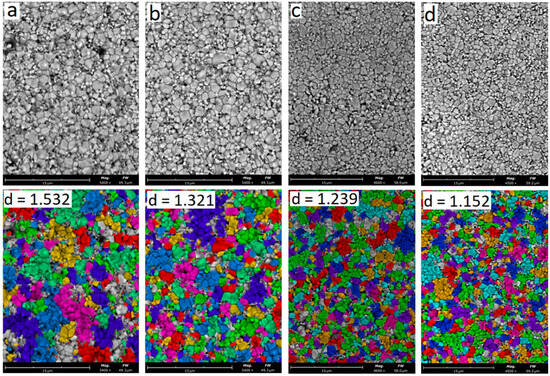
Figure 9.
SEM analysis of particle size and shape of samples: (a) 3Y; (b) 4Y; (c) 5Y; (d) 6Y. T = 1570 °C.
With an increase in the yttrium oxide content at 1570 °C, the average grain size decreases slightly from 1.773 μm with Y2O3 of 3.0 wt.% to 1.297 μm with Y2O3 of 6.0 wt.%. The number of small particles of 0.5–1.0 μm in size increases with an increase in the yttrium oxide content.
Analysis of SEM images of the ceramic surface sintered at 1530 °C with yttrium oxide content of 3.0 wt.% indicates the presence of a microcracked structure of the ceramic surface. The microcracks are 15–80 μm long, the crack width is several nanometers (Figure 10a). On the surface of the sample without special preparation, immediately after sintering, it is possible to detect elements of microcrack initiation (Figure 10b), represented by clearly defined cracks propagating along grain boundaries, having a similar propagation pattern and length.
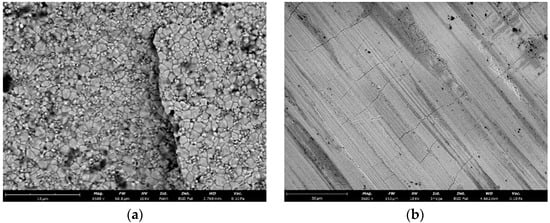
Figure 10.
SEM image of the surface of sample 3Y (sintering temperature 1530 °C): (a) sample surface after sintering (without special preparation); (b) sample surface after polishing and etching.
As a result of the analysis of the SEM image of the microstructure of ceramics with an increase in the yttrium oxide content at 1570 °C, the average grain size decreases from 1.773 μm with Y2O3 of 3.0 wt.% to 1.297 μm with Y2O3 of 6.0 wt.%. At the same time, the microstructure of ceramics with an yttrium oxide content of 6.0 wt.% in the sintering temperature range of 1530–1570 °C has the most uniform grain composition, microcracks and other defects are practically absent (Figure 11).
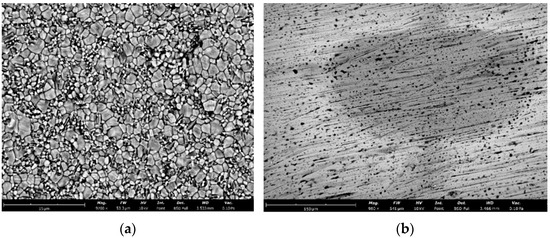
Figure 11.
Microstructure of ceramics sample 6Y (sintering temperature 1550 °C): (a) ceramics after sintering; (b) ceramics after polishing.
The microstructure of the surface of 3Y, 4Y and 5Y samples after sintering at 1550, 1570 °C and polishing has a similar nature of the distribution of microcracks, with a tendency to decrease their number and size with an increase in the content of yttrium oxide.
YSZ–Al2O3 based ceramics showed that increasing the yttrium oxide (Y2O3) content from 3.0 to 6.0 wt.% leads to a decrease in the average grain size at all sintering temperatures: at 1530 °C—from 1.032 to 0.913 μm, at 1550 °C—from 1.532 to 1.152 μm, at 1570 °C—from 1.773 to 1.297 μm. This is explained by the formation of oxygen vacancies, which slow down grain growth by reducing boundary mobility. An increase in the sintering temperature promotes grain growth due to the activation of diffusion processes, but the effect is limited with an increase in the Y2O3 content, which prevents excessive coarsening of particles. The grain size distribution showed that with an increase in the Y2O3 content, the proportion of small particles (0.5–1.0 μm) increases, which is associated with a slowdown in coalescence. At 3.0 wt. % Y2O3 (Figure 8), microcracks 15–80 μm long are observed, forming due to internal stresses, whereas at 6.0 wt.% Y2O3 (Figure 9), microcracks are virtually absent, and the structure becomes more homogeneous. A decrease in the number of cracks with an increase in the Y2O3 content is associated with several mechanisms: firstly, stabilization of the tetragonal phase of ZrO2 reduces the likelihood of phase transformations accompanied by volumetric changes leading to crack formation; secondly, a decrease in grain size leads to a more uniform distribution of residual stresses; thirdly, yttrium oxide contributes to an increase in the viscosity of grain boundaries, which reduces the risk of intergranular fracture. The optimal microstructure is achieved at 6.0 wt.% Y2O3 and a sintering temperature of 1550 °C, which ensures minimal grain size, absence of defects and high mechanical properties of the ceramics.
The results obtained in this study—specifically, the decrease in the average grain size from 1.773 µm to 1.297 µm with an increase in Y2O3 content from 3.0 to 6.0 wt.% (at 1570 °C) and the corresponding reduction in microcrack density—are in good agreement with current understanding of the role of dopants in microstructural control. The observed grain growth inhibition effect of yttrium oxide can be explained in terms of the grain-boundary pinning mechanism. As shown in Ref. [10], even a small addition of dispersed Al2O3 particles into a ZrO2 matrix leads to their localization at grain boundaries, creating a mechanical barrier to boundary migration during sintering. This “pinning effect” is synergistically enhanced in the presence of Y2O3. Moreover, as demonstrated by combined experimental and computational studies [9], Y3+ ions segregating to grain boundaries reduce their energy and mobility, while the presence of Al2O3 particles further increases the energy barrier for boundary motion. Together, these effects result in the formation of a fine-grained and homogeneous microstructure, as observed in samples with higher yttria content (e.g., the 6Y composition).
The results of the study of the X-ray phase composition of the samples are presented in Figure 12, Figure 13, Figure 14 and Figure 15. Thus, Figure 12 shows the results of the X-ray phase analysis of sample 3Y, sintered in the temperature range of 1530–1570 °C.
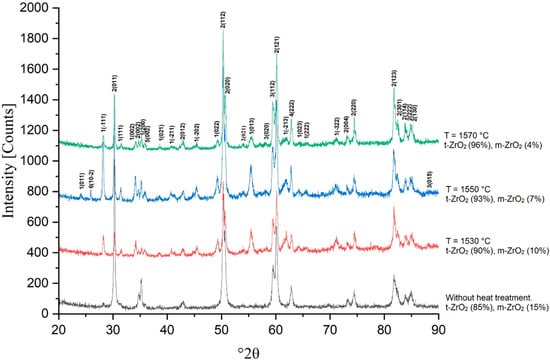
Figure 12.
X-ray phase analysis of sample 3Y sintered at 1530, 1550 and 1570 °C.
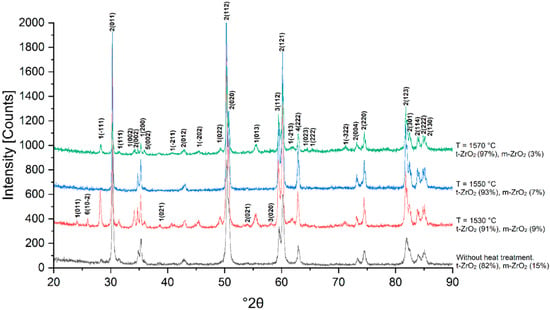
Figure 13.
X-ray phase analysis of sample 4Y sintered at 1530, 1550 and 1570 °C.
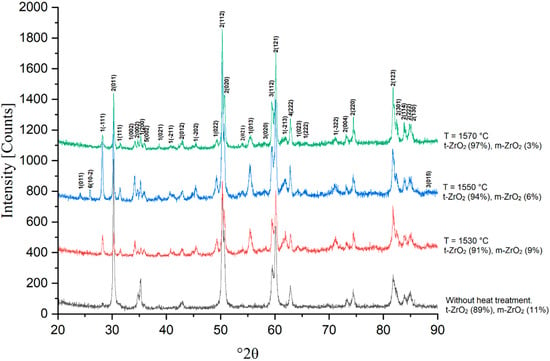
Figure 14.
X-ray phase analysis of sample 5Y sintered at 1530, 1550 and 1570 °C.
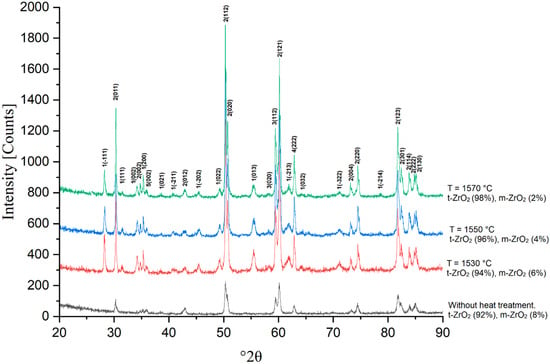
Figure 15.
X-ray phase analysis of sample 6Y sintered at 1530, 1550 and 1570 °C.
With increasing concentration of Y2O3, a consistent change in the phase composition of the material is observed. At a low content of the stabilizer (3Y), the phase composition is represented predominantly by the tetragonal modification (t-ZrO2) with a small amount of the monoclinic phase (m-ZrO2). At 1530 °C, a noticeable proportion of the monoclinic phase is retained, which indicates insufficient stabilization of the structure. With an increase in temperature to 1570 °C, a decrease in the proportion of the monoclinic phase occurs due to the dissolution of an additional amount of yttrium in the zirconium crystal lattice, but its complete disappearance is not observed.
With an increase in the Y2O3 content to 4.0 wt.% (4Y), further stabilization of the tetragonal phase is observed. Already at 1530 °C, the main phase component is t-ZrO2, and the proportion of the monoclinic phase decreases compared to 3Y. With an increase in temperature to 1570 °C, the tetragonal phase becomes predominant, and the residual monoclinic structure is retained only in small quantities. This indicates a more effective stabilization of the material; however, complete suppression of m-ZrO2 does not occur.
At 5.0 wt.% Y2O3 (5Y), the monoclinic phase is present in a minimal amount already at 1530 °C, and at 1570 °C it is practically not recorded in the X-ray diffraction patterns. This confirms that an increase in the yttrium concentration contributes to the stabilization of the tetragonal modification, making it the main phase in the material. It is important to note that at this composition, no signs of the formation of a cubic structure were detected, which indicates the preservation of phase stability in the studied temperature range.
At 6.0 wt.% Y2O3 (6Y), the material is almost completely stabilized in the tetragonal phase even at 1530 °C. Increasing the sintering temperature leads to greater homogeneity of the structure, and the residual monoclinic phase is not recorded in the X-ray phase analysis at 1570 °C. This confirms that increasing the Y2O3 content to 6.0 wt.% ensures maximum stabilization of the material in the tetragonal form. Increasing the sintering temperature contributes to additional stabilization of high-temperature phases and a decrease in the content of the residual monoclinic phase. X-ray phase analysis showed that increasing the Y2O3 content to 6.0 wt.% ensures almost complete stabilization of the tetragonal phase, while compositions with lower yttria content exhibit minor amounts of the monoclinic modification.
The results of the study of the physical and mechanical properties of ceramics are presented in Table 2. To obtain reliable results, at least 5 samples from each composition were tested.

Table 2.
Physical and mechanical properties of the samples depending on the composition and sintering temperature.
The introduction of aluminum oxide prevents abnormal growth of individual grains, and the homogeneous microstructure obtained by adding Al2O3 improves the mechanical properties of ceramics.
The decrease in hardness with increasing Y2O3 content is not directly caused by oxygen vacancies themselves, but rather by structural changes resulting from their distribution and the phase evolution of the material. A higher Y2O3 concentration increases the number of oxygen vacancies and enhances diffusion, which promotes densification and structural homogeneity. However, this also increases the fraction of the cubic phase and decreases that of the tetragonal phase, thereby reducing the contribution of transformation toughening and leading to a decrease in microhardness.
At the same time, segregation of Y3+ ions at grain boundaries forms oxygen-depleted space-charge regions, which hinder boundary migration and stabilize the fine-grained structure. Thus, the observed combination of “grain refinement and hardness reduction” results from the competing effects of phase composition, diffusion processes, and defect–boundary interactions.
In addition, studies were carried out on the dependence of the relative change in specimen length in the high-temperature sintering region on temperature (Figure 16).
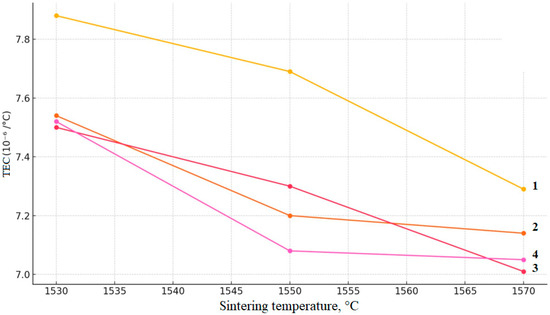
Figure 16.
Dependence of thermal expansion coefficient of ceramic samples on the sintering temperature at different concentration of yttrium: (1) 3Y; (2) 4Y; (3) 5Y; (4) 6Y.
Figure 16 shows the graph of the obtained values of the relative change in specimen length in the high-temperature sintering region for different ceramic compositions at temperatures of 1530, 1550, and 1570 °C. As can be seen, this dependence exhibits a decreasing trend with increasing temperature, which is consistent with the densification of the material’s structure. The obtained coefficients fall within the range of 7.0–7.9 × 10−6 °C−1. It was established that with increasing temperature, a moderate decrease in the measured coefficient is observed for all four compositions. The observed decrease in this parameter with temperature increase is apparently not associated with true thermal expansion but rather with final densification processes, such as closure of residual porosity and relaxation of internal stresses within the material’s structure. The detected differences in the coefficient of linear length change among the compositions are likely related to variations in yttria content and its influence on phase composition and microstructure. Since the Al2O3 content is constant across all samples, its contribution to the thermal behavior is systematic. The lower values observed for the compositions with higher Y2O3 content (5Y and 6Y) can be explained by the greater stabilization of the tetragonal phase, smaller grain size, and consequently, a more balanced interplay between lattice thermal expansion and high-temperature stress relaxation processes within the studied temperature range. The highest values are recorded for the 3Y composition, while the introduction of alumina (in the 5Y and 6Y compositions) leads to a decrease in elongation, which may be attributed to the formation of a more thermally stable Al2O3 phase and an increased degree of densification.
The apparent density of ceramics sintered at 1530 °C has a minimum value of 6.08 g/cm3 for 3Y and a maximum value of 6.10 g/cm3 for 6Y. Water absorption for 6Y becomes practically zero. At 1550 °C, the apparent density value for 3Y and 4Y stabilizes at a level of 6.09 g/cm3, and for 5Y and 6Y it increases up to 6.1 g/cm3, open porosity and water absorption are noticeably reduced. At 1570 °C, the density value stabilizes at 6.09–6.10 g/cm3, water absorption becomes practically zero, which indicates that the maximum density of ceramics has been achieved.
The hardness of the synthesized ceramics appeared to be dependent on the content of Y2O3 and stabilization of the tetragonal and cubic phases. With a low content of yttrium oxide (3Y–4Y), the hardness value has the highest indicators at sintering temperature of 1550 °C. With an increase in the content of yttrium oxide, the hardness value slightly decreases despite stabilization in the tetragonal phase.
Despite the fact that at 1570 °C, the density of the 6Y sample is practically the same as at 1550 °C (both are close to 6.10 g/cm3), it was the temperature of 1550 °C that was defined as optimal for two main reasons. First, at 1550 °C, almost full density is achieved (water absorption tends to zero), and a further increase in temperature to 1570 °C does not provide a noticeable gain in this parameter, but increases the risk of more intensive grain growth and, as a result, can potentially reduce hardness and increase the likelihood of defects. Second, in industrial production, energy costs and the risk of overbaking of ceramics are taken into account, so the choice of a lower sintering temperature, if it ensures the same level of density and high mechanical properties, becomes economically feasible. Thus, 1550 °C provides a balance between achieving maximum density, maintaining a fine-grained structure and production costs.
In addition, according to Table 2, the hardness decreases with increasing Y2O3 content (for example, for 6Y at 1550 °C it is 13.24 GPa, which is lower than that of 3Y—13.72 GPa), this phenomenon can be explained as follows. Additional oxygen vacancies arising from the substitution of Zr4+ by Y3+ can contribute to an increase in the diffusion mobility of atoms in the lattice, which also affects the hardness. From a practical point of view, a higher yttrium content (5–6 wt.%) is beneficial for improving bending strength and reducing the tendency to cracking, but this is accompanied by some decrease in hardness compared to 3Y.
Thus, yttrium oxide has a positive effect on the sintering process by increasing the mobility of grain boundaries. The introduction of Y2O3 reduces intergranular diffusion resistance, which facilitates the redistribution of the substance and promotes more uniform compaction. Ceramic grains in the presence of yttrium oxide sinter more effectively, forming a dense structure with a minimum number of defects.
Another important aspect is the change in the grain boundary structure. With an increase in the yttrium oxide content, the grain boundary energy decreases, making them more stable and resistant to loosening during heat treatment. This prevents the formation of isolated pores and increases the density of the material. In addition, a decrease in the number of open pores leads to a decrease in water absorption, which is also confirmed by the experimental results. If for the 3Y composition at 1530 °C, water absorption remains at a level of 0.09%, then with an increase in the Y2O3 content to 6.0 wt.%, it becomes equal to zero. This indicates the absence of capillary channels through which water could penetrate, which once again confirms the maximum density of the structure.
The observed suppression of microcrack formation in samples with 6.0 wt% Y2O3 sintered at 1550 °C is the result of a synergistic interaction of several factors, including phase stability, grain size, and sintering temperature. X-ray diffraction confirms that at 6.0 wt% Y2O3 the material is almost fully stabilized in the tetragonal phase. This eliminates volumetric changes associated with the martensitic tetragonal-to-monoclinic transformation upon cooling, which is the main source of stresses and cracking in less stabilized compositions (e.g., 3Y). SEM data show that increasing the Y2O3 content at all sintering temperatures leads to a reduction in the average grain size. Fine grains promote a more uniform distribution of residual stresses and enhance the fracture toughness of the material, thereby hindering crack propagation.
The optimal temperature of 1550 °C ensures nearly full densification without triggering abnormal grain growth. Thus, the “threshold” of crack suppression is achieved within an optimal composition–temperature window, where the combined effect of phase purity, fine grain size, and high density minimizes internal stresses.
4. Conclusions
The study showed that an increase in the yttrium oxide (Y2O3) content from 3.0 to 6.0 wt.% promotes stabilization of the tetragonal phase of zirconium dioxide (t-ZrO2), a decrease in the average grain size and a decrease in the number of defects. At 3.0 wt.% Y2O3, a significant proportion of the monoclinic phase is retained, which can cause internal stresses and microcracks, whereas at 6.0 wt.% the structure is completely stabilized. The optimal sintering temperature is 1550–1570 °C, while the material density reaches 6.10 g/cm3, and the porosity and water absorption decrease to zero. The hardness of the synthesized ceramics decreases with an increase in the yttrium oxide content. The introduction of aluminum oxide (0.5 wt.% Al2O3) improves the mechanical properties and prevents grain growth. At 6.0 wt.% Y2O3 has the most uniform microstructure, a minimum number of cracks and high phase stability.
The conducted studies show that the introduction of 6 wt.% yttrium oxide (Y2O3) and 0.5 wt.% aluminum oxide (Al2O3) into the zirconium dioxide (ZrO2) matrix, in combination with sintering at 1550 °C, ensures the formation of the most homogeneous and fine-grained structure, almost complete stabilization of the tetragonal phase and a minimum number of defects, which in total leads to a significant increase in bending strength (up to 1300 MPa). This result is associated with a combination of stabilization of the tetragonal modification, reducing the risk of microcracks during phase transitions, and the absence of abnormal grain growth due to the presence of Al2O3. This effect has high practical value, since the obtained ceramics combine high mechanical characteristics and chemical inertness, which makes it promising for use in critical units of mechanical engineering, medicine, electronics and other high-tech areas.
The obtained values of the relative change in specimen length in the high-temperature sintering region for different ceramic compositions at 1530, 1550, and 1570 °C lie within the range of 7.0–7.9 × 10−6 °C−1, which is generally characteristic of oxide technical ceramics. At the same time, the coefficient of length change shows a tendency to decrease with increasing temperature, which is consistent with the densification of the material’s structure. By varying the composition and heat treatment regime, it is possible to purposefully control the relative change in specimen length in the high-temperature sintering region, which is important for the development of heat-resistant and thermal-shock-resistant materials.
The combination of 6.0 wt.% Y2O3 and 0.5 wt.% Al2O3 at 1550–1570 °C provides optimal properties of ceramics, making it promising for use in mechanical engineering, medicine, and electronics.
The obtained results and identified regularities provide a foundation for further improvement of the ceramic properties. As a promising direction, the use of a two-step sintering process is considered, which includes preliminary sintering at a reduced temperature followed by densification using low-temperature hot isostatic pressing (HIP). This approach makes it possible to achieve nearly theoretical density without abnormal grain growth and ensures more precise control over the stability of the tetragonal phase, thereby maximizing the contribution of martensitic transformation to the mechanical strengthening of the material.
The conducted study not only established the relationship between Y2O3 content and the structural characteristics of the ceramics but also clarified the mechanisms responsible for the formation of their properties. It was shown that increasing the Y2O3 concentration up to 6.0 wt.% leads to stabilization of the tetragonal phase and suppression of grain growth due to the segregation of Y3+ ions at the grain boundaries, forming space-charge layers. These effects promote the formation of a fine-grained, dense, and homogeneous microstructure.
The observed decrease in microhardness with increasing Y2O3 content is interpreted as a consequence of the reduced contribution of transformation toughening due to stabilization of the tetragonal structure. At the same time, the preservation of high bending strength (up to 1300 MPa) and low porosity indicates a well-balanced combination of phase composition and grain structure.
From a practical standpoint, the obtained results confirm the possibility of energy-efficient sintering of zirconia ceramics at 1550 °C without deterioration of their mechanical properties, making this regime promising for the production of structural and functional components in mechanical engineering, electronics, and biomedical applications.
Author Contributions
Conceptualization, M.A., M.S. and A.Z.; methodology, M.A., D.Y. and A.Z.; software, D.Y. and A.P.; validation, M.A., M.S. and A.P.; formal analysis, A.Z.; investigation, D.Y., A.P., A.Z. and I.K.; resources, M.A.; data curation, M.A., D.Y., A.Z. and A.P.; writing—original draft preparation, D.Y. and A.Z.; writing—review and editing, M.S. and I.K.; visualization, M.A., D.Y. and A.P.; supervision, M.S. and A.Z.; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP19677974.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
During the preparation of this manuscript, the authors used GPT-5 for the purposes of grammar check. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kou, B.; Zhou, W.; Lin, Y.; Han, F.; Ouyang, J. Wettability and interfacial reaction of ZrO2 and Y2O3 doped Al2O3-based ceramic shells in K417G superalloy investment casting. Ceram. Int. 2025, 51, 40087–40097. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Sitnikov, A.I.; Val′yano, G.E.; Borodina, T.I.; Afonina, M.B. Alumina ceramics sintered from hollow corundum microspheres with Al2O3 and ZrO2–Y2O3 as sintering activators. Ceram. Int. 2023, 49, 1496–1501. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, K.; Li, J.; Chen, B.; Huang, C.J.; Soboleva, N. A comprehensive review on the rare earth elements improving microstructure and properties of laser cladded coatings. J. Alloys Compd. 2025, 1036, 181761. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Mekgwe, G.N.; Akinwamide, S.O.; Gamaoun, F.; Abeykoon, C.; Johnson, O.T.; Olubambi, P.A. A review on optical properties and application of transparent ceramics. J. Mater. Res. Technol. 2022, 21, 712–738. [Google Scholar] [CrossRef]
- Xia, X.; Li, X.; Zhang, M.; Zheng, D. Transitional/eutectic microstructure of Al2O3–ZrO2 (Y2O3) ceramics prepared by spark plasma sintering. Mater. Lett. 2016, 175, 212–214. [Google Scholar] [CrossRef]
- Taurino, R.; Martinuzzi, S.; Padovano, E.; Caporali, S.; Bondioli, F. Laser additive manufacturing of Al2O3 and ZrO2-based eutectic ceramic oxide: An overview. J. Eur. Ceram. Soc. 2025, 45, 117133. [Google Scholar] [CrossRef]
- Shwailiya, S.A.; Hameed, M.R. Multi-material functionally graded additive manufacturing of zirconia ceramic: A systematic review. Open Ceram. 2025, 21, 100741. [Google Scholar] [CrossRef]
- Chitoria, A.K.; Mir, A.; Shah, M.A. A review of ZrO2 nanoparticles applications and recent advancements. Ceram. Int. 2023, 49, 32343–32358. [Google Scholar] [CrossRef]
- Fu, L.; Li, B.; Xu, G.; Huang, J.; Engqvist, H.; Xia, W. Size-driven phase transformation and microstructure evolution of ZrO2 nanocrystallites associated with thermal treatments. J. Eur. Ceram. Soc. 2021, 41, 5624–5633. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, H.; Zhang, Y.; Zhang, F.; Gao, L.; Omran, M.; Chen, G. Microstructure and phase transformation behavior of Al2O3–ZrO2 under microwave sintering. Ceram. Int. 2023, 49, 4855–4862. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, H.; Zhang, Y.; Nan, L.; Gao, L.; Chen, J.; Omran, M.; Chen, G. Preparation of nano zirconia by binary doping: Effect of controlled sintering on structure and phase transformation. Ceram. Int. 2022, 48, 25374–25381. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, J.; Ren, C.; Omran, M.; Gao, L.; Tang, J.; Zhang, F.; Chen, G. Research on the drying kinetics for the microwave drying of Y2O3–ZrO2 ceramic powder. J. Mater. Res. Technol. 2023, 26, 4563–4580. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Yuan, Y.; Wang, R.; Zhu, S.; Bai, Y.; Fang, C.; Chen, C.; Zhang, Z.; Zheng, Y. Additive-free preparation of high-toughness Al2O3/ZrO2 ceramics with platelet grain through spontaneously formed dendrite. Ceram. Int. 2024, 50, 31780–31791. [Google Scholar] [CrossRef]
- Du, W.; Ai, Y.; He, W.; Chen, W.; Liang, B.; Lv, C. Formation and control of “intragranular” ZrO2 strengthened and toughened Al2O3 ceramics. Ceram. Int. 2020, 46, 8452–8461. [Google Scholar] [CrossRef]
- Sunil Kumar, K.; Kalos, P.S.; Akhtar, M.N.; Shaik, S.; Sundara, V.; Fayaz, H.; Khan, S.A.; Asif, M. Experimental and theoretical analysis of exhaust manifold by uncoated and coated ceramics (Al2O3, TiO2 and ZrO2). Case Stud. Therm. Eng. 2023, 50, 103465. [Google Scholar]
- Dong, X.; An, Q.; Zhang, S.; Yu, H.; Wang, M. Porous ceramics based on high-thermal-stability Al2O3–ZrO2 nanofibers for thermal insulation and sound absorption applications. Ceram. Int. 2023, 49, 31035–31045. [Google Scholar]
- Abilev, M.; Yerbolat, D.; Skakov, M.; Zhilkashinova, A.; Pavlov, A.; Gert, S.; Zhambakin, D.; Kantay, N.; Zhilkashinova, A. Structure and properties of composite 6YSZ-Al2O3-HfO2 ceramics depending on the sintering mode. J. Mater. Eng. Perform. 2025, 34, 12247–12255. [Google Scholar]
- Wang, H.; Huang, H.; Liang, J.; Liu, J. Preparation of ZrO2/Gd2O3 composite ceramic materials by coprecipitation method. Ceram. Int. 2014, 40, 3995–3999. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.; Liu, H.; Xiong, X.; Cheng, H. Synthesis and high-temperature phase transformation behavior of Dy2O3–Sc2O3 co-stabilized ZrO2 powders prepared by cocurrent chemical coprecipitation. Ceram. Int. 2024, 50, 22395–22404. [Google Scholar] [CrossRef]
- Zhong, J.; Li, N.; Xu, X.; Wei, L.; Liu, H.; Li, H.; Fang, Z.; Bao, H. The effects of multiphase composition on structure and mechanical properties of ZrO2/Si–C–O ceramic fibers by sol-gel process. Ceram. Int. 2023, 49, 16283–16289. [Google Scholar]
- Lawal, S.O.; Takahashi, Y.; Moriyama, N.; Nagasawa, H.; Tsuru, T.; Kanezashi, M. Fabricating hydrothermally robust ceramic membranes from amorphous yttrium-doped SiO2-ZrO2 composites. J. Eur. Ceram. Soc. 2025, 45, 116826. [Google Scholar] [CrossRef]
- Zhou, D.; Ding, C.; Han, W.; Li, H.; Zhang, H.; Xu, W.; Zhou, Y. Low-temperature fabrication of zirconia transparent ceramics via hydrothermal nanopowder. Ceram. Int. 2024, 50, 49210–49216. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. J. Eur. Ceram. Soc. 2008, 28, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskiy, A.L.; Konyukhova, M.; Borgekov, D.B.; Anatoliy, I.P. Investigation of the effect of irradiation temperature on the mechanisms of radiation-induced polymorphic transformations in ZrO2 ceramics. Opt. Mater. 2024, 156, 1159–1194. [Google Scholar] [CrossRef]
- Smits, K.; Grigorjeva, L.; Millers, D.; Kundzins, K.; Ignatans, R.; Grabis, J.; Monti, C. Luminescent properties of zirconia nanocrystals prepared by solar physical vapor deposition. Opt. Mater. 2014, 37, 251–256. [Google Scholar] [CrossRef]
- ISO 6872:2024; Dentistry—Ceramic materials. ISO: Geneva, Switzerland, 2024.
- ASTM C20-00(2022); Standard test methods for apparent porosity, water absorption, apparent specific gravity, and bulk density of burned refractory brick and shapes by boiling water. ASTM International: West Conshohocken, PA, USA, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).