Halogens On, H-Bonds Off—Insights into Structure Control of Dihalogenated Imidazole Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. General Aspects
2.2. Synthetic Procedures
2.3. X-Ray
3. Results
3.1. Synthesis of Halogenated and Protected Imidazole Derivatives
3.2. Molecular Structures of the Dihalogenated Derivatives
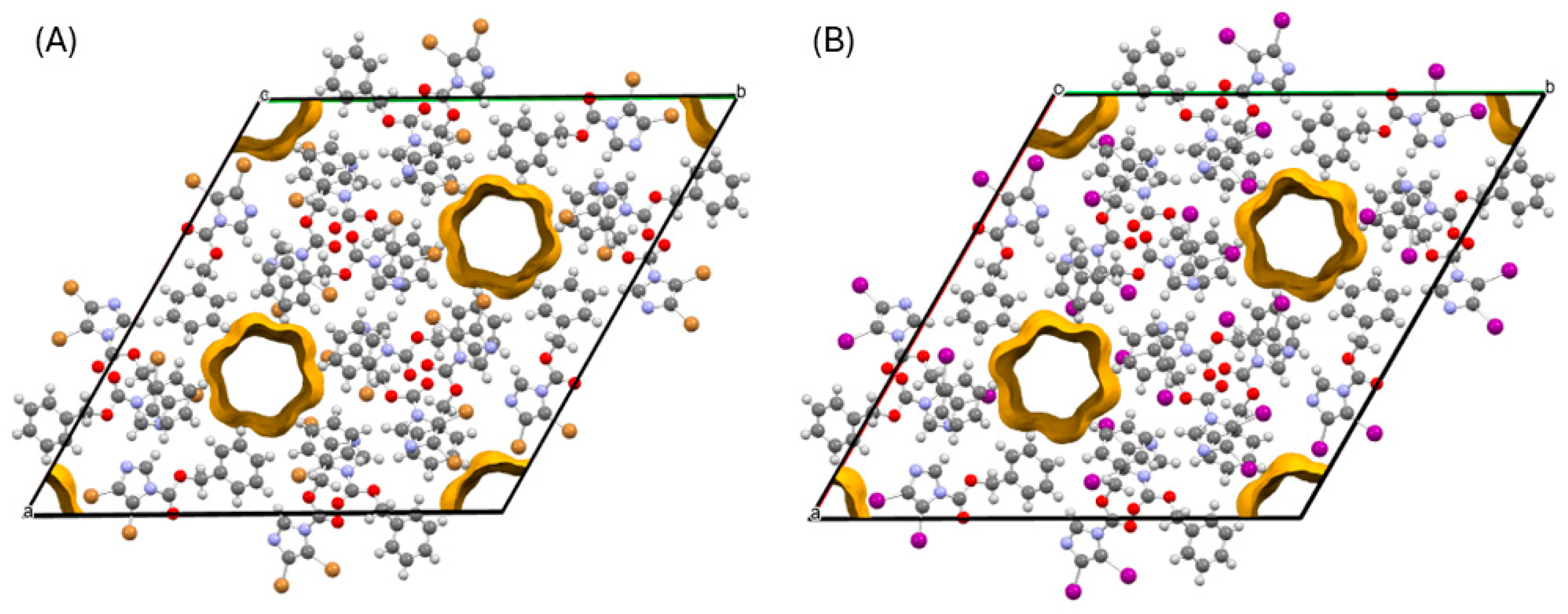
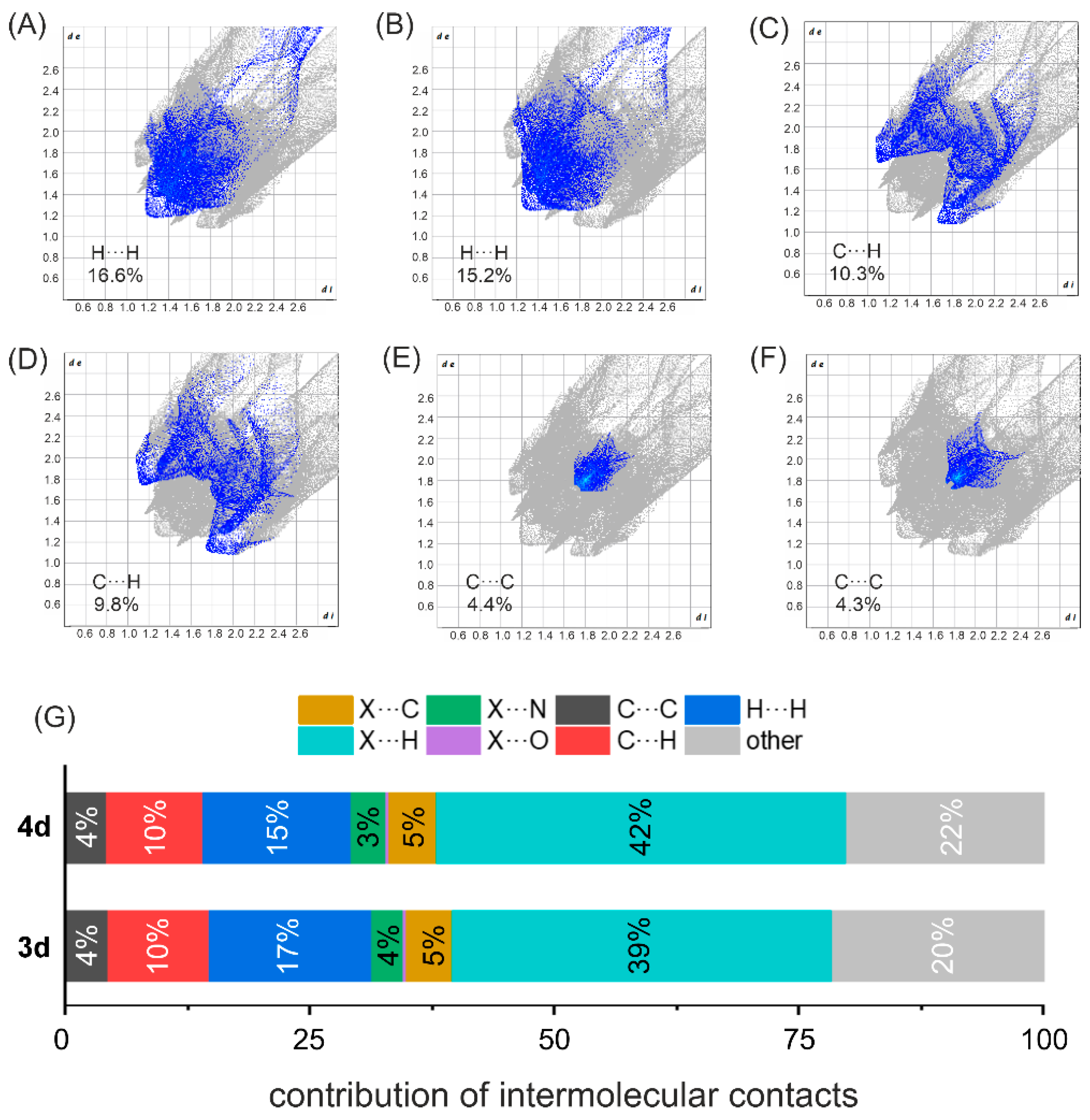
3.3. Supramolecular Features of the Dihalogenated Derivatives
4. Discussion
Comparison of Halogen-Bonding Related Effects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aq. | aqueous |
| Boc | tert-butyloxycarbonyl |
| Bn | benzyl |
| Cbz | benzyloxycarbonyl |
| Cmpd | compound |
| DCM | dichloromethane |
| DMSO | dimethyl sulfoxide |
| EtOAc | ethyl acetate |
| na | not available |
| PMB | p-methoxybenzyl |
| sat. | saturated |
| THF | tetrahydrofuran |
References
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef]
- Bulfield, D.; Engelage, E.; Mancheski, L.; Stoesser, J.; Huber, S.M. Crystal Engineering with Multipoint Halogen Bonding: Double Two-Point Donors and Acceptors at Work. Chem. Eur. J. 2020, 26, 1567–1575. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Shaashua, O.; Braunstein, S.; Pappo, D. Halogen Bond-Driven Ligand Displacement: Co-Crystal Lattice Versus Coordination Bonds. Chem. Eur. J. 2025, 31, e202404784. [Google Scholar] [CrossRef]
- Keuper, A.C.; Fengler, K.; Ostler, F.; Danelzik, T.; Piekarski, D.G.; Garcia Mancheno, O. Fine-Tuning Substrate-Catalyst Halogen-Halogen Interactions for Boosting Enantioselectivity in Halogen-Bonding Catalysis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202304781. [Google Scholar] [CrossRef]
- Staron, J.; Pietrus, W.; Bugno, R.; Kurczab, R.; Satala, G.; Warszycki, D.; Lenda, T.; Wantuch, A.; Hogendorf, A.S.; Hogendorf, A.; et al. Tuning the activity of known drugs via the introduction of halogen atoms, a case study of SERT ligands—Fluoxetine and fluvoxamine. Eur. J. Med. Chem. 2021, 220, 113533. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.J.; Gaultier de Claubry, H. Sur le combinaisons de l’iode avec les substances végétales et animales. Ann. Chim. 1814, 90, 87–100. [Google Scholar]
- Guthrie, F. XXVIII.—On the iodide of iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef]
- Mulliken, R.S. Structures of Complexes Formed by Halogen Molecules with Aromatic and with Oxygenated Solvents1. J. Am. Chem. Soc. 1950, 72, 600–608. [Google Scholar] [CrossRef]
- Hassel, O.; Hvoslef, J.; Vihovde, E.H.; Sörensen, N.A. The Structure of Bromine 1,4-Dioxanate. Acta Chem. Scand. 1954, 8, 873. [Google Scholar] [CrossRef]
- Dumas, J.; Peurichard, H.; Gomel, M. CX4··· base interaction as models of weak charge-transfer interactions: Comparison with strong charge-transfer and hydrogen-bond interactions. J. Chem. Res. 1978, 2, 54–55. [Google Scholar]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Scharf, P.; Müller, J. Nucleic Acids with Metal-Mediated Base Pairs and Their Applications. ChemPlusChem 2013, 78, 20–34. [Google Scholar] [CrossRef]
- Jash, B.; Müller, J. Metal-Mediated Base Pairs: From Characterization to Application. Chem. Eur. J. 2017, 23, 17166–17178. [Google Scholar] [CrossRef]
- Chien, T.C.; Saluja, S.S.; Drach, J.C.; Townsend, L.B. Synthesis and antiviral evaluation of polyhalogenated imidazole nucleosides: Dimensional analogues of 2,5,6-trichloro-1-(beta-D-ribofuranosyl)benzimidazole. J. Med. Chem. 2004, 47, 5743–5752. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.B.; Devivar, R.V.; Turk, S.R.; Nassiri, M.R.; Drach, J.C. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 1995, 38, 4098–4105. [Google Scholar] [CrossRef]
- Mikula, M.; Hanusek, K.; Paziewska, A.; Dzwonek, A.; Rubel, T.; Bomsztyk, K.; Ostrowski, J. Halogenated imidazole derivatives block RNA polymerase II elongation along mitogen inducible genes. BMC Mol. Biol. 2010, 11, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdulazeez, I.; Zeino, A.; Kee, C.W.; Al-Saadi, A.A.; Khaled, M.; Wong, M.W.; Al-Sunaidi, A.A. Mechanistic studies of the influence of halogen substituents on the corrosion inhibitive efficiency of selected imidazole molecules: A synergistic computational and experimental approach. Appl. Surf. Sci. 2019, 471, 494–505. [Google Scholar] [CrossRef]
- Dadou, S.; Elyoussfi, A.; Dagdag, O.; Koudad, M.; Isaad, J.; Kim, H.; Berisha, A.; Azghay, I.; Salhi, A.; Ahari, M.H.; et al. The impact of halogen substitution on the corrosion inhibition of imidazothiazole derivatives for mild steel in corrosive media (Part A). Colloids Surf. A 2024, 687, 133451. [Google Scholar] [CrossRef]
- Chaplais, G.; Fraux, G.; Paillaud, J.-L.; Marichal, C.; Nouali, H.; Fuchs, A.H.; Coudert, F.-X.; Patarin, J. Impacts of the Imidazolate Linker Substitution (CH3, Cl, or Br) on the Structural and Adsorptive Properties of ZIF-8. J. Phys. Chem. C 2018, 122, 26945–26955. [Google Scholar] [CrossRef]
- Namsani, S.; Yazaydin, A.O. Electric field induced rotation of halogenated organic linkers in isoreticular metal–organic frameworks for nanofluidic applications. Mol. Syst. Des. Eng. 2018, 3, 951–958. [Google Scholar] [CrossRef]
- Hou, J.; Rios Gomez, M.L.; Krajnc, A.; McCaul, A.; Li, S.; Bumstead, A.M.; Sapnik, A.F.; Deng, Z.; Lin, R.; Chater, P.A.; et al. Halogenated Metal-Organic Framework Glasses and Liquids. J. Am. Chem. Soc. 2020, 142, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Elejalde-Cadena, N.R.; García-Olave, M.; Macías, M.A.; Portilla, J. Influence of halogen atoms and hydrogen bonds in the crystal structure of 1,2,4-trisubstituted imidazoles having haloaryl groups. J. Mol. Struct. 2023, 1286, 135662. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- García-Olave, M.; Elejalde-Cadena, N.R.; Portilla, J.; Macías, M.A. C H‧‧‧X (X = F, Cl) and Cl‧‧‧Cl halogen-mediated interactions driving the crystal packing in N-substituted 4-arylimidazoles. J. Mol. Struct. 2023, 1272, 134181. [Google Scholar] [CrossRef]
- Andrzejewski, M.; Marciniak, J.; Rajewski, K.W.; Katrusiak, A. Halogen and Hydrogen Bond Architectures in Switchable Chains of Di- and Trihaloimidazoles. Cryst. Growth Des. 2015, 15, 1658–1665. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Rajbanshi, A.; Desper, J.; Moore, C.; Urbina, J.F. Structural Competition between Hydrogen Bonds and Halogen Bonds. J. Am. Chem. Soc. 2007, 129, 11824–11825. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Aakeröy, C.B.; Sinha, A.S.; Desper, J. Switching Between Halogen and Hydrogen Bonding via Stoichiometric Variations in Co-Crystals. CrystEngComm. 2012, 14, 6110–6114. [Google Scholar] [CrossRef]
- Robertson, C.C.; Perutz, R.N.; Brammer, L.; Hunter, C.A. Hydrogen Bonding Versus Halogen Bonding: The Solvent Decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef]
- Topić, F.; Rissanen, K. Cocrystal Trimorphism and Stoichiomorphism of mdppo and tfib: Orthogonality of Hydrogen- and Halogen-Bonding Motifs. Chem. Commun. 2019, 55, 8663–8666. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Chopade, P.D.; Desper, J. Mapping the Supramolecular Landscape of Competing Hydrogen- and Halogen-Bond Donors in Crystal Engineering. CrystEngComm 2013, 15, 4720–4730. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Spartz, C.L.; Dembowski, S.; Dwyre, S.; Desper, J. A systematic structural study of halogen bonding versus hydrogen bonding within competitive supramolecular systems. IUCrJ 2015, 2, 498–510. [Google Scholar] [CrossRef]
- Abeysekera, A.M.; Aakeröy, C.B.; Sinha, A.S.; Desper, J. Mapping out the Relative Influence of Hydrogen and Halogen Bonds in Crystal Structures of a Family of Amide-Substituted Pyridines. Cryst. Growth Des. 2020, 20, 6078–6088. [Google Scholar] [CrossRef]
- Gamekkanda, J.; Sinha, A.S.; Desper, J.; Đaković, M.; Aakeröy, C.B. Competition between hydrogen bonds and halogen bonds: A structural study. New J. Chem. 2018, 42, 10512–10520. [Google Scholar] [CrossRef]
- Meier, C.; Seichter, W.; Mazik, M. Combination of Hydrogen and Halogen Bonds in the Crystal Structures of 5-Halogeno-1H-isatin-3-oximes: Involvement of the Oxime Functionality in Halogen Bonding. Molecules 2024, 29, 1174. [Google Scholar] [CrossRef]
- Iddon, B.; Khan, N. Azoles. Part 5. Metal-halogen exchange reactions of polybromoimidazoles. J. Chem. Soc. Perkin Trans. 1 1987, 1987, 1445–1451. [Google Scholar] [CrossRef]
- Lovely, C.; Du, H.; He, Y.; Sivappa, R. New Methods of Imidazole Functionalization—From Imidazole to Marine Alkaloids. Synlett 2006, 2006, 0965–0992. [Google Scholar] [CrossRef]
- Lima, H.M.; Garcia-Barboza, B.J.; Khatibi, N.N.; Lovely, C.J. Total syntheses of isonaamine C and isonaamidine E. Tetrahedron Lett. 2011, 52, 5725–5727. [Google Scholar] [CrossRef][Green Version]
- Lovely, C.J.; Du, H.; Sivappa, R.; Bhandari, M.R.; He, Y.; Dias, H.V. Preparation and Diels-Alder chemistry of 4-vinylimidazoles. J. Org. Chem. 2007, 72, 3741–3749. [Google Scholar] [CrossRef]
- Heller, S.T.; Sarpong, R. Chemoselective esterification and amidation of carboxylic acids with imidazole carbamates and ureas. Org. Lett. 2010, 12, 4572–4575. [Google Scholar] [CrossRef]
- APEX5, Version 2023.9.2; Bruker AXS Inc.: Madison, WI, USA, 2023.
- SAINT, Version 8.40B; Includes Xprep and SADABS, Eventuell TWINABS; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Burnett, M.N.; Johnson, C.K. ORTEP-III Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations; Oak Ridge National Laboratory Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1996. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, 1987, S1–S19. [Google Scholar] [CrossRef]
- Noland, W.E.; Cole, K.P.; Britton, D. 4,5-Dibromo-1-methyl-1H-imidazole. Acta Crystallogr. E 2003, 59, o458–o460. [Google Scholar] [CrossRef]
- Jimbo, T.; Tsuji, M.; Taniguchi, R.; Sada, K.; Kokado, K. Control of Aggregation-Induced Emission from a Tetraphenylethene Derivative Through the Components in the Co-crystal. Cryst. Growth Des. 2018, 18, 3863–3869. [Google Scholar] [CrossRef]
- Hosten, E.C.; Betz, R. Crystal structure of 4-bromo-1H-imidazole, at 200 K, C3H3BrN2. Z. Kristallogr. N. Cryst. Struct. 2015, 230, 27–28. [Google Scholar] [CrossRef][Green Version]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Troff, R.W.; Mäkelä, T.; Topić, F.; Valkonen, A.; Raatikainen, K.; Rissanen, K. Alternative Motifs for Halogen Bonding. Eur. J. Org. Chem. 2013, 2013, 1617–1637. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Gamekkanda, J.; Sinha, A.; Desper, J.; Ðaković, M.; Aakeröy, C. The Role of Halogen Bonding in Controlling Assembly and Organization of Cu(II)-Acac Based Coordination Complexes. Crystals 2017, 7, 226. [Google Scholar] [CrossRef]
- Węcławik, M.; Gągor, A.; Piecha, A.; Jakubas, R.; Medycki, W. Synthesis, crystal structure and phase transitions of a series of imidazolium iodides. CrystEngComm 2013, 15, 5633–5640. [Google Scholar] [CrossRef]
- Nwachukwu, C.I.; Bowling, N.P.; Bosch, E. C-I···N and C-I···pi halogen bonding in the structures of 1-benzyliodoimidazole derivatives. Acta Crystallogr. C 2017, 73, 2–8. [Google Scholar] [CrossRef]
- Wu, Y.; Izquierdo, S.; Vidossich, P.; Lledos, A.; Shafir, A. NH-Heterocyclic Aryliodonium Salts and Their Selective Conversion into N1-Aryl-5-iodoimidazoles. Angew. Chem. Int. Ed. Engl. 2016, 55, 7152–7156. [Google Scholar] [CrossRef]
- Riwar, L.J.; Trapp, N.; Kuhn, B.; Diederich, F. Substituent Effects in Parallel-Displaced pi-pi Stacking Interactions: Distance Matters. Angew. Chem. Int. Ed. 2017, 56, 11252–11257. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Nishio, M. The CH/pi hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef]
- Chernysheva, M.V.; Bulatova, M.; Ding, X.; Haukka, M. Influence of Substituents in the Aromatic Ring on the Strength of Halogen Bonding in Iodobenzene Derivatives. Cryst. Growth Des. 2020, 20, 7197–7210. [Google Scholar] [CrossRef]
- Padgett, C.W.; Dean, R.; Cobb, A.; Miller, A.; Goetz, A.; Bailey, S.; Hillis, K.; McMillen, C.; Toney, S.; Guillet, G.L.; et al. Comparison of N···I and N···O Halogen Bonds in Organoiodine Cocrystals of Heterocyclic Aromatic Diazine Mono-N-oxides. Cryst. Growth Des. 2024, 24, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Knight, K.S.; Marshall, W.G.; Coles, S.J.; Horton, P.N.; Pitak, M.B. The competition between halogen bonds (Br···O) and C–H···O hydrogen bonds: The structure of the acetone–bromine complex revisited. CrystEngComm 2013, 15, 8572–8577. [Google Scholar] [CrossRef]
- Kikkawa, S.; Takeno, M.; Nakayama, T.; Koike, D.; Saito, Y.; Tashiro, M.; Aoyama, Y.; Hikawa, H.; Azumaya, I. Effect of Br···O Bonding on the Chiral Assembly of Brominated Amides in the Crystalline Phase. Cryst. Growth Des. 2024, 24, 9564–9570. [Google Scholar] [CrossRef]
- Fotović, L.; Bedeković, B.; Pičuljan, K.; Stilinović, V. Order versus Disorder in the Cocrystals of m-Halogenopyridines with m-Halogenobenzoic Acids: The Effects of the I···O Halogen Bond. Cryst. Growth Des. 2022, 12, 7508–7517. [Google Scholar] [CrossRef]

 , N: ●, O: ●, I: ●).
, N: ●, O: ●, I: ●).
 , N: ●, O: ●, I: ●).
, N: ●, O: ●, I: ●).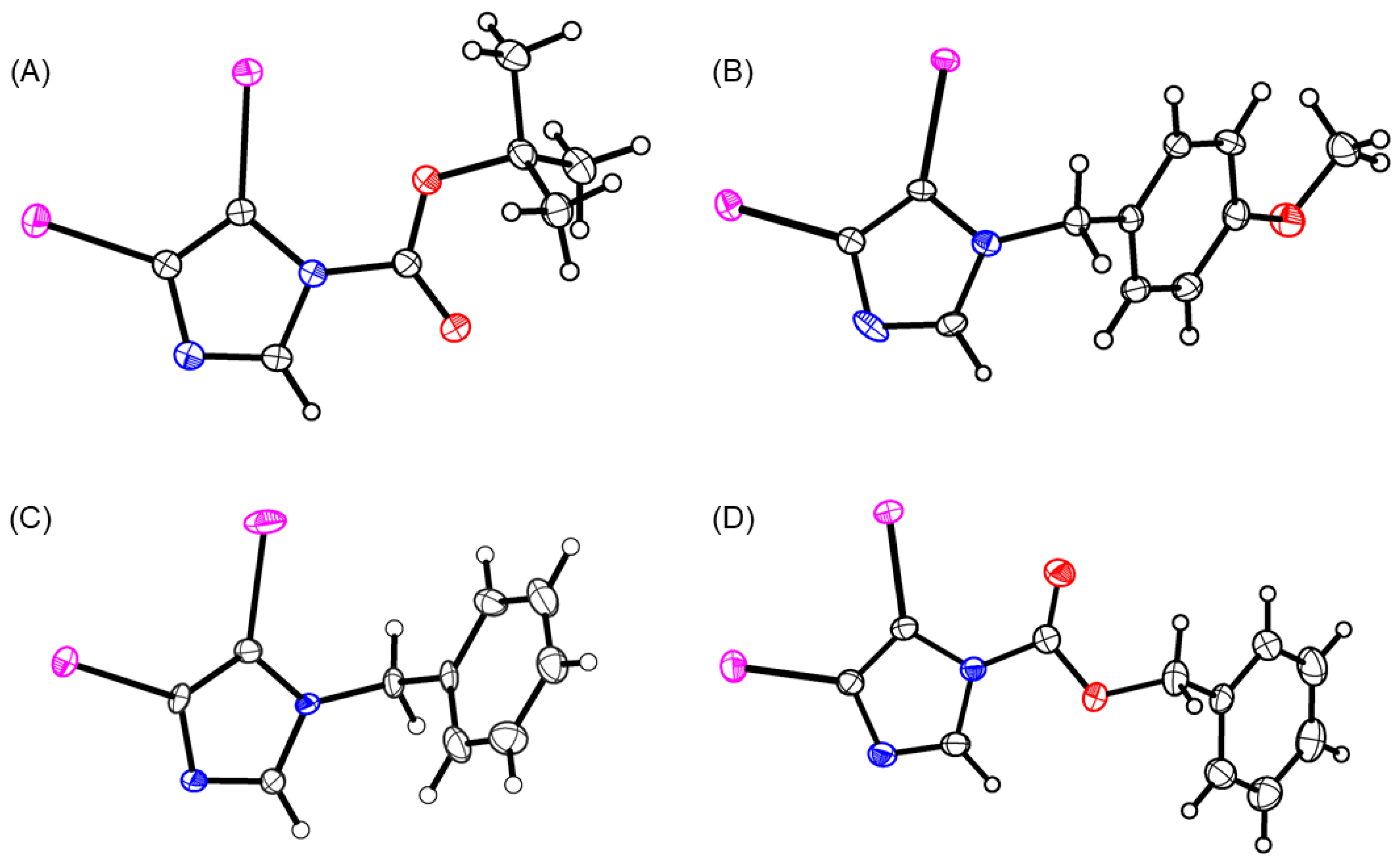
 , N: ●, O: ●, Br: ●).
, N: ●, O: ●, Br: ●).
 , N: ●, O: ●, Br: ●).
, N: ●, O: ●, Br: ●).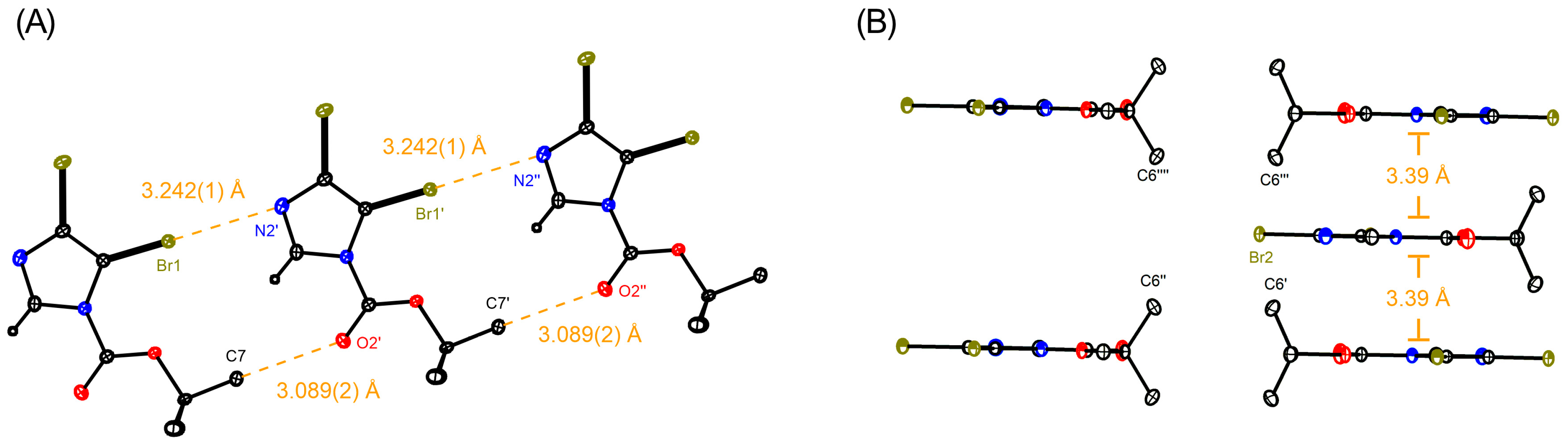
 , N: ●, O: ●, I: ●).
, N: ●, O: ●, I: ●).
 , N: ●, O: ●, I: ●).
, N: ●, O: ●, I: ●).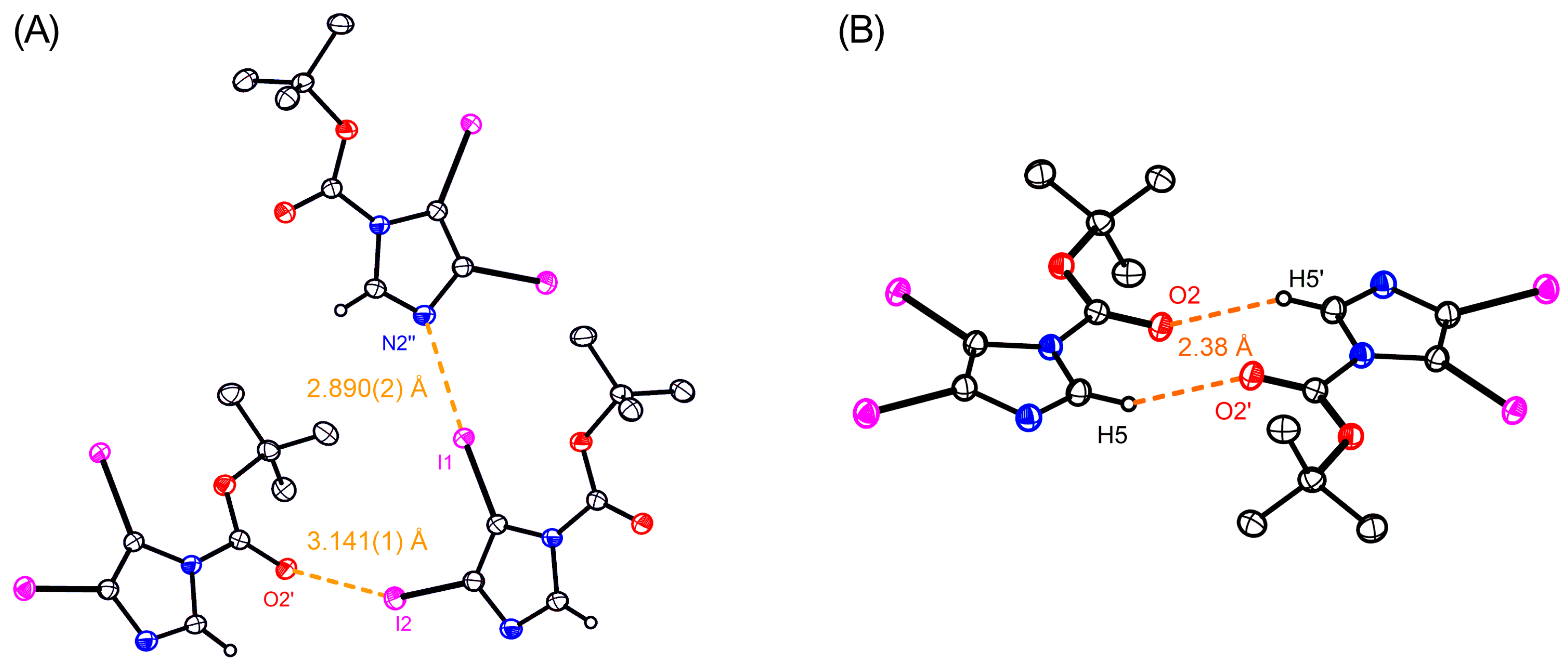
 , N: ●, O: ●, Br: ●, I: ●).
, N: ●, O: ●, Br: ●, I: ●).
 , N: ●, O: ●, Br: ●, I: ●).
, N: ●, O: ●, Br: ●, I: ●).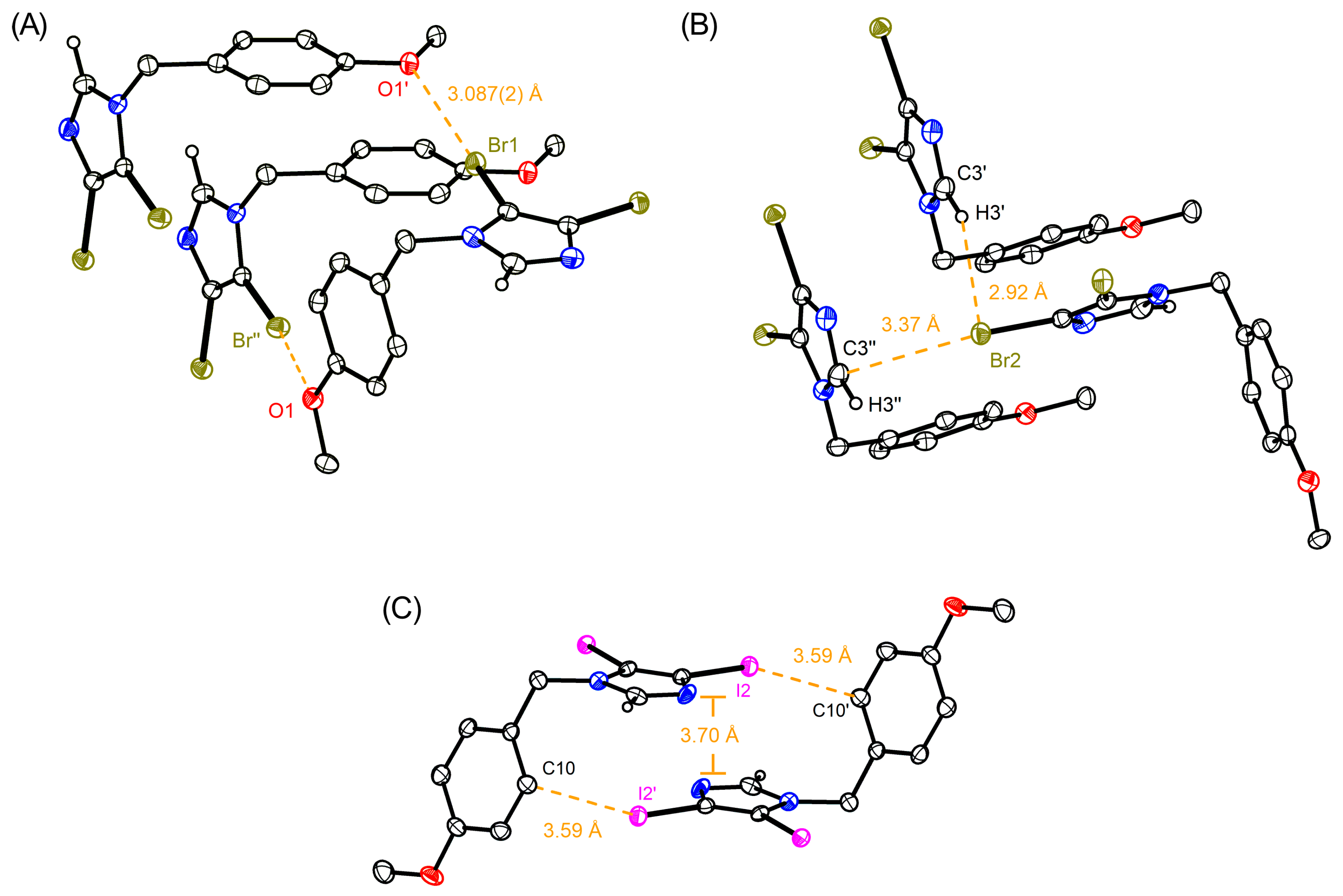
| Cmpd 3a | Cmpd 3b | Cmpd 3c | Cmpd 3d | Cmpd 4a | Cmpd 4b | Cmpd 4c | Cmpd 4d | |
|---|---|---|---|---|---|---|---|---|
| CCDC | 2499326 | 2499330 | 2499331 | 2499327 | 2499329 | 2499334 | 2499328 | 2499335 |
| Empirical formula | C8H10Br2N2O2 | C11H10Br2N2O | C10H8Br2N2 | C11H8Br2N2O2 | C8H10I2N2O2 | C11H10I2N2O | C10H8I2N2 | C11H8I2N2O2 |
| Formula weight | 326.00 | 346.03 | 316.00 | 360.01 | 419.98 | 440.01 | 409.98 | 453.99 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Trigonal | Orthorhombic | Orthorhombic | Monoclinic | Trigonal |
| Space group | Pnma | P212121 | P212121 | R-3 | Pbca | Pbca | P21 | R-3 |
| a, Å | 7.2871(2) | 4.82760(10) | 6.64120(10) | 28.4883(3) | 13.8801(2) | 14.1428(8) | 11.5334(17) | 29.2203(6) |
| b, Å | 6.7709(2) | 10.0063(3) | 12.3670(3) | 28.4883(3) | 11.0928(2) | 8.4991(5) | 14.1707(19) | 29.2203(6) |
| c, Å | 21.5274(5) | 24.5484(6) | 12.6667(3) | 8.38630(10) | 15.9440(2) | 21.0899(12) | 14.215(3) | 8.5898(3) |
| α, deg. | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| β, deg. | 90 | 90 | 90 | 90 | 90 | 90 | 92.286(6) | 90 |
| γ, deg. | 90 | 90 | 90 | 120 | 90 | 90 | 90 | 120 |
| V, Å3 | 1062.17(5) | 1185.85(5) | 1040.34(4) | 5894.32(14) | 2454.88(6) | 2535.0(3) | 2321.5(7) | 6351.6(3) |
| Z | 4 | 4 | 4 | 18 | 8 | 8 | 8 | 18 |
| ρcalc, g cm−3 | 2.039 | 1.938 | 2.018 | 1.826 | 2.273 | 2.306 | 2.346 | 2.136 |
| μ(MoKα), mm−1 | 7.608 | 6.815 | 7.751 | 6.180 | 5.104 | 4.943 | 5.383 | 4.448 |
| Crystal size, mm3 | 0.19 × 0.17 × 0.06 | 0.19 × 0.13 × 0.08 | 0.22 × 0.17 × 0.07 | 0.29 × 0.17 × 0.12 | 0.19 × 0.18 × 0.13 | 0.21 × 0.07 × 0.05 | 0.13 × 0.07 × 0.04 | 0.56 × 0.05 × 0.05 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 123(2) | 145(2) | 145(2) |
| θ range, deg | 2.951–30.117 | 2.198–30.044 | 2.302–30.090 | 2.477–30.105 | 2.555–34.669 | 2.409–27.488 | 1.767–30.048 | 2.504–27.500 |
| hkl range, deg | −10:10, −9:9, −30:30 | −6:6, −14:14, −34:34 | −9:9, −17:17, −17:17 | −40:40, −40:40, −11:11 | −22:22, −17:17, −25:25 | −18:18, −11:11, −27:27 | −16:15, −19:19, −20:19 | −37:37, −37:37, −11:11 |
| Total/unique data/Rint | 18227/1687/0.0248 | 19884/3466/0.0463 | 18798/3053/0.0300 | 35924/3854/0.0298 | 141626/4969/0.0423 | 22199/2906/0.0342 | 7011/7011/0.0201 | 30218/3229/0.0276 |
| Data/restraints/parameters | 1687/0/84 | 3466/0/146 | 3053/475/200 | 3854/0/154 | 4969/0/130 | 2906/0/146 | 7011/1/505 | 3229/0/154 |
| R1/wR2 [I > 2σ(I)] | 0.0123/0.0311 | 0.0237/0.0556 | 0.0134/0.0315 | 0.0150/0.0359 | 0.0187/0.0486 | 0.0312/0.0854 | 0.0434/0.1065 | 0.0129/0.0314 |
| R1/wR2 [all data] | 0.0129/0.0314 | 0.0261/0.0564 | 0.0140/0.0317 | 0.0166/0.0363 | 0.0240/0.0506 | 0.0339/0.0868 | 0.0446/0.1073 | 0.0135/0.0316 |
| Flack parameter | na | 0.004(6) | 0.003(5) | na | na | na | 0.12(5) | na |
| S | 1.122 | 1.031 | 1.047 | 1.074 | 1.096 | 1.249 | 1.133 | 1.100 |
| Min./max. res. dens., eÅ−3 | 0.464/−0.299 | 0.945/−0.423 | 0.211/−0.231 | 0.463/−0.385 | 0.596/−1.351 | 1.070/−1.046 | 2.498/−1.233 | 0.561/−0.568 |
| Compound | N···X [Å] | O···X [Å] | X···X [Å] |
|---|---|---|---|
| 3a | 3.242(1) | 3.6241(4) | nd a |
| 4a | 2.890(2) | 3.141(1) | nd a |
| 3b | 3.458(3) | 3.087(2) | nd a |
| 4b | 2.818(4) | nd a | nd a |
| 3c | nd a | na b | nd a |
| 4c | 2.96(1) | na b | 3.866(1) |
| 3d | 2.921(1) | nd a | 3.9404(7) |
| 4d | 2.936(1) | nd a | nd a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensing, L.; Layh, M.; Hebenbrock, M. Halogens On, H-Bonds Off—Insights into Structure Control of Dihalogenated Imidazole Derivatives. Crystals 2025, 15, 1000. https://doi.org/10.3390/cryst15111000
Mensing L, Layh M, Hebenbrock M. Halogens On, H-Bonds Off—Insights into Structure Control of Dihalogenated Imidazole Derivatives. Crystals. 2025; 15(11):1000. https://doi.org/10.3390/cryst15111000
Chicago/Turabian StyleMensing, Luca, Marcus Layh, and Marian Hebenbrock. 2025. "Halogens On, H-Bonds Off—Insights into Structure Control of Dihalogenated Imidazole Derivatives" Crystals 15, no. 11: 1000. https://doi.org/10.3390/cryst15111000
APA StyleMensing, L., Layh, M., & Hebenbrock, M. (2025). Halogens On, H-Bonds Off—Insights into Structure Control of Dihalogenated Imidazole Derivatives. Crystals, 15(11), 1000. https://doi.org/10.3390/cryst15111000






