Study on Preparation and Properties of Industrial Lignin Metallized Pellets for Ironmaking
Abstract
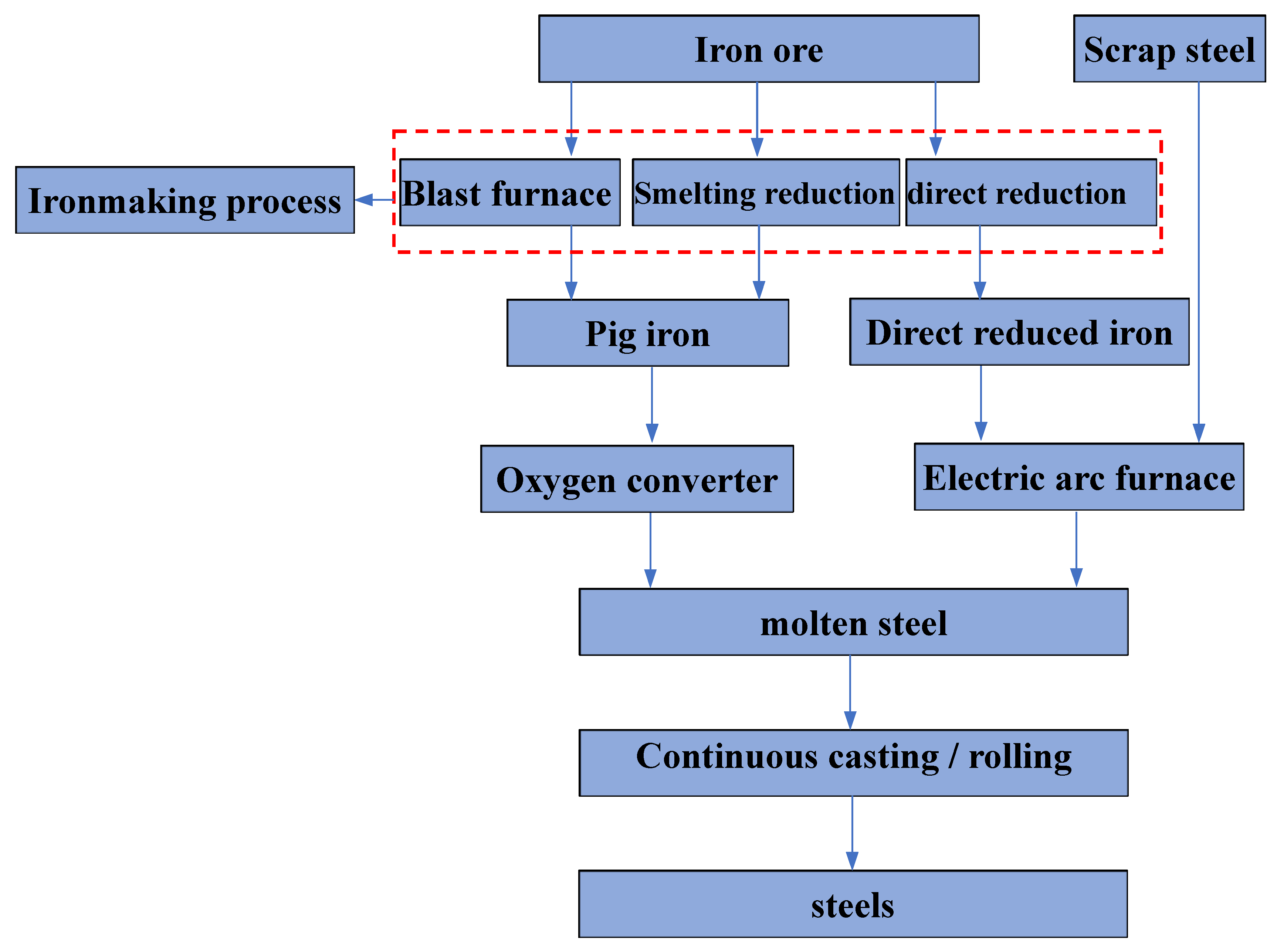
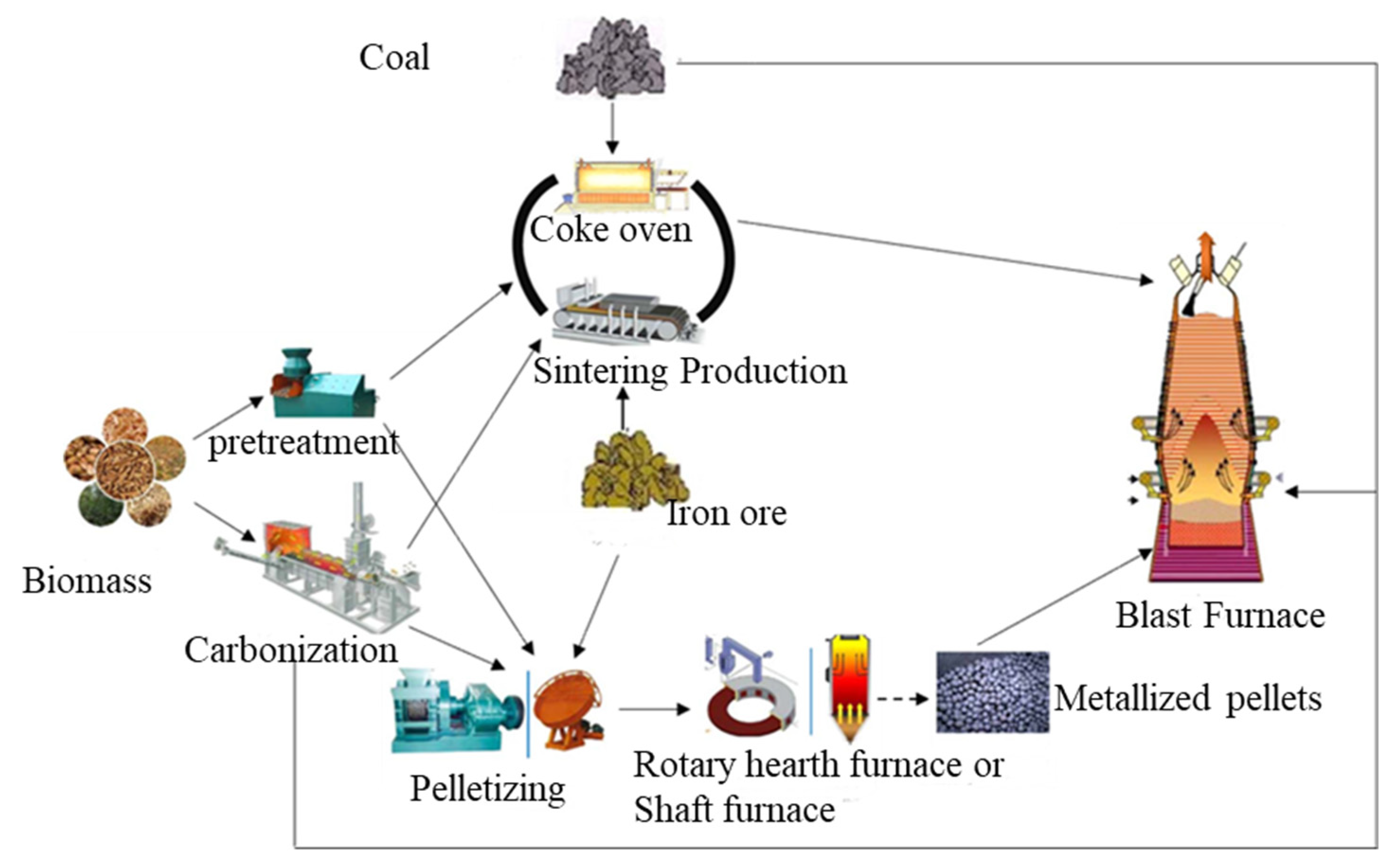
1. Introduction
2. Raw Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Element and Component Analysis
2.2.2. The Preparation of Green Pellets
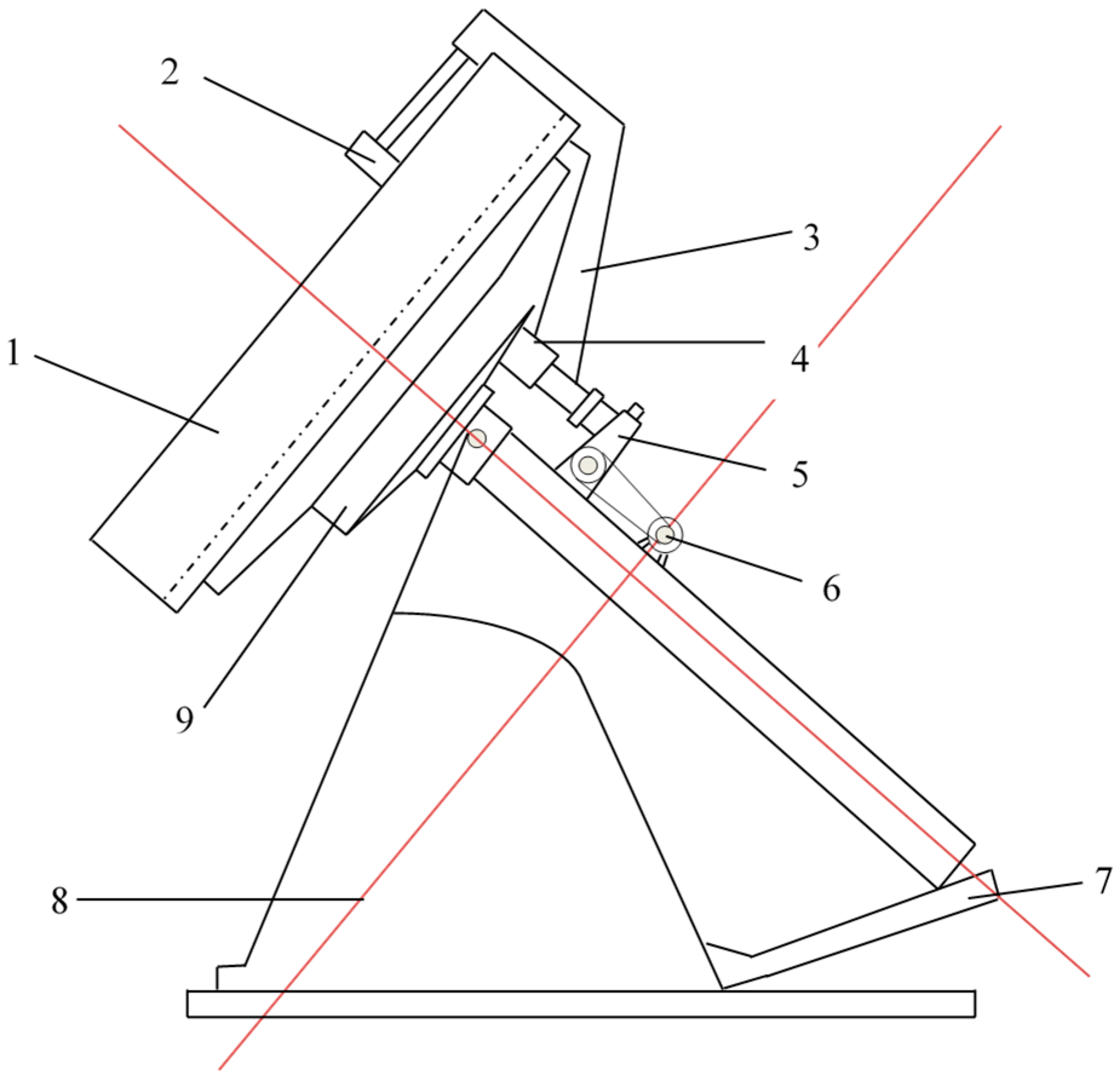
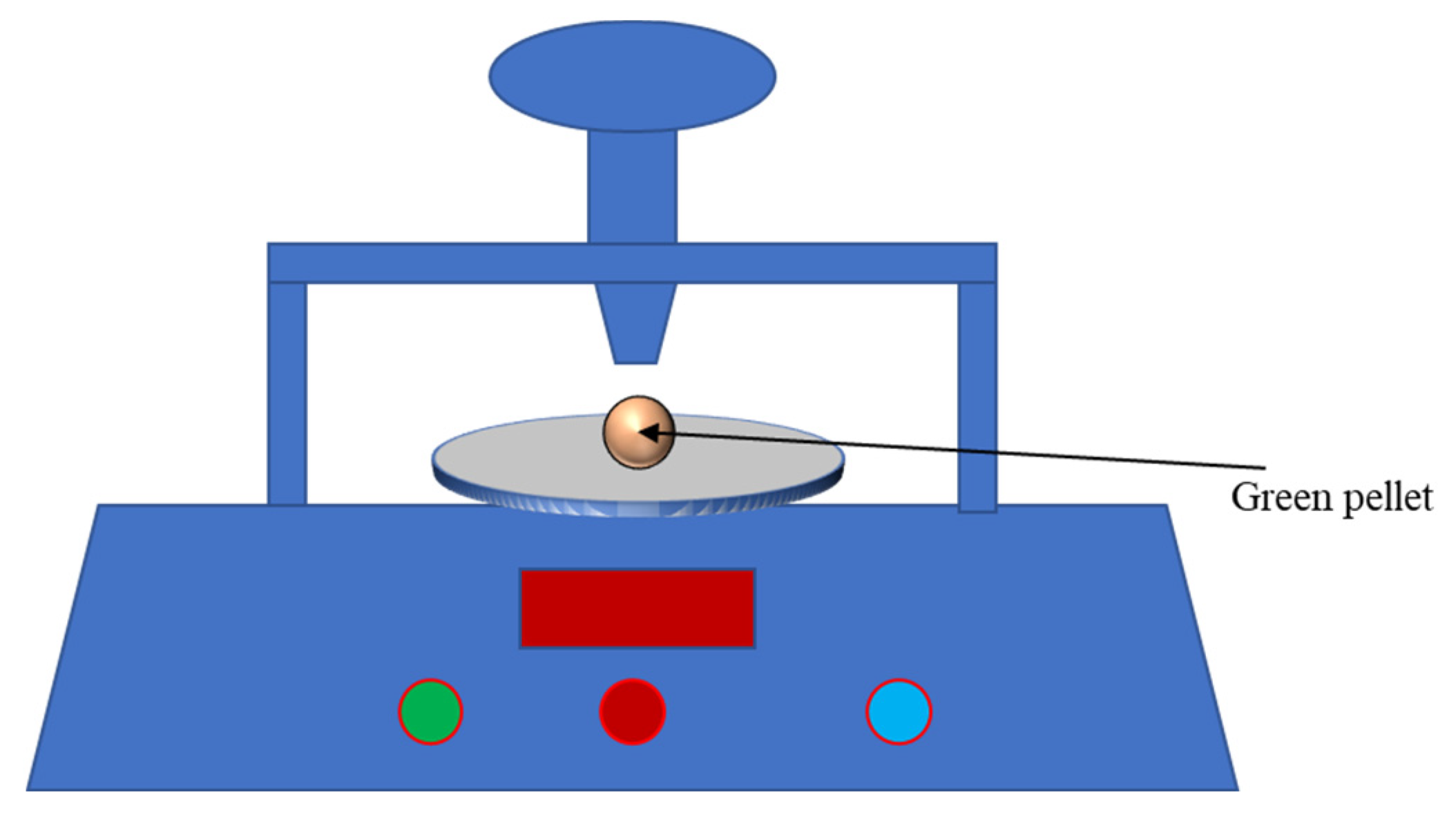
2.2.3. The Falling Strength of Green Pellets
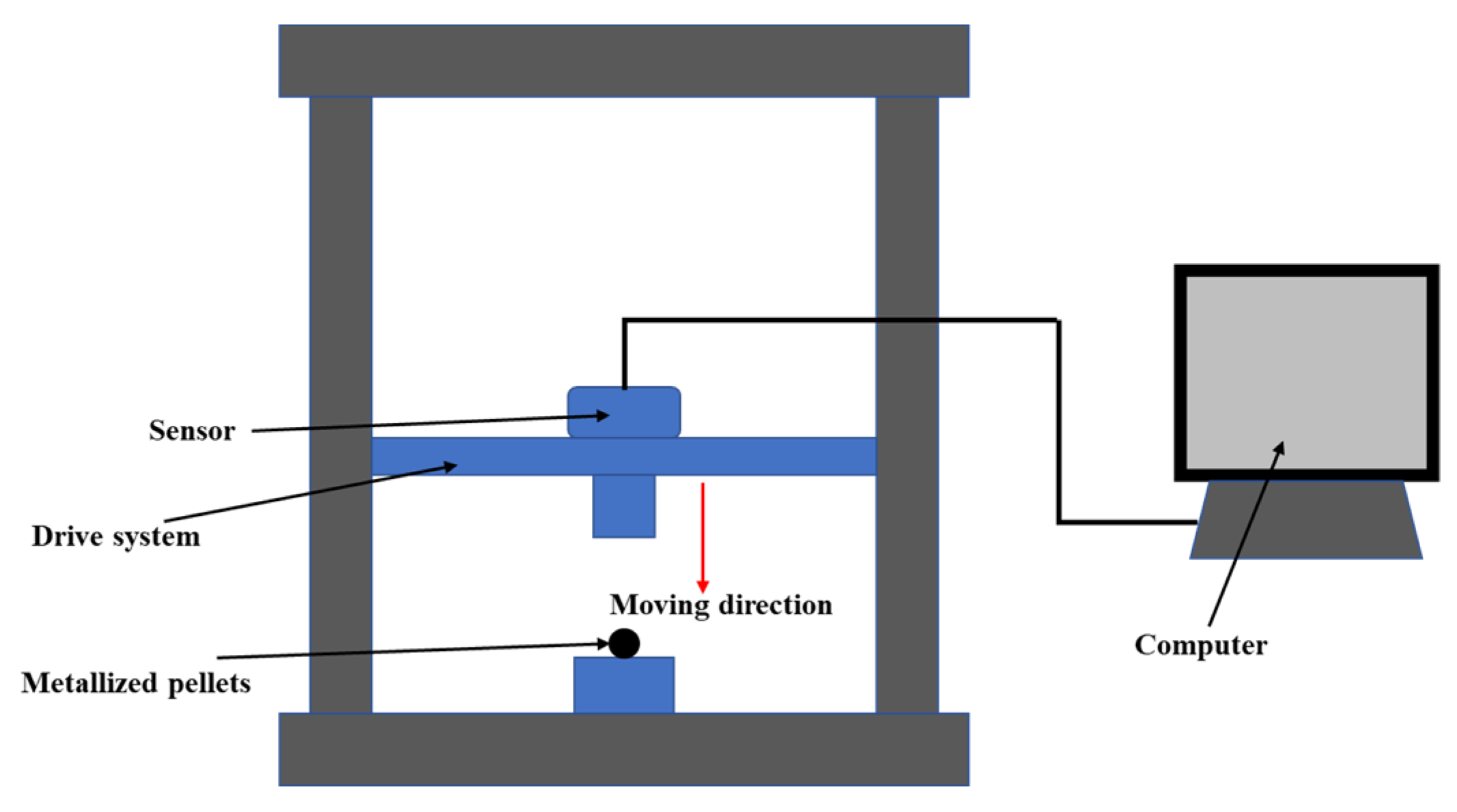
2.2.4. The Compressive Strength of Green Pellets
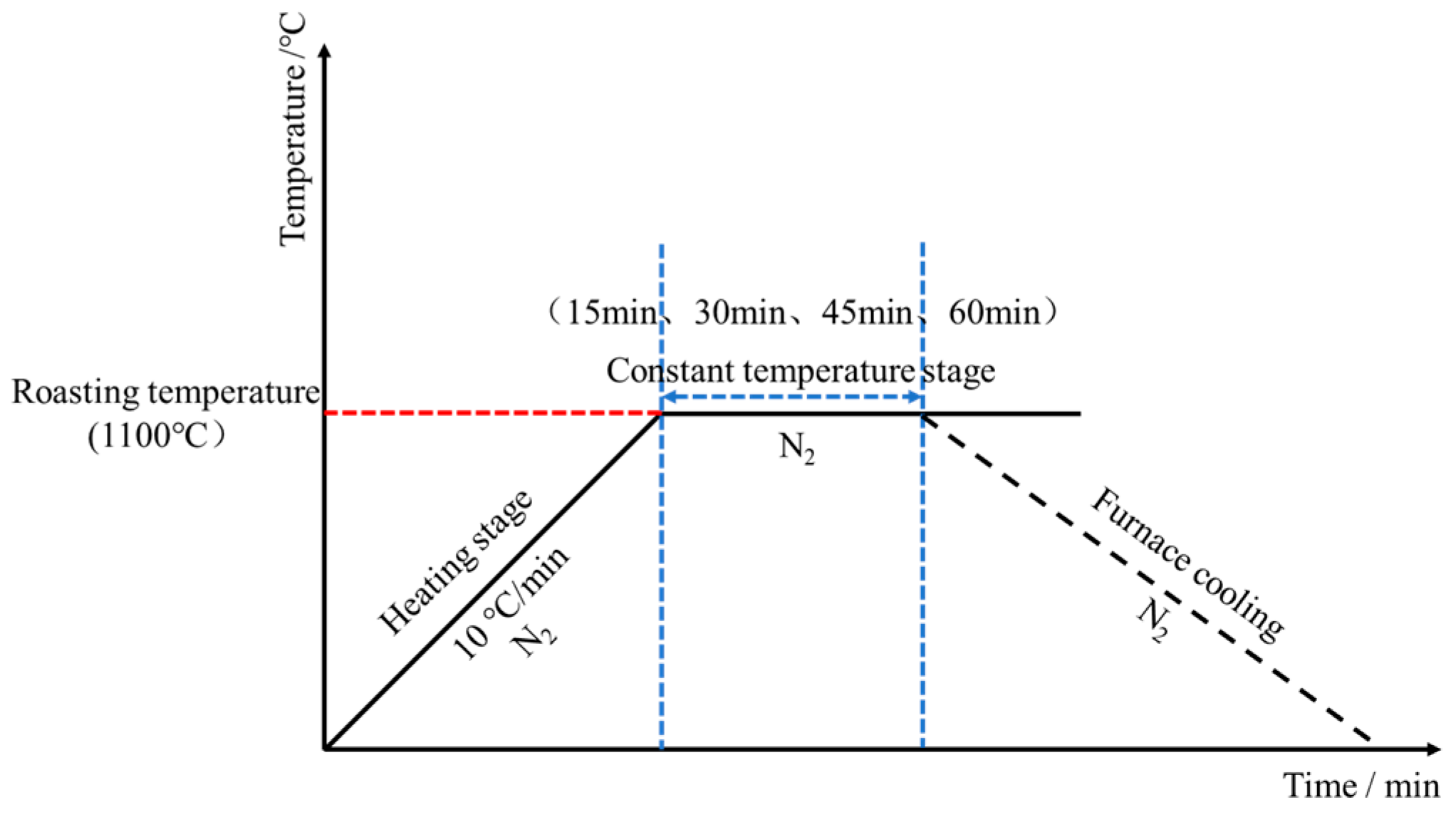
2.2.5. The Roasting of Green Pellets
2.2.6. The Compressive Strength of Green Pellets After Roasting
3. Results and Discussion
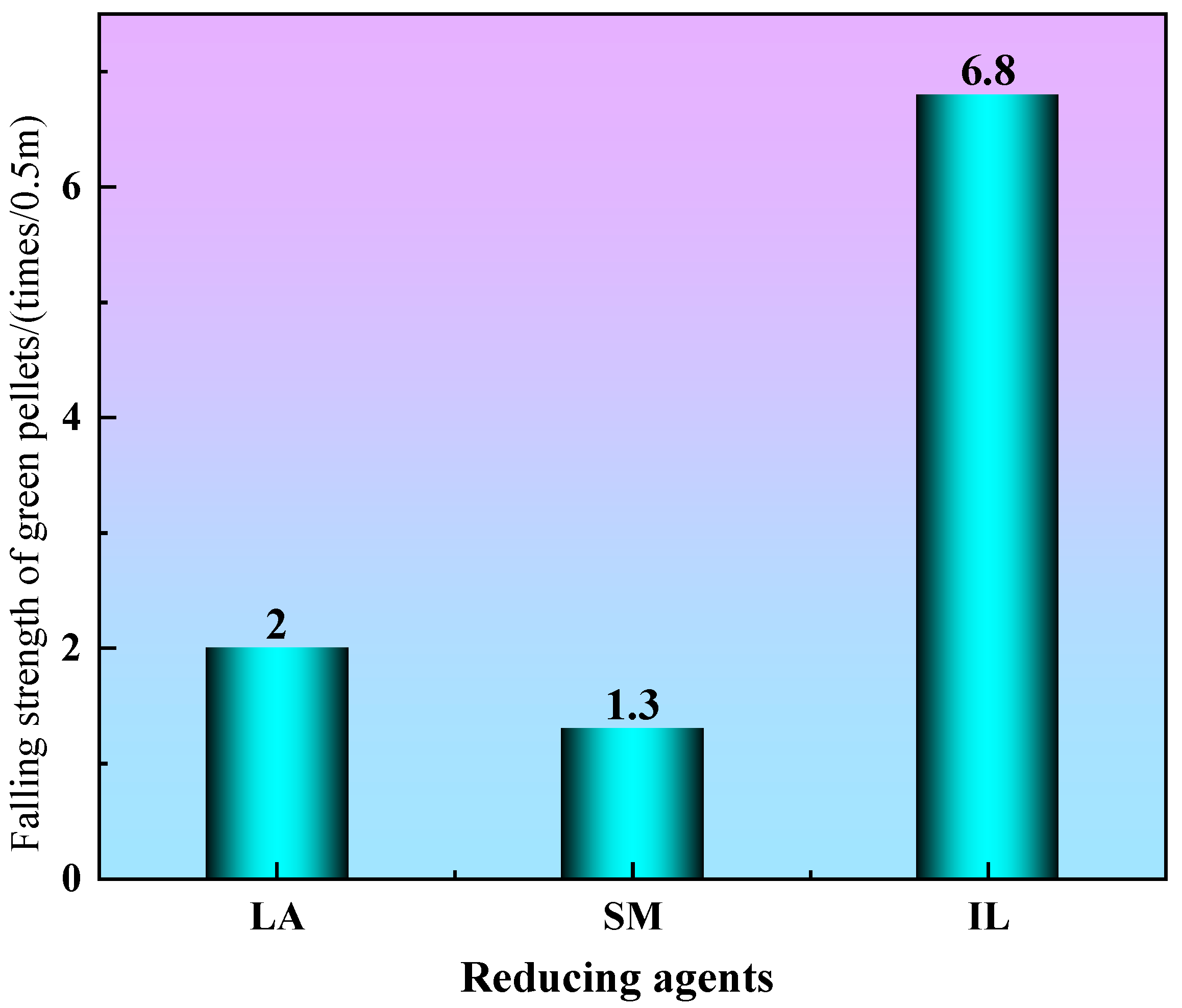
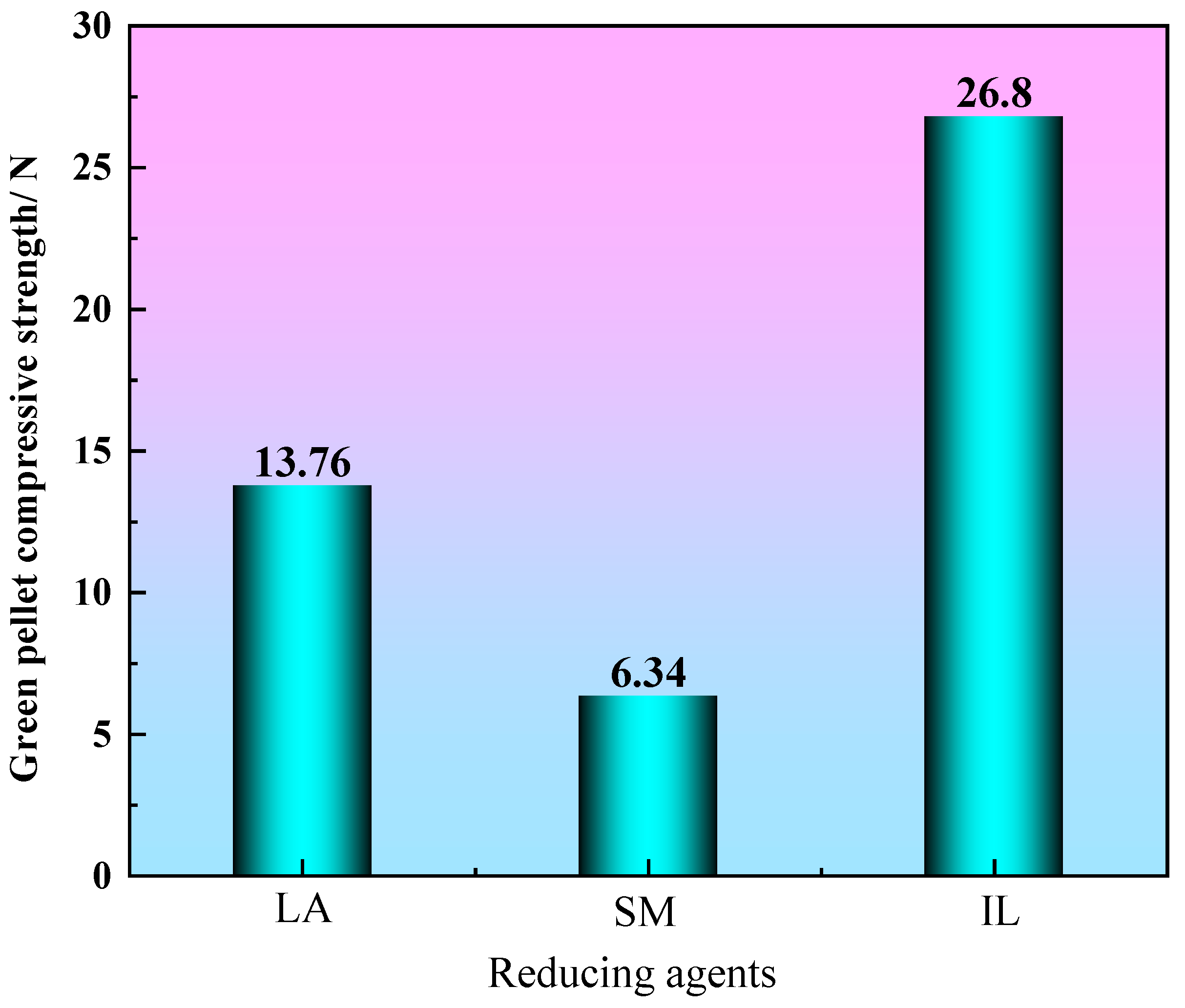
3.1. The Strength of Green Pellets
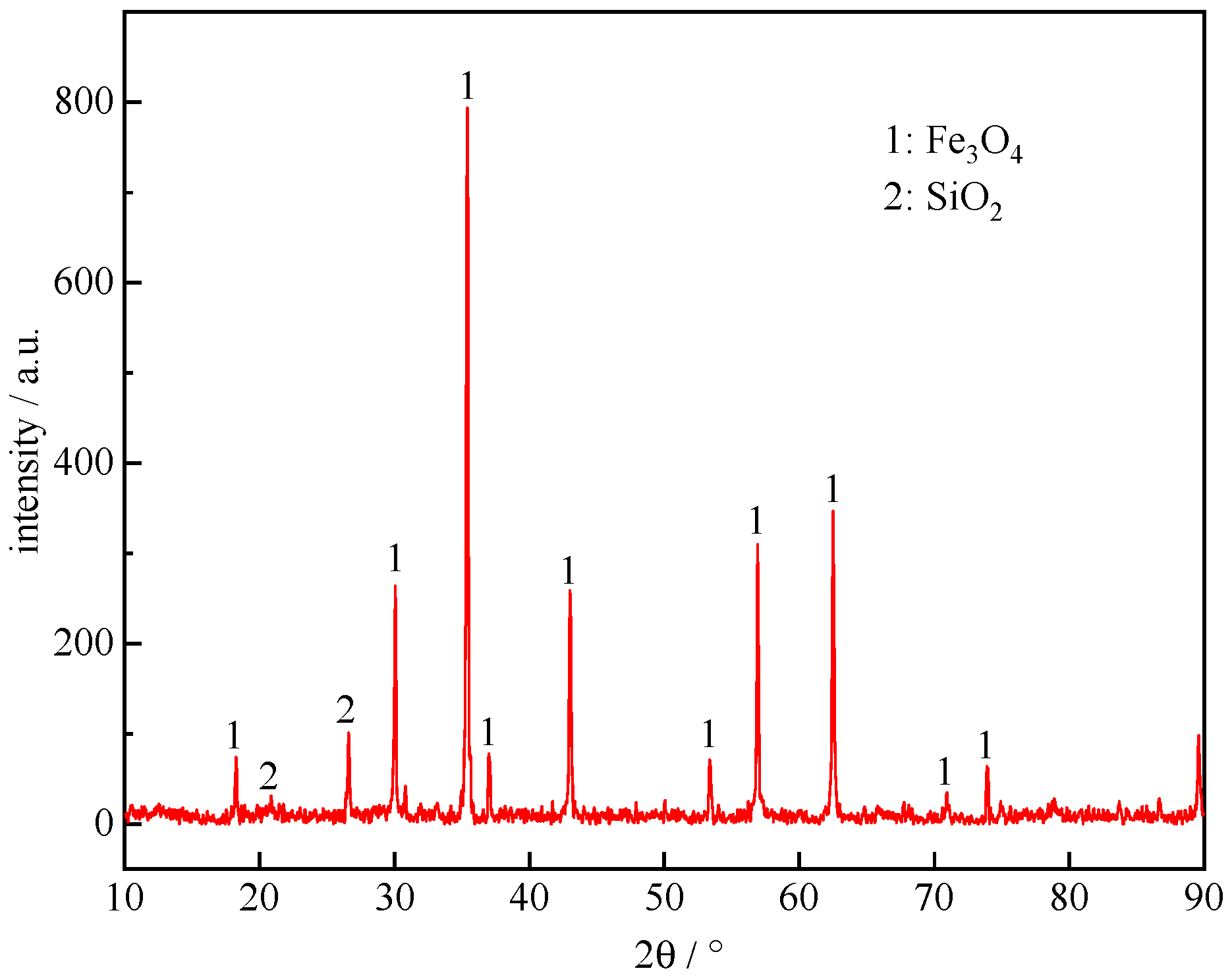
3.2. The Strength of Metallized Pellets
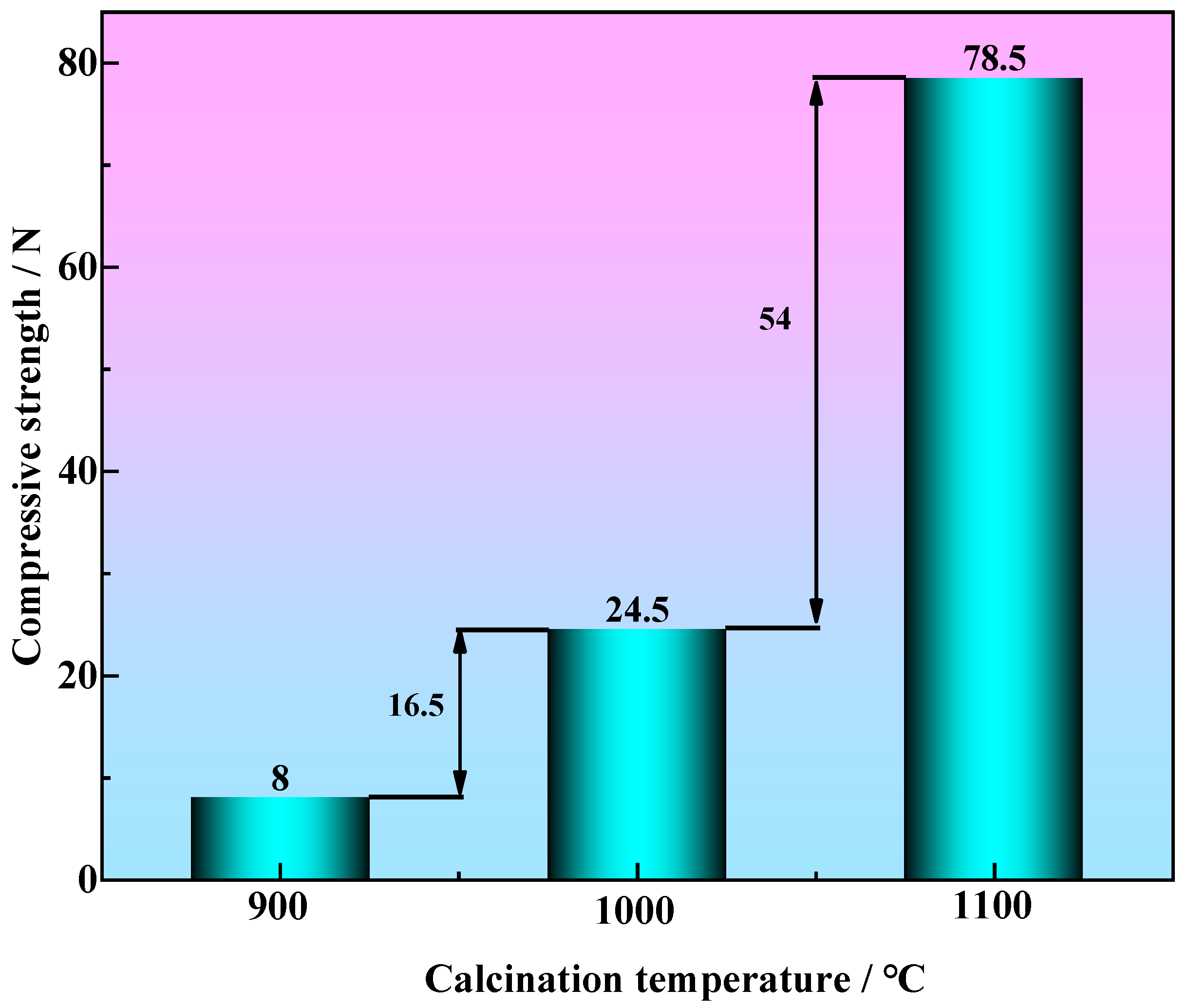
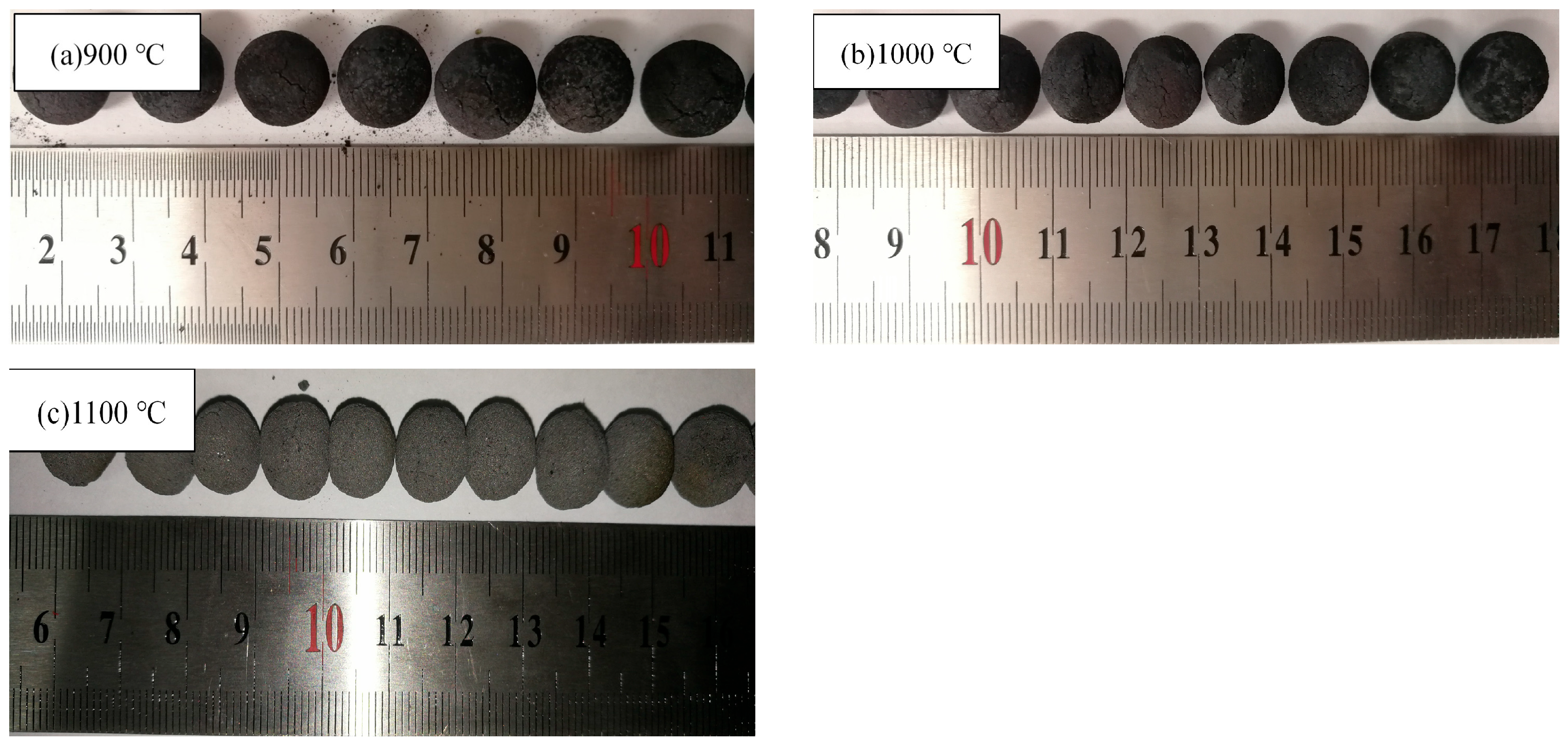
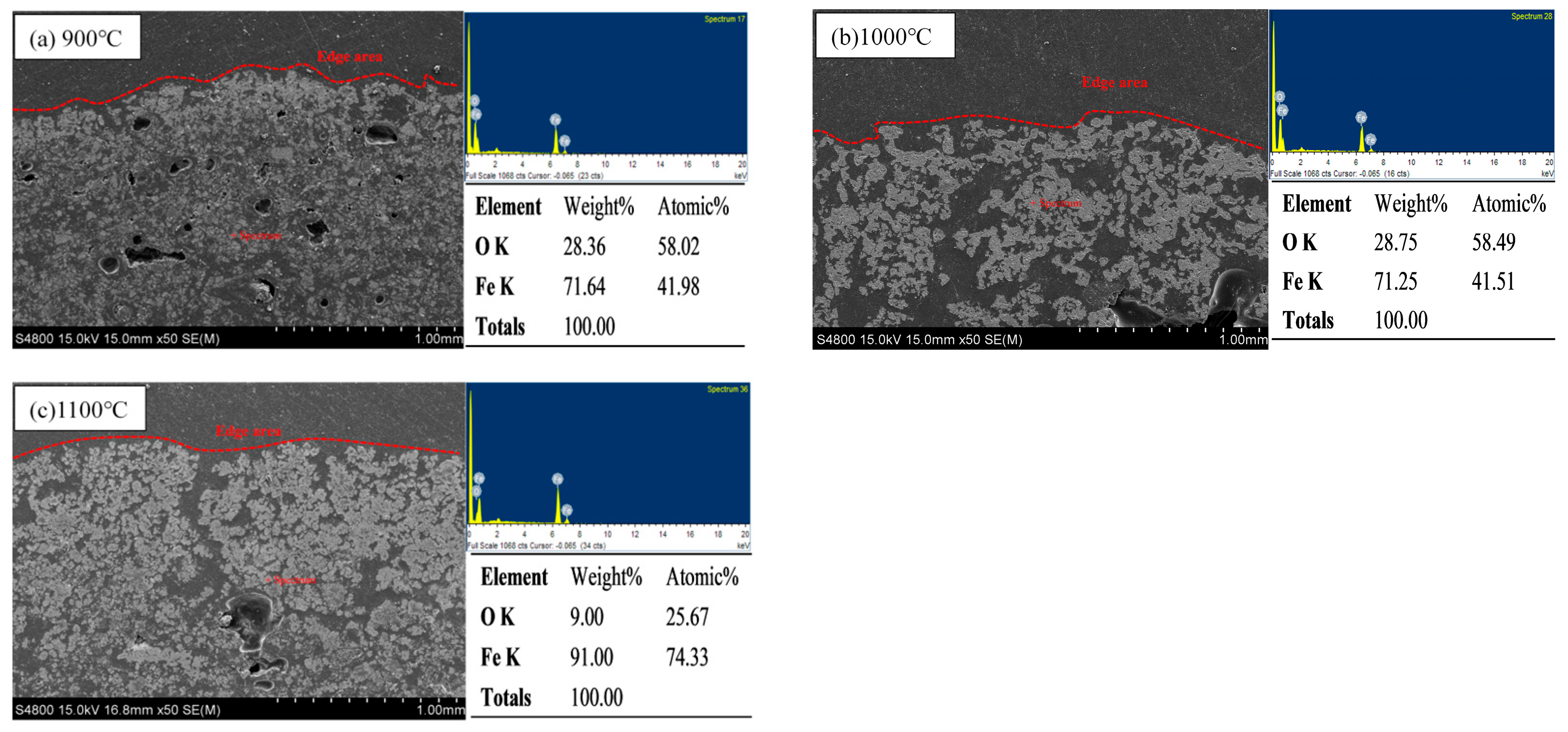
3.2.1. The Effect of Roasting Temperature on the Compressive Strength of Metallized Pellets
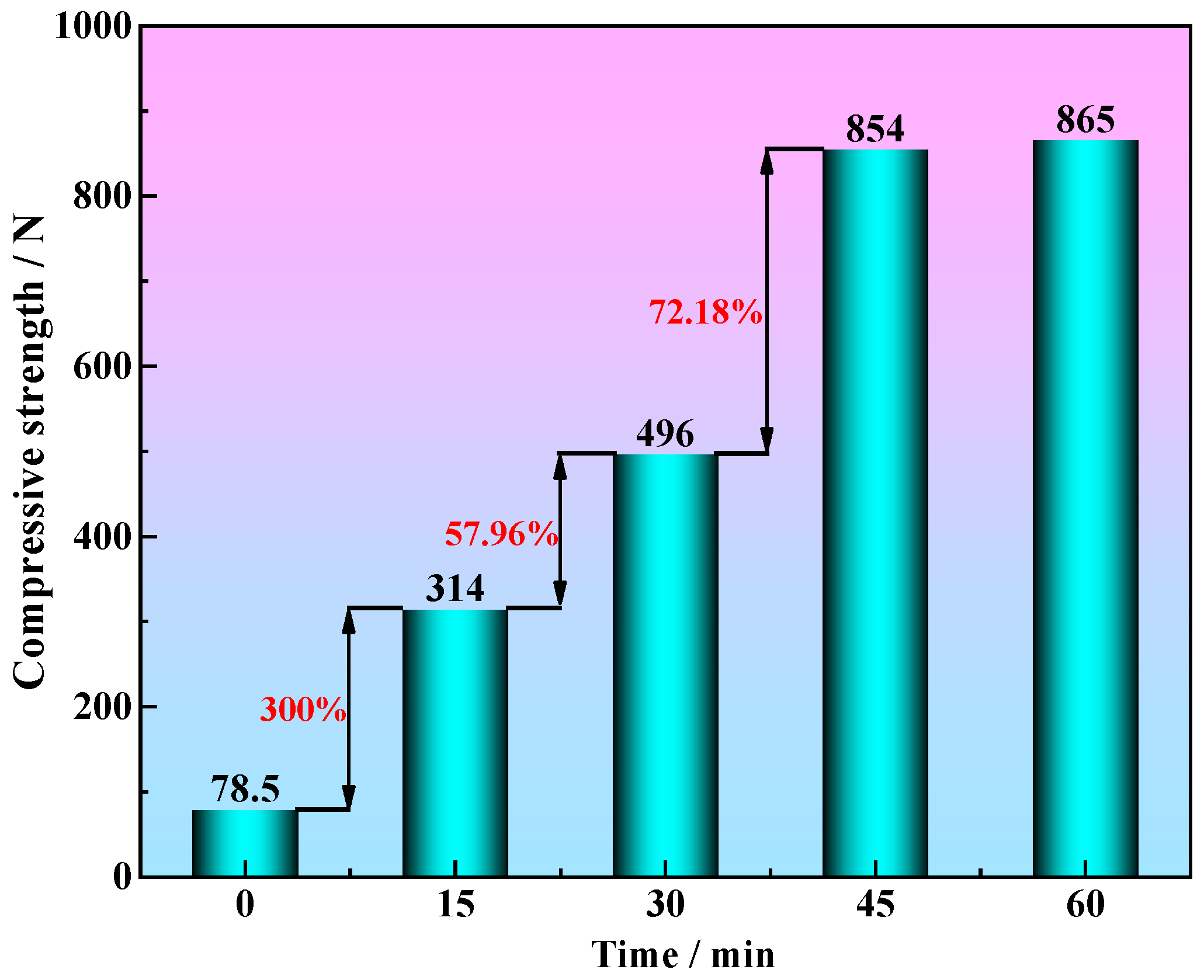
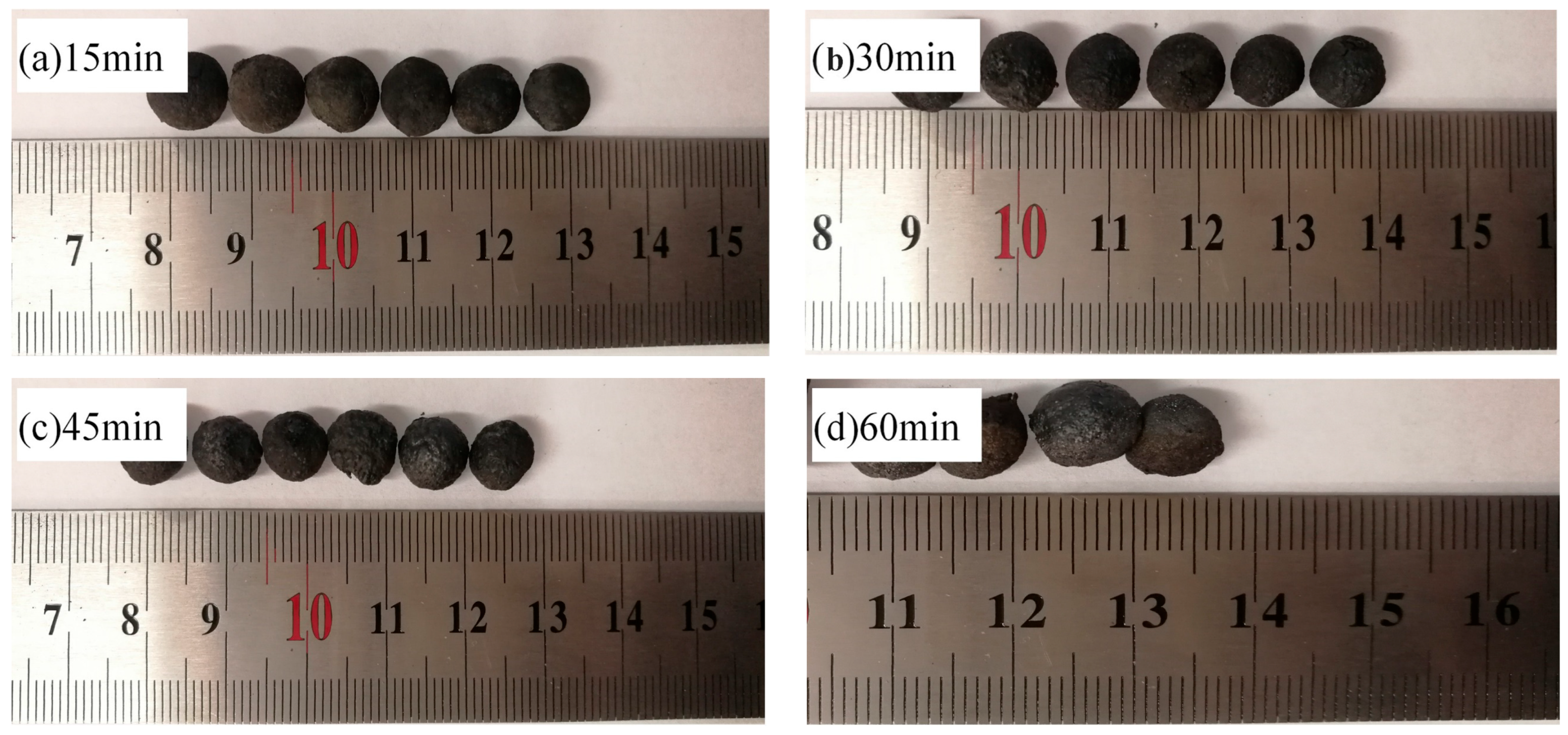
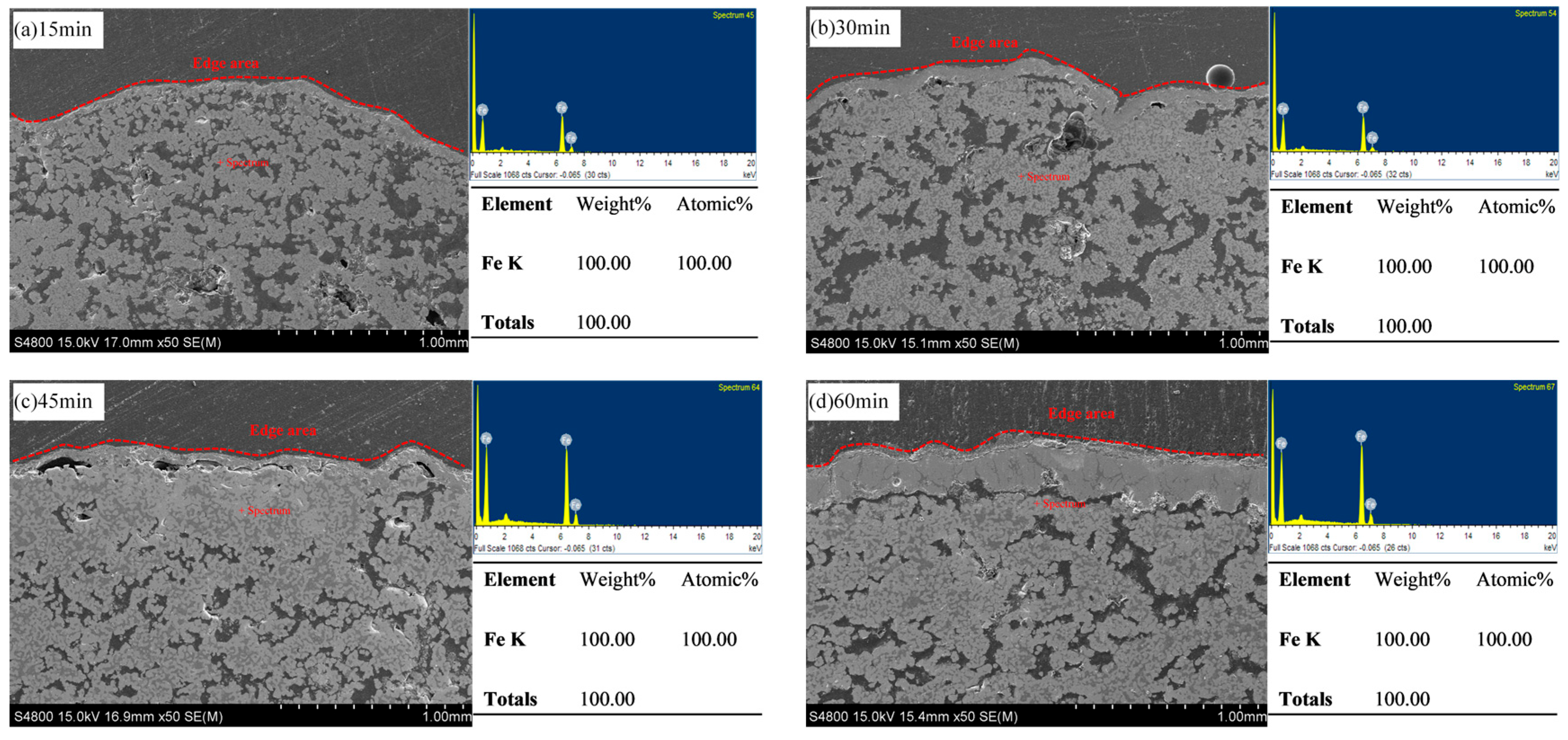
3.2.2. The Effect of Holding Time on the Compressive Strength of Metallized Pellets
4. Conclusions
- 1.
- Because industrial lignin contains a certain amount of hydroxyl, the falling strength and compressive strength of industrial lignin green pellets are 6.8 times/0.5 m and 26.8 N, respectively, which can meet the requirements of industrial production.
- 2.
- When the molar ratio of industrial lignin to iron ore powder is nc/no = 1.2, the roasting temperature is increased from 900 °C to 1100 °C and the compressive strength of the metallized pellets is increased from 8 N to 78.5 N. This is mainly because the higher reduction temperature can make the pellet structure more compact, with less voids, and the metal iron crystals can be connected into sheets.
- 3.
- When the molar ratio of industrial lignin to iron ore powder is nc/no = 1.2 and the roasting time is extended from 0 min to 45 min, the compressive strength of metallized pellets increases from 78.5 N to 854 N, which is an increase of about 988%. This is mainly due to the continuous formation of a relatively dense metal iron shell on the surface of the industrial lignin pellets.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bataille, C.; Åhman, M.; Neuhoff, K.; Nilsson, L.J.; Fischedick, M.; Lechtenböhmer, S.; Solano-Rodriquez, B.; Denis-Ryan, A.; Stiebert, S.; Waisman, H.; et al. A review of technology and policy deep decarbonization pathway options for making energy-intensive industry production consistent with the Paris Agreement. J. Clean. Prod. 2018, 187, 960–973. [Google Scholar] [CrossRef]
- Åhman, M.; Nilsson, L.J.; Johansson, B. Global climate policy and deep decarbonization of energy-intensive industries. Clim. Policy 2017, 17, 634–649. [Google Scholar] [CrossRef]
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies. Available online: https://www.worldsteel.org/en/dam/jcr:66fed386-fd0b-485e-aa23-b8a5e7533435/Position_paper_climate_2018.pdf (accessed on 20 May 2021).
- World Steel Association. Steel’s Contribution to a Low Carbon Future. Available online: https://www.worldsteel.org/zh/dam/jcr:2306a7a7-331c-491a-99a6-a093fb6ca96a/Position_paper_climate_2020_vfinal_CN.pdf (accessed on 20 May 2021).
- Zhang, X.; Wu, G.M.; Wu, S.H.; Xiang, X.D. Determination of carbon dioxide emission factors in typical processes for large iron-steel companies. Acta Sci. Circumst. 2012, 32, 2024–2027. [Google Scholar]
- Li, J.Y. Energy saving and environmental protection situation analysis and countermeasures of iron and steel enterprises. China Steel 2014, 03, 20–23. [Google Scholar]
- World Steel Association. 2024 World Steel in Figures. Available online: https://worldsteel.org/wp-content/uploads/WSIF-2024_CN.pdf (accessed on 10 December 2024).
- Han, J.; Huang, Z.; Qin, L.; Chen, W.; Zhao, B.; Xing, F. Refused derived fuel from municipal solid waste used as an alternative fuel during the iron ore sinter process. J. Clean. Prod. 2021, 278, 123594. [Google Scholar] [CrossRef]
- Zandi, M.; Martinez-Pacheco, M.; Fray, T. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Hara, M. Utilization of biomass for iron ore sintering. ISIJ Int. 2013, 53, 1599–1606. [Google Scholar] [CrossRef]
- Lu, L.; Adam, M.; Kilburn, M.; Hapugoda, S.; Somerville, M.; Jahanshahi, S.; Mathieson, J.G. Substitution of charcoal for coke breeze in iron ore sintering. ISIJ Int. 2013, 53, 1607–1616. [Google Scholar] [CrossRef]
- Fan, X.H.; Ji, Z.Y.; Gan, M.; Jiang, T.; Chen, X.L.; Li, W.Q.; Wang, Q.; Yu, Z.Y.; Huang, X.X.; Yuan, L.S. Application of biomass fuel in iron ore sintering. J. Cent. South Univ. (Sci. Technol.) 2013, 44, 1747–1753. [Google Scholar]
- Fu, J.X.; Zhang, C.; Hwang, W.S.; Liau, Y.T.; Lin, Y.T. Exploration of biomass char for CO2 reduction in RHF process for steel production. Int. J. Greenh. Gas Control 2012, 8, 143–149. [Google Scholar] [CrossRef]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Inoue, R.; Ariyama, T. Improvement of reactivity of carbon iron ore composite with biomass char for blast furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef]
- Strezov, V. Iron ore reduction using sawdust: Experimental analysis and kinetic modelling. Renew. Energy 2006, 31, 1892–1905. [Google Scholar] [CrossRef]
- Srivastava, U.; Kawatra, S.K.; Eisele, T.C. Production of pig iron by utilizing biomass as a reducing agent. Int. J. Miner. Process. 2013, 119, 51–57. [Google Scholar] [CrossRef]
- Li, D.W.; Yue, C.S.; Han, H.L.; Duan, D.P. Application of straw fiber as the reducing agent in production of metallic pellets. Environ. Eng. 2016, 34, 119–122. [Google Scholar]
- Yuan, P.; Yue, C.S.; Han, H.L.; Duan, D.P. Impact of biomass reducing agents on RHF direct reduction process. Environ. Eng. 2015, 33, 113–117. [Google Scholar]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant-Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Fernandez, M. Charcoal behaviour by its injection into the modern blast furnace. ISIJ Int. 2010, 50, 81–88. [Google Scholar] [CrossRef]
- Mathieson, J.G.; Rogers, H.; Somerville, M.A.; Jahanshahi, S. Reducing net CO2 emissions using charcoal as a blast furnace tuyere injectant. ISIJ Int. 2012, 52, 1489–1496. [Google Scholar] [CrossRef]
- Hu, J.J.; Zhang, Q.G.; Lee, D.J. Kraft lignin biorefinery: A perspective. Bioresour. Technol. 2018, 247, 1181–1183. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- John, M.J.; Lefatle, M.C.; Sithole, B. Lignin fractionation and conversion to bio-based functional products. Sustain. Chem. Pharm. 2022, 25, 100594. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Crestini, C. Fractionation of industrial lignins: Opportunities and challenges. Green Chem. 2020, 22, 4722–4746. [Google Scholar] [CrossRef]
- Cai, M.L.; Bai, S.J.; Liu, M.H. Present Situation of Treatment and Resource Utilization of Papermaking Black Liquor. East China Pulp Pap. Ind. 2016, 47, 39–42. [Google Scholar]
- Qian, Y.; Lou, H.M.; Liu, W.F.; Yang, D.J.; Ouyang, X.P.; Li, Y.; Qiu, X.Q. Lignin—A promising biomass resource. Tappi J. 2018, 17, 125–141. [Google Scholar] [CrossRef]
- Mousa, E.A.; Ahmed, H.M.; Wang, C. Novel Approach Towards Biomass Lignin Utilization in Ironmaking Blast Furnace. ISIJ Int. 2017, 57, 1788–1796. [Google Scholar] [CrossRef]
- Cai, D.W.; Chen, Q.Y.; Pan, J.; Zhu, D.Q.; Yang, C.C. Research status of low carbon smelting for metallized burden in blast furnace. China Metall. 2024, 34, 9–20. [Google Scholar] [CrossRef]
- Wang, D.Z. Basic Research on Efficient Utilization of Scrap Steel in Converter Steelmaking. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2023. [Google Scholar]
- Leng, C.M.; Jiang, X.; Xue, Q.B.; Long, F.; Huo, H.Y.; Shen, F.M. Effects of high proportion of acid burden on blast furnace operation and its countermeasures. Iron Steel 2022, 57, 16. [Google Scholar]
- Di Cecca, C.; Barella, S.; Mapelli, C.; Ciuffini, A.F.; Gruttadauria, A.; Mombelli, D.; Bondi, E. Use of DRI/HBI in ironmaking and steelmaking furnaces. Metall. Ital. 2016, 108, 37–42. Available online: https://re.public.polimi.it/handle/11311/997783 (accessed on 25 August 2025).
- Cai, Y.J.; Shi, J.X.; Chen, H.; Jin, Q.F.; Peng, G.X. Application Test of Metallized Pellets in Electric Furnace Steelmaking. Shandong Metall. 2022, 44, 40–42. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. Beijing COC Tech Co., Ltd.: Beijing, China, 2008.
- Zhou, W.; Xiang, D.; Zhang, Q.; Wu, G.; Wang, Y.; Li, D.; Zhang, Q.; Hu, H. Kinetics of Reduction of Iron Ore Powder by Industrial Lignin from Pulping and Papermaking Waste Biomass Energy. Crystals 2025, 15, 193. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, Q.; Wu, G.; Wang, Y.; Li, D.; Zhang, Q.; Hu, H. Study on Reduction Mechanism of Iron Oxide by Industrial Lignin. Metals 2024, 14, 1467. [Google Scholar] [CrossRef]
- Fan, J.J.; Guo, Y.F.; Zang, L.; Shi, Y.L.; Li, H.K.; Zheng, F.Q. Effect of basicity on properties of pellets produced by ultra fine-sized iron concentrate. J. Iron Steel Res. 2019, 31, 440–445. [Google Scholar]
- Han, H.L.; Yuan, P.; Duan, D.P.; Li, D.W. Application of biomass to the RHF direct reduction process. J. Chongqing Univ. 2015, 38, 164–170. [Google Scholar] [CrossRef]
- Zhao, M.X.; Liu, J.W.; Jia, G.L.; Wang, G.W.; Yu, X.B. Preparation and performance optimization for biomass carbon-bearing pellets with high strength. China Metall. 2024, 34, 96–105. [Google Scholar] [CrossRef]
- Liang, C.; Li, J.X.; Long, H.M.; Wang, P.; Jie, C.C. Effect of double layer structure on the strength of iron ore pellets containing carbon. J. Iron Steel Res. 2016, 28, 8–12. [Google Scholar]
- Xiang, D.W.; Shen, F.M.; Jiang, X.; An, H.W.; Zheng, H.Y.; Gao, Q.J. Pyrolysis Characteristics of Industrial Lignin for Use as a Reductant and an Energy Source for Future Iron Making. ACS Omega 2021, 6, 3578–3586. [Google Scholar] [CrossRef]
- Wu, C.W.; Zhang, P.C.; Zhou, P. Research on mechanical behavior of super-lightweight structure made of thin-walled spheres. J. Dalian Univ. Technol. 2008, 48, 625–630. [Google Scholar]
- Konishi, H.; Ichikawa, K.; Usui, T. Effect of residual volatile matter on reduction of iron oxide in semi-charcoal composite pellets. Trans. Iron Steel Inst. Jpn. 2010, 50, 386–389. [Google Scholar] [CrossRef]

| Samples | Proximate Analysis (ad.) | Elemental Analysis (ad.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Carbon | Ash | Volatile | Moisture | C | H | O | N | S | |
| IL | 26.35 | 8.31 | 60.44 | 4.89 | 59.56 | 6.82 | 29.67 | 0.20 | 1.82 |
| LA | 75.90 | 10.01 | 13.38 | 0.71 | 82.00 | 3.41 | 8.50 | 1.79 | 0.20 |
| SM | 62.41 | 7.46 | 28.28 | 1.85 | 75.90 | 4.14 | 16.50 | 1.38 | 0.63 |
| TFe | FeO | SiO2 | CaO | Al2O3 | MgO | Others |
|---|---|---|---|---|---|---|
| 68.04 | 24.88 | 5.50 | 0.14 | 0.28 | 0.36 | 0.8 |
| Al2O3 | CaO | MgO | SiO2 | TFe | MFe | FeO | |
|---|---|---|---|---|---|---|---|
| Metallized pellets | 0.46 | 1.04 | 0.36 | 7.3 | 87.99 | 87.74 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Guo, H.; Zhang, Q.; Wu, G.; Wang, Y.; Li, D.; Zhang, Q.; Hu, H. Study on Preparation and Properties of Industrial Lignin Metallized Pellets for Ironmaking. Crystals 2025, 15, 878. https://doi.org/10.3390/cryst15100878
Xiang D, Guo H, Zhang Q, Wu G, Wang Y, Li D, Zhang Q, Hu H. Study on Preparation and Properties of Industrial Lignin Metallized Pellets for Ironmaking. Crystals. 2025; 15(10):878. https://doi.org/10.3390/cryst15100878
Chicago/Turabian StyleXiang, Dongwen, He Guo, Qiang Zhang, Guoqing Wu, Yajie Wang, Dong Li, Qinghua Zhang, and Huaxin Hu. 2025. "Study on Preparation and Properties of Industrial Lignin Metallized Pellets for Ironmaking" Crystals 15, no. 10: 878. https://doi.org/10.3390/cryst15100878
APA StyleXiang, D., Guo, H., Zhang, Q., Wu, G., Wang, Y., Li, D., Zhang, Q., & Hu, H. (2025). Study on Preparation and Properties of Industrial Lignin Metallized Pellets for Ironmaking. Crystals, 15(10), 878. https://doi.org/10.3390/cryst15100878






