X-Ray Crystallography, Hirshfeld Surface Analysis, and Molecular Docking Studies of Two Sulfonamide Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Substances and Equipment
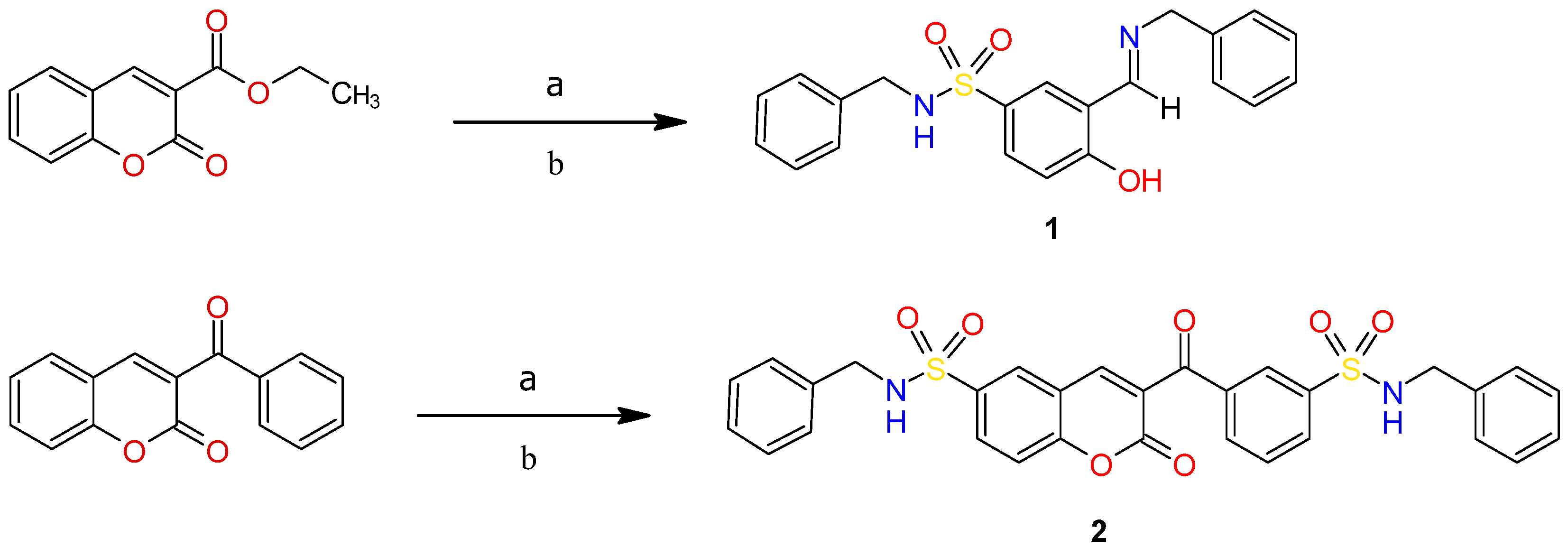
2.2. Synthesis of Compounds
2.3. Single Crystal X-Ray Diffraction
2.4. Hirshfeld Surfaces, Interaction Energies, and Energy Framework Diagrams Calculations
2.5. Molecular Docking Modeling
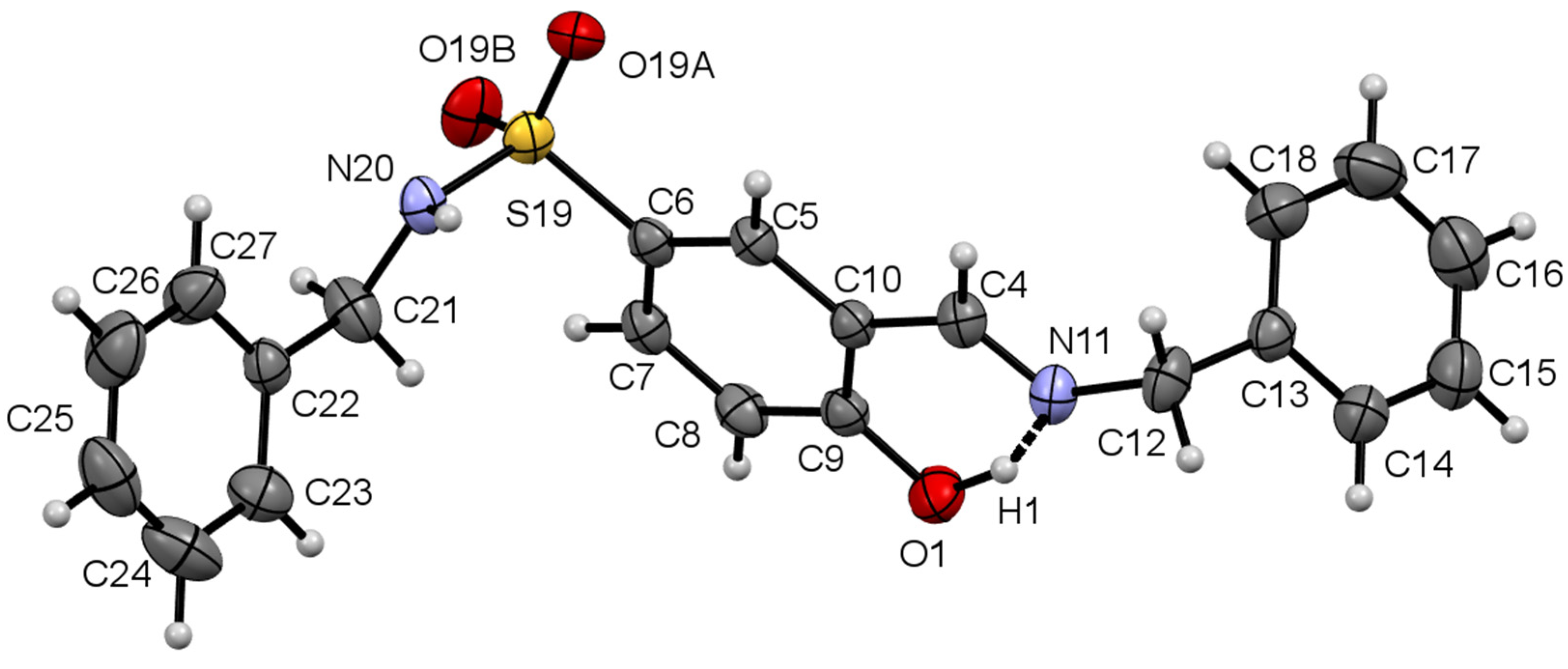
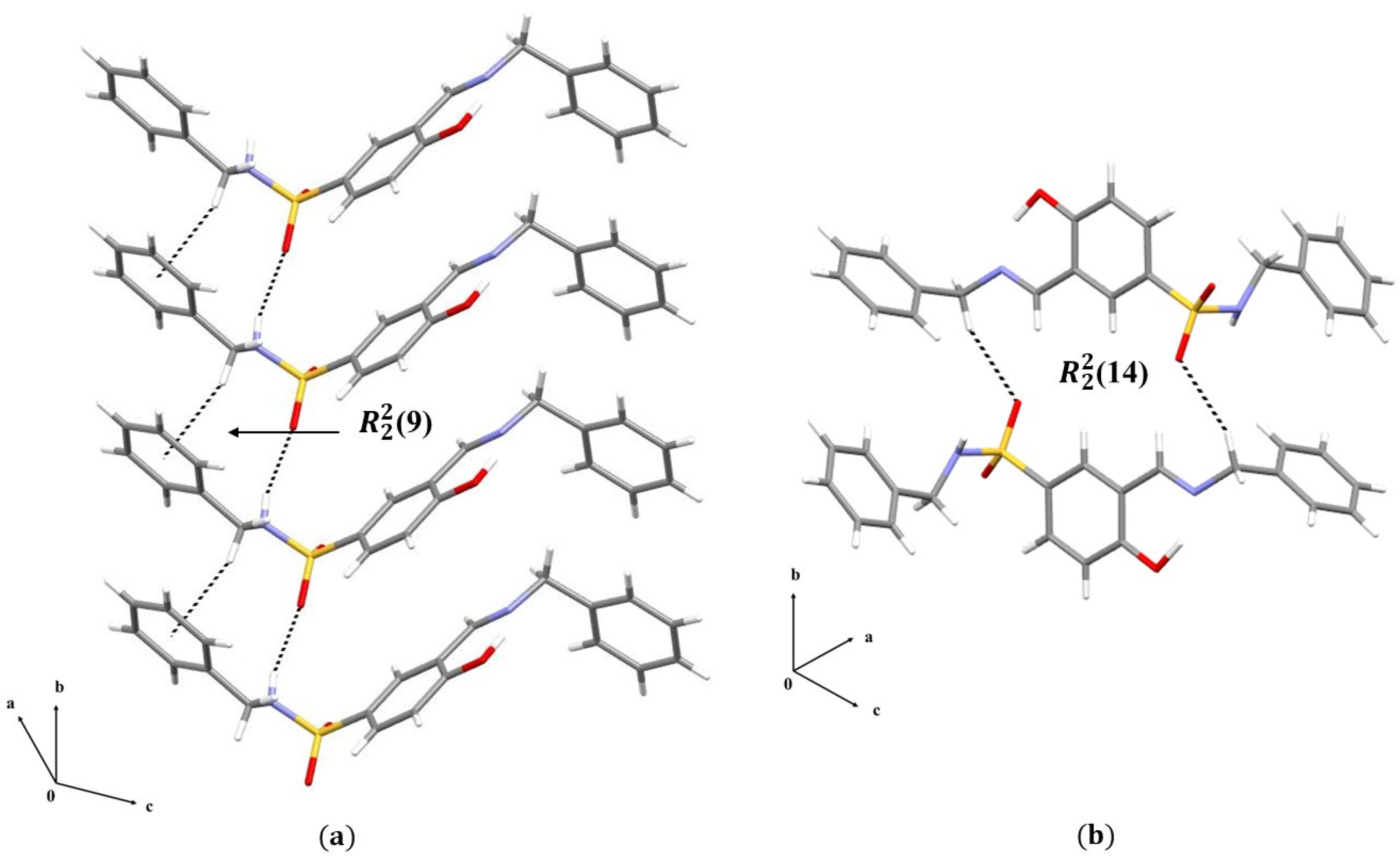
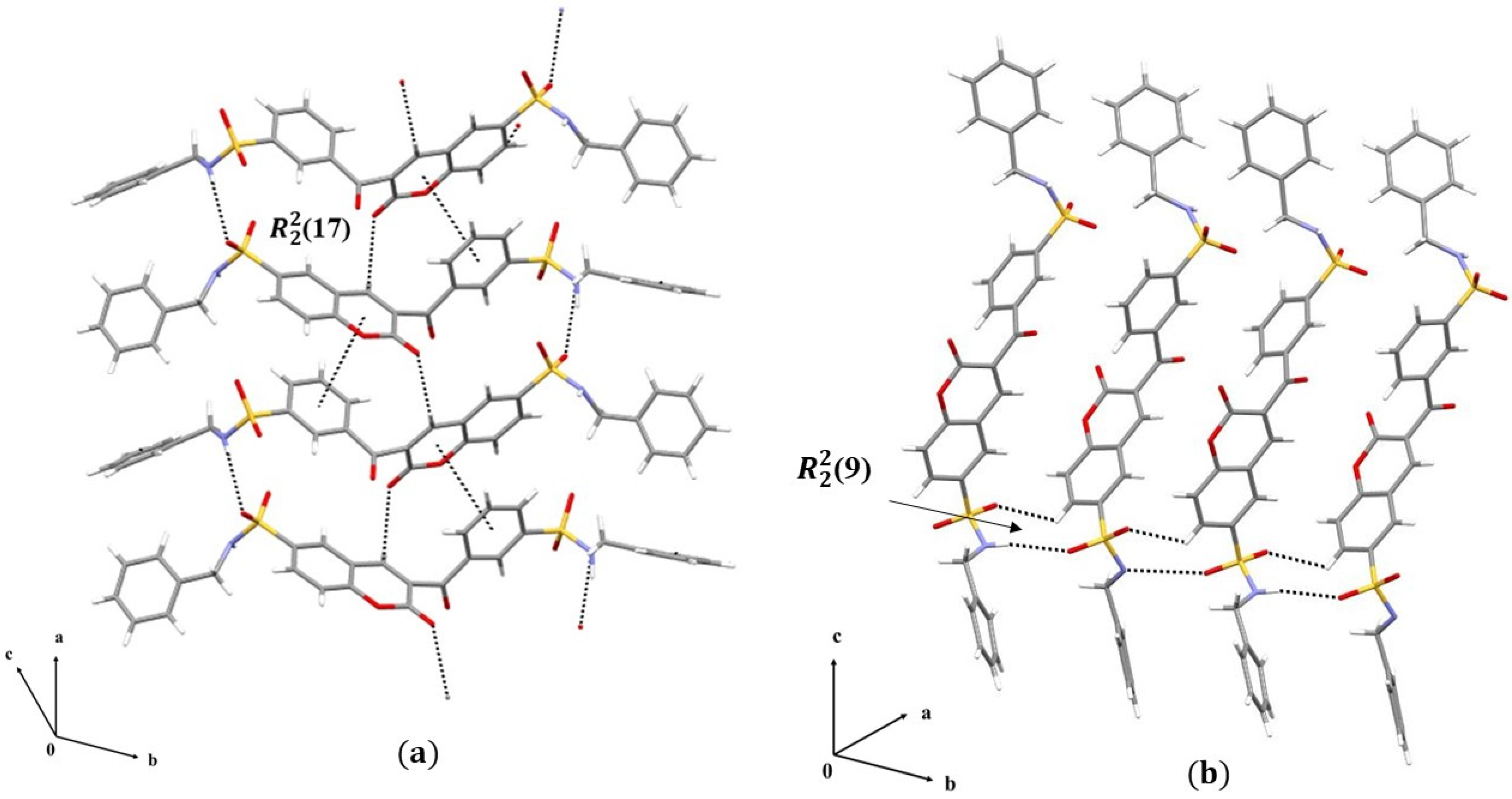
3. Results and Discussions
3.1. Single Crystal X-Ray Diffraction Analysis
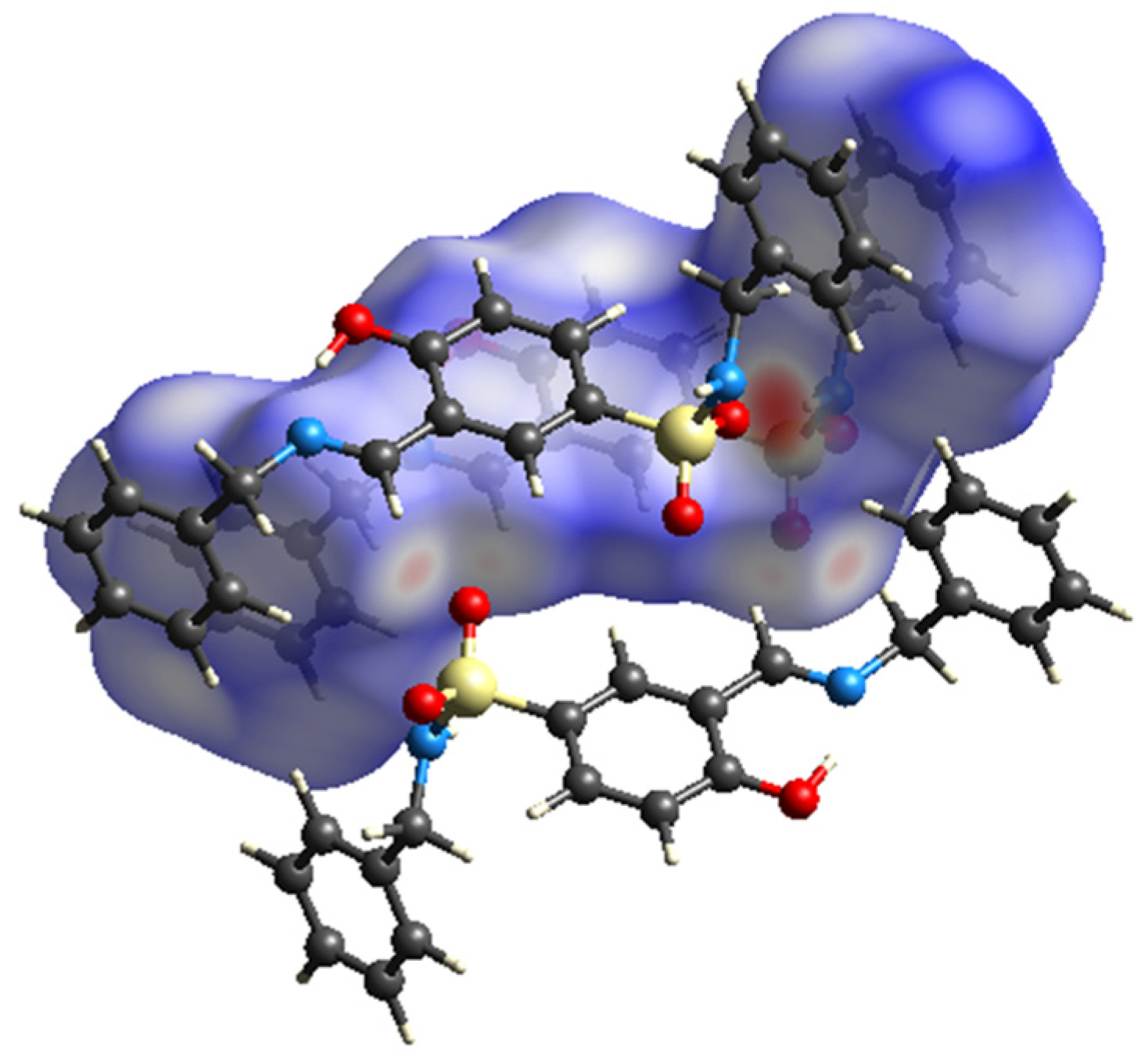
3.1.1. Molecular Structure of Compounds 1 and 2
3.1.2. Crystal Packing of Compounds 1 and 2
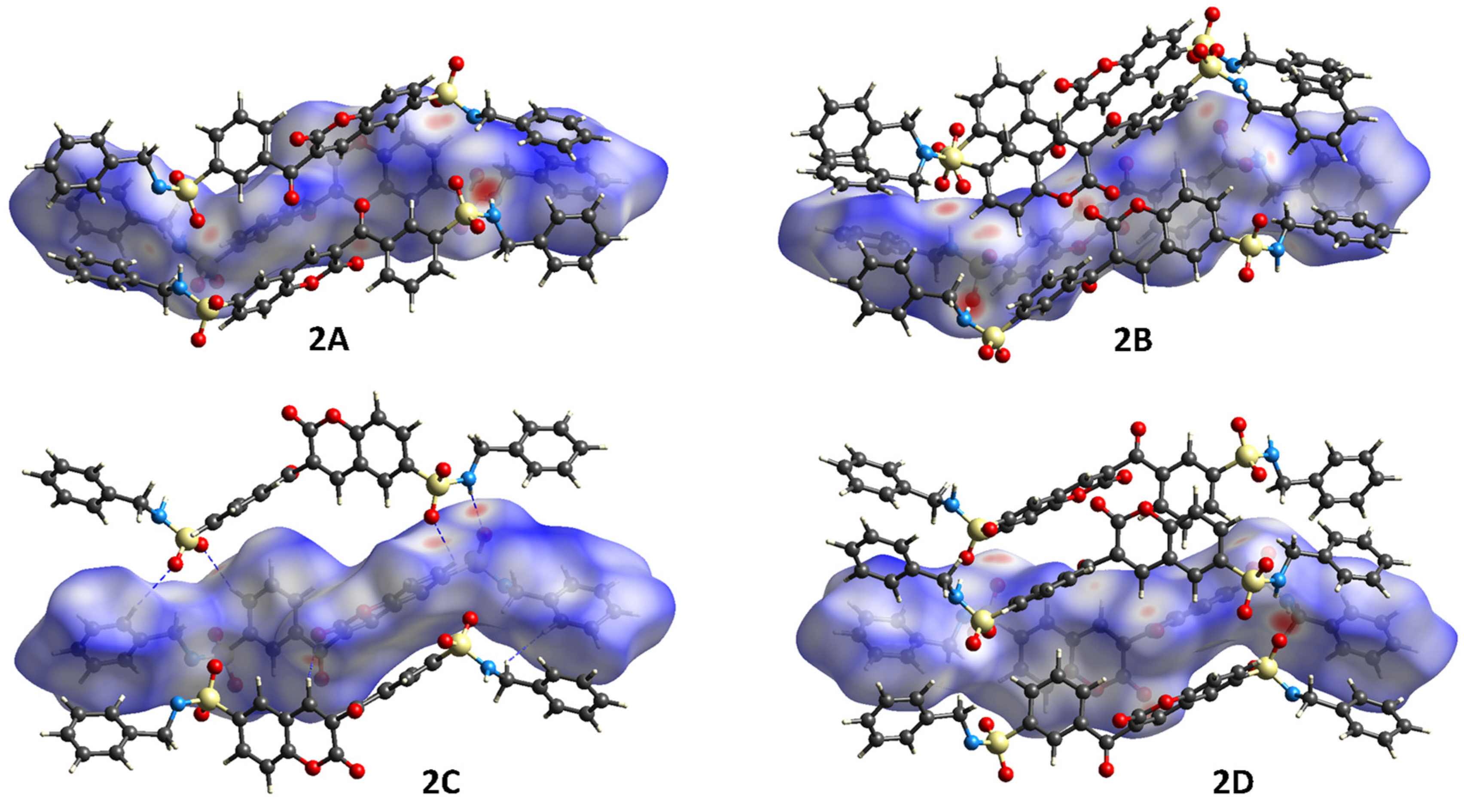
3.2. Hirshfeld Surface (HS) Analysis
3.3. Interaction Energies
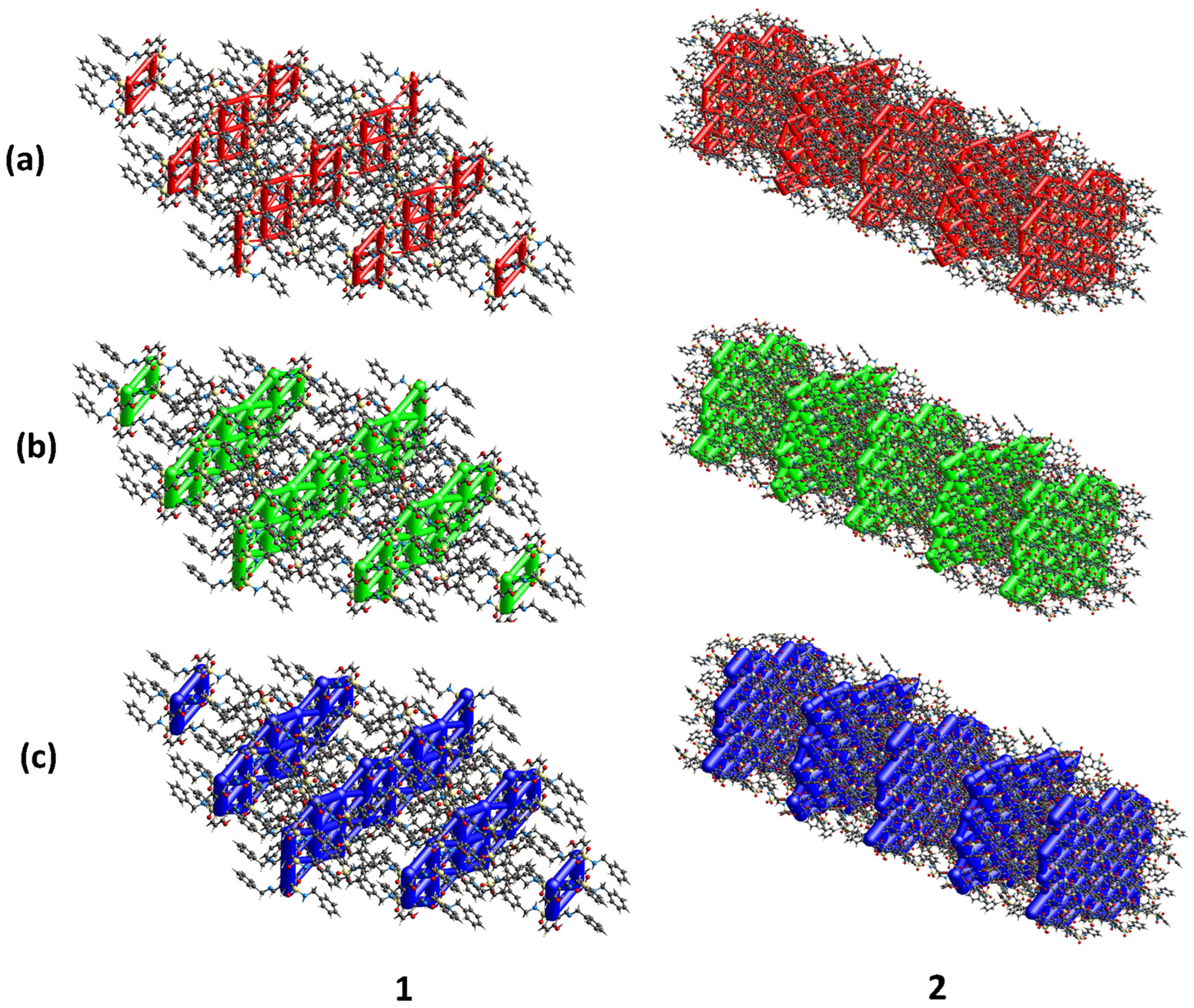
3.4. Energy Framework Diagrams
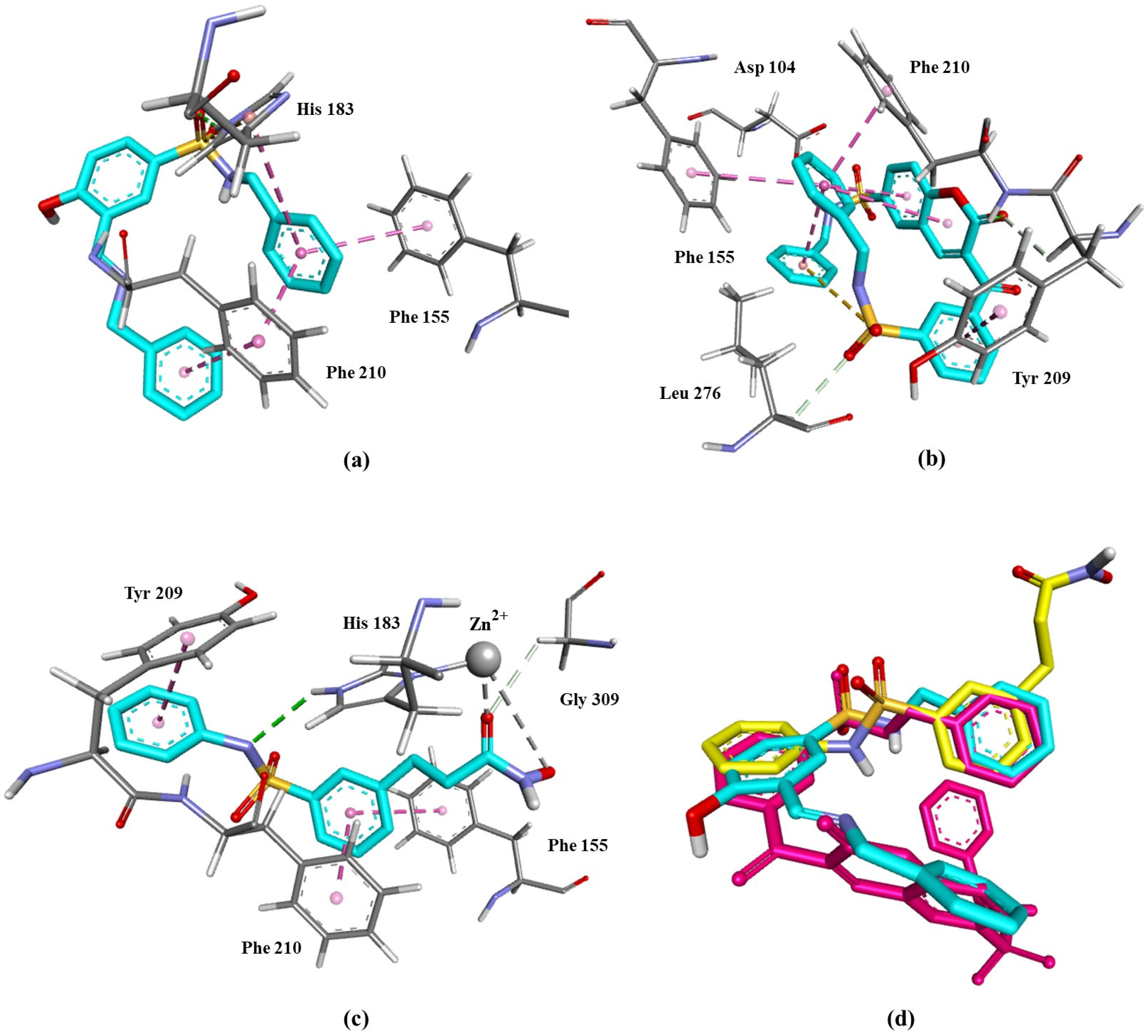
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mondal, S.; Malakar, S. Synthesis of Sulfonamide and Their Synthetic and Therapeutic Applications: Recent Advances. Tetrahedron 2020, 76, 131662. [Google Scholar] [CrossRef]
- Adsmond, D.A.; Grant, D.J. Hydrogen Bonding in Sulfonamides. J. Pharm. Sci. 2001, 90, 2058–2077. [Google Scholar] [CrossRef]
- Irfan, A.; Rubab, L.; Rehman, M.U.; Anjum, R.; Ullah, S.; Marjana, M.; Qadeer, S.; Sana, S. Coumarin Sulfonamide Derivatives: An Emerging Class of Therapeutic Agents. Heterocycl. Commun. 2020, 26, 46–59. [Google Scholar] [CrossRef]
- Madrigal-Angulo, J.L.; Hernández-Fuentes, G.A.; Parra-Delgado, H.; Olvera-Valdéz, M.; Padilla-Martínez, I.I.; Cabrera-Licona, A.; Espinosa-Gil, A.S.; Delgado-Enciso, I.; Martínez-Martínez, F.J. Design, Synthesis, Biological and in Silico Evaluation of 3-carboxy-coumarin Sulfonamides as Potential Antiproliferative Agents Targeting HDAC6. Biomed. Rep. 2024, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Thomas, C.M. Belinostat for the Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma. J. Oncol. Pharm. Pract. 2017, 23, 143–147. [Google Scholar] [CrossRef]
- Pullagurla, M.R.; Valsan, N.K.; Pitta, B.R.; Rangisetty, J.B. Novel Process for the Preparation of Belinostat. U.S. Patent US 2019/0256459 A1, 22 August 2019. [Google Scholar]
- Li, Z.; Ouyang, R.; Shi, P.; Du, S.; Gong, J.; Wu, S. Rationalizing the Formation of Belinostat Solvates with Experimental Screening and Computational Predictions. Cryst. Growth Des. 2021, 21, 4986–4996. [Google Scholar] [CrossRef]
- Li, Z.; Ouyang, R.; Cao, Y.; Han, D.; Wu, S.; Gong, J. Solubility Enhancement of Anticancer Drug Belinostat via the Co-Crystallization Strategy: Synthesis, Characterization, and Antitumor Activity In Vitro Evaluation. Cryst. Growth Des. 2023, 23, 792–800. [Google Scholar] [CrossRef]
- Bruker APEX2, Version 2012.10-0; Bruker AXS, Inc.: Madison, WI, USA, 2012.
- SAINT, Version 8.27B; Bruker AXS, Inc.: Madison, WI, USA, 2012.
- SADABS, Version 8.27B; Bruker AXS, Inc.: Madison, WI, USA, 2012.
- Blessing, R.H. An Empirical Correction for Absorption Anisotropy. Acta Crystallogr. A 1995, 51 Pt 1, 33–38. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Morales-Santana, M.; Chong, S.; Santiago-Quintana, J.M.; Martínez-Martínez, F.; García-Báez, E.; Cruz, A.; Rojas-Lima, S.; Padilla-Martínez, I. Microcrystalline Solid–Solid Transformations of Conformationally-Responsive Solvates, Desolvates and a Salt of N, N′-(1,4-Phenylene)Dioxalamic Acid: The Energetics of Hydrogen Bonding and n/π → Π* Interactions. CrystEngComm 2024, 24, 1017–1034. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy Frameworks: Insights into Interaction Anisotropy and the Mechanical Properties of Molecular Crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef]
- Lauffer, B.E.; Mintzer, R.; Fong, R.; Mukund, S.; Tam, C.; Zilberleyb, I.; Flicke, B.; Ritscher, A.; Fedorowicz, G.; Vallero, R.; et al. Histone Deacetylase (HDAC) Inhibitor Kinetic Rate Constants Correlate with Cellular Histone Acetylation but Not Transcription and Cell Viability. J. Biol. Chem. 2013, 288, 26926–26943. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes. BIOVIA Discovery Studio Visualizer 2021; Dassault Systèmes: Paris, France, 2025; Available online: https://www.3ds.com/products/biovia (accessed on 25 February 2025).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Schrodinger, L. Maestro, Version 13.3; Schodinger Release 2025-1; Schrodinger: New York, NY, USA, 2025. Available online: https://www.schrodinger.com (accessed on 25 February 2025).
- Ortegon-Reyna, D.; Garcias-Morales, C.; García-Báez, E.V.; Ariza-Castolo, A.; Martínez-Martínez, F.J. (E)-4-Hydroxy-2-{[(2-Phenylethyl)Iminiumyl]Methyl}phenolate. Acta Crystallogr. E Struct. Rep. Online 2012, 68, o2075. [Google Scholar] [CrossRef]
- Santos-Contreras, R.J.; Martínez-Martínez, F.J.; Mancilla-Margalli, N.A.; Peraza-Campos, A.L.; Morín-Sánchez, L.M.; García-Báez, E.V.; Padilla-Martínez, I.I. Competition between OH⋯O and Multiple Halogen–Dipole Interactions on the Formation of Intramolecular Three-Centred Hydrogen Bond in 3-Acyl Coumarins. CrystEngComm 2009, 11, 1451–1461. [Google Scholar] [CrossRef]
- Sunil Kumar, A.; Kudva, J.; Lahtinen, M.; Peuronen, A.; Sadashiva, R.; Naral, D. Synthesis, Characterization, Crystal Structures and Biological Screening of 4-Amino Quinazoline Sulfonamide Derivatives. J. Mol. Struct. 2019, 1190, 29–36. [Google Scholar] [CrossRef]
- Danish, M.; Bibi, A.; Gilani, K.; Raza, M.A.; Ashfaq, M.; Arshad, M.N.; Asiri, A.M.; Ayub, K. Antiradical, Antimicrobial and Enzyme Inhibition Evaluation of Sulfonamide Derived Esters; Synthesis, X-Ray Analysis and DFT Studies. J. Mol. Struct. 2019, 1175, 379–388. [Google Scholar] [CrossRef]
- Özbek, N.; Özdemir, Ü.Ö.; Altun, A.F.; Şahin, E. Sulfonamide-Derived Hydrazone Compounds and Their Pd (II) Complexes: Synthesis, Spectroscopic Characterization, X-Ray Structure Determination, in Vitro Antibacterial Activity and Computational Studies. J. Mol. Struct. 2019, 1196, 707–719. [Google Scholar] [CrossRef]
- Male, S.R.; Upadhyay, S.; Sharma, S.K.; Acharya, H.; Singh, G.; Lahiri, S.; Cabri, W. Polymorphic Forms of Belinostat and Processes for Preparation Thereof. WO 2018020406 A1, 1 February 2018. [Google Scholar]
- Stenfors, B.A.; Staples, R.J.; Biros, S.M.; Ngassa, F.N. Crystal Structure of 4-Methyl-N-(4-Methyl benz yl)Benzene sulfonamide. Acta Crystallogr. E 2020, 76, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Prohens, R.; Portell, A.; Font-Bardia, M.; Bauzá, A.; Frontera, A. Experimental and Theoretical Study of Aromaticity Effects in the Solid State Architecture on Squaric Acid Derivatives. Cryst. Growth Des. 2014, 14, 2578–2587. [Google Scholar] [CrossRef]
- García-Báez, E.V.; Martínez-Martínez, F.J.; Höpfl, H.; Padilla-Martínez, I.I. π-Stacking Interactions and CH···X (X = O, Aryl) Hydrogen Bonding as Directing Features of the Supramolecular Self-Association in 3-Carboxy and 3-Amido Coumarin Derivatives. Cryst. Growth Des. 2003, 3, 35–45. [Google Scholar] [CrossRef]
- Malathy Sony, S.M.; Ponnuswamy, M.N. Nature of π-Interactions in Nitrogen-Containing Heterocyclic Systems: A Structural Database Analysis. Cryst. Growth Des. 2006, 6, 736–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yang, Y.; Guo, J.; Ma, J.-F. Syntheses, Structures, and Luminescent Properties of a Series of Coordination Compounds Based on (4-Carboxylphenyl) (4-(2′-Carboxylphenyl)Benzyl) Ether and Different N-Donor Ligands. Cryst. Growth Des. 2012, 12, 4060–4071. [Google Scholar] [CrossRef]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl-Carbonyl Interactions Can Be Competitive with Hydrogen Bonds. Acta Crystallogr. Sect. B 1998, 54, 320–329. [Google Scholar] [CrossRef]
- Shan, W.; Jiang, Y.; Yu, H.; Huang, Q.; Liu, L.; Guo, X.; Li, L.; Zhang, K.; Yang, Z. HDAC2 Overexpression Correlates with Aggressive Clinicopathological Features and DNA-Damage Response Pathway of Breast Cancer. Am. J. Cancer Res. 2017, 7, 1213. [Google Scholar]
- Zhao, H.; Yu, Z.; Zhao, L.; He, M.; Ren, J.; Wu, H.; Chen, Q.; Yao, W.; Wei, M. HDAC2 Overexpression Is a Poor Prognostic Factor of Breast Cancer Patients with Increased Multidrug Resistance-Associated Protein Expression Who Received Anthracyclines Therapy. Jpn. J. Clin. Oncol. 2016, 46, 893–902. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Dimitroulis, D.; Kouraklis, G.; Garmpi, A.; Sarantis, P.; Koustas, E.; Patsouras, A.; Psilopatis, I.; Antoniou, E.A.; et al. Clinical Significance of the Histone Deacetylase 2 (HDAC-2) Expression in Human Breast Cancer. J. Pers. Med. 2022, 12, 1672. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Abdelsalam, M.; Zeyn, Y.; Zessin, M.; Mustafa, A.-H.M.; Fischer, M.A.; Zeyen, P.; Sun, P.; Bülbül, E.F.; Vecchio, A.; et al. Synthesis, Molecular Docking and Biological Characterization of Pyrazine Linked 2-Aminobenzamides as New Class I Selective Histone Deacetylase (HDAC) Inhibitors with Anti-Leukemic Activity. Int. J. Mol. Sci. 2022, 23, 369. [Google Scholar] [CrossRef]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular Docking Simulations on Histone Deacetylases (HDAC)-1 and -2 to Investigate the Flavone Binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, X.; Yu, W.; Hu, X.; Zhang, Y.; Yang, K.; You, Z.; Liu, Z.; Qiao, X.; Song, Y. Pyrazolo [3,4-d]Pyrimidine-Based Dual HDAC/Topo II Inhibitors: Design, Synthesis, and Biological Evaluation as Potential Antitumor Agents. J. Mol. Struct. 2023, 1272, 134221. [Google Scholar] [CrossRef]



| Compound | 1 | 2 |
|---|---|---|
| CCDC deposition number | 2384467 | 2385727 |
| Chemical formula | C21H20N2O3S | C30H24N2O7S2 |
| Formula weight | 380.45 | 588.63 |
| Crystal size (mm) | 0.3 × 0.2 × 0.1 | 0.35 × 0.30 × 0.28 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | Cc |
| a (Å) | 20.303 (5) | 14.9003 (13) |
| b (Å) | 4.9211 (12) | 14.9110 (14) |
| c (Å) | 20.945 (5) | 50.4691 (5) |
| α (°) | 90 | 90 |
| β (°) | 113.88 (1) | 93.138 (3) |
| γ (°) | 90° | 90° |
| V (Å3) | 1913.6 (8) | 11196.3 (18) |
| Z | 4 | 16 |
| Dx (mg/m3) | 1.321 | 1.397 |
| F (000) | 800 | 4896 |
| µ (mm−1) | 0.193 | 0.24 |
| Measured, independent, and observed reflections [I > 2σ(I)] | 46857, 3191, 2178 | 160036, 20868, 12259 |
| R (int) | 0.156 | 0.106 |
| GOOF | 1.139 | 1.09 |
| No. of reflections | 3141 | 20868 |
| No. of parameters | 257 | 1510 |
| R[F2 > 2σ(F2)], wR(F2) | 0.064, 0.100 | 0.072, 0.136 |
| Bond Lengths (Å) | |||
|---|---|---|---|
| 1 | 2 | ||
| O1–C9 | 1.337 (4) | C2–O2 | 1.204 (7) |
| C4–N11 | 1.275 (5) | C3–C4 | 1.340 (8) |
| C6–S19 | 1.752 (3) | C11–O11 | 1.204 (8) |
| S19–O19A | 1.435 (2) | C6–S27 | 1.752 (4) |
| S19–N20 | 1.614 (3) | C14–S18 | 1.766 (5) |
| N20–C21 | 1.466 (5) | S18–O18A | 1.438 (5) |
| S27–O27A | 1.428 (3) | ||
| S27–N28 | 1.605 (4) | ||
| N28–C29 | 1.447 (7) | ||
| Torsion angles (deg) | |||
| C5–C6–S19–O19A | −21.1 (3) | O1–C9–C10–C4 | 1.025 (7) |
| C5–C6–S19–O19B | −150.9 (3) | C2–C3–C11–O11 | −82.7 (7) |
| C5–C6–S19–N20 | 94.5 (3) | C3–C4–C10–C9 | 0.62 (7) |
| C5–C10–C4–N11 | 179.8 (3) | C4–C3–C11–O11 | −96.975 (8) |
| C9–C10–C4–N11 | 0.9 (5) | C4–C3–C11–C12 | 83.475 (7) |
| C5–C6–S27–O7A | 17.925 (4) | ||
| C5–C6–S27–N28 | 98.275 (4) | ||
| C13–C14–S18–O18A | 19.825 (4) | ||
| C13–C14–S18–N19 | 94.375 (4) | ||
| Comp. | D–H…A | D–H (Å) | H…A (Å) | D….A (Å) | D–H…A (°) |
|---|---|---|---|---|---|
| 1 | N20–H20···O19B i | 0.79 (3) | 2.08 (3) | 2.844 (4) | 163 (3) |
| C12–H12B….O19A ii | 0.97 | 2.52 | 3.450 (4) | 160 | |
| 2A | C4A–H4A…O2D iii | 0.93 | 2.49 | 3.210 (13) | 135 |
| C7A–H7A…O27E iv | 0.93 | 2.46 | 3.019 (11) | 119 | |
| N19A–H19A…O27G v | 0.67 (6) | 2.37 (6) | 3.032 (12) | 172 (8) | |
| N28A–H28A…O27F iii | 0.98 (10) | 2.28 (10) | 3.258 (11) | 178 (10) | |
| 2B | C4B–H4B…O2C iii | 0.93 | 2.46 | 3.202 (13) | 137 |
| C7B–H7B…O27H vi | 0.93 | 2.51 | 3.001 (11) | 113 | |
| N19B–H19B…O27F vii | 1.00 (7) | 2.07 (7) | 3.032 (11) | 161 (5) | |
| N28B–H28B…O27G iii | 0.94 (9) | 2.52 (9) | 3.198 (11) | 130 (7) | |
| 2C | C4C–H4C…O2B v | 0.93 | 2.46 | 3.195 (12) | 136 |
| C7C–H7C…O27A iii | 0.93 | 2.52 | 3.003 (10) | 112 | |
| N19C–H19C…O27D iii | 1.01 (9) | 2.06 (9) | 3.040 (11) | 164 (7) | |
| N28C–H28C…O27B vi | 0.92 (12) | 2.32 (11) | 3.179 (11) | 156 (10) | |
| 2D | C4D–H4D…O2A vii | 0.93 | 2.45 | 3.190 (12) | 136 |
| C7D–H7D…O27C iii | 0.93 | 2.46 | 2.995 (11) | 117 | |
| N19D–H19D…O27B iii | 0.91 (15) | 2.11 (17) | 2.994 (10) | 165 (15) | |
| N28D–H28D…O27D iv | 0.75 (7) | 2.55 (8) | 3.252 (11) | 157 (6) |
| Interaction | Molecules | −Eelec | −Epol | −Edisp | Erep | −Etot | %Eelec | %Edisp | R c/Å |
|---|---|---|---|---|---|---|---|---|---|
| Comp. 1 | |||||||||
| N20–H20∙∙∙O19B C21–H21B∙∙∙Cg(3) d | 37.6 | 7.0 | 63.8 | 37.5 | 71.0 | 34.7 | 58.9 | 4.92 | |
| C12–H12B∙∙∙O19A C4–H4∙∙∙O19A | 21.4 | 8.7 | 33.3 | 11.6 | 51.7 | 33.7 | 52.5 | 6.02 | |
| Comp. 2 | |||||||||
| N19B–H19B∙∙∙O27F C4C–H4C∙∙∙O2B | B∙∙∙C | 58.6 | 13.3 | 86.3 | 45.4 | 112.8 | 37.0 | 54.6 | 5.248 |
| N19C–H19C∙∙∙O27D C4B–H4B∙∙∙O2C | C∙∙∙B | 56.2 | 12.8 | 83.3 | 42.8 | 109.5 | 36.9 | 54.7 | 5.297 |
| N19A–H19A∙∙∙O27G C4D–H4D∙∙∙ O2A C11A–O11A∙∙∙Cg(17) e | A∙∙∙D | 54.2 | 12.5 | 85.5 | 44.1 | 108.2 | 35.6 | 56.2 | 5.260 |
| N19D–H19D∙∙∙O27B C4A–H4A∙∙∙ O2D C11D–O11D∙∙∙Cg(2) f | D∙∙∙A | 59.6 | 13.8 | 84.5 | 47.2 | 110.7 | 37.7 | 53.5 | 5.282 |
| C2A–O2A∙∙∙Cg(6) g C8A–H8A∙∙∙Cg(8) h C2B–O2B∙∙∙ Cg(1) i C8B–H8B∙∙∙Cg(3) j | A∙∙∙B B∙∙∙A | 51.6 | 10.1 | 62.5 | 24.7 | 99.5 | 41.5 | 50.3 | 5.527 |
| C2C–O2C∙∙∙Cg(16) k C8C–H8C∙∙∙Cg(18) l C2C–O2C∙∙∙C2D C2D–O2D∙∙∙ Cg(11) m C8D–H8D∙∙∙Cg(13) n C2D–O2D∙∙∙C2C | C D D∙∙∙C | 47.4 | 9.5 | 62.2 | 23.2 | 95.8 | 39.8 | 52.3 | 5.556 |
| C4A–H4A∙∙∙O11B C29B–H29D∙∙∙O18A | A∙∙∙B | 19.1 | 8.0 | 75.0 | 29.3 | 72.8 | 18.7 | 73.4 | 5.593 |
| N28C–H28C∙∙∙O27B C7A–H7A∙∙∙O27E C15A–H15A∙∙∙ O18E | C∙∙∙A | 49.8 | 9.7 | 43.3 | 27.6 | 75.2 | 48.4 | 42.1 | 6.283 |
| N28A–H28A∙∙∙O27F C7C–H7C∙∙∙O27A C15C–H15C∙∙∙O18A | A∙∙∙C | 46.4 | 9.5 | 41.9 | 25.2 | 72.6 | 47.5 | 42.9 | 6.345 |
| N28B–H28B∙∙∙O27G C7D–H7D∙∙∙O27C C15D–H15D∙∙∙ O18C | B∙∙∙D | 43.3 | 9.3 | 42.7 | 25.0 | 70.4 | 45.5 | 44.8 | 6.311 |
| N28D–H28D∙∙∙O27D C7B–H7B∙∙∙O27H C15B–H15B∙∙∙ O18G | D∙∙∙B | 46.5 | 9.3 | 43.0 | 25.8 | 73.0 | 47.1 | 43.6 | 6.331 |
| Compound | ΔG (kJ/mol) |
| 1 | −32.2 |
| 2 | −34.7 |
| Belinostat | −36.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrigal-Angulo, J.L.; Magaña-Vergara, N.E.; González-González, J.S.; Santiago-Quintana, J.M.; García-Báez, E.V.; Padilla-Martínez, I.I.; Martínez-Martínez, F.J. X-Ray Crystallography, Hirshfeld Surface Analysis, and Molecular Docking Studies of Two Sulfonamide Derivatives. Crystals 2025, 15, 854. https://doi.org/10.3390/cryst15100854
Madrigal-Angulo JL, Magaña-Vergara NE, González-González JS, Santiago-Quintana JM, García-Báez EV, Padilla-Martínez II, Martínez-Martínez FJ. X-Ray Crystallography, Hirshfeld Surface Analysis, and Molecular Docking Studies of Two Sulfonamide Derivatives. Crystals. 2025; 15(10):854. https://doi.org/10.3390/cryst15100854
Chicago/Turabian StyleMadrigal-Angulo, José Luis, Nancy E. Magaña-Vergara, Juan Saulo González-González, José Martín Santiago-Quintana, Efrén V. García-Báez, Itzia I. Padilla-Martínez, and Francisco J. Martínez-Martínez. 2025. "X-Ray Crystallography, Hirshfeld Surface Analysis, and Molecular Docking Studies of Two Sulfonamide Derivatives" Crystals 15, no. 10: 854. https://doi.org/10.3390/cryst15100854
APA StyleMadrigal-Angulo, J. L., Magaña-Vergara, N. E., González-González, J. S., Santiago-Quintana, J. M., García-Báez, E. V., Padilla-Martínez, I. I., & Martínez-Martínez, F. J. (2025). X-Ray Crystallography, Hirshfeld Surface Analysis, and Molecular Docking Studies of Two Sulfonamide Derivatives. Crystals, 15(10), 854. https://doi.org/10.3390/cryst15100854









