Nanoscale Cu2ZnSnSxSe(4−x) (CZTS/Se) for Sustainable Solutions in Renewable Energy, Sensing, and Nanomedicine
Abstract
1. Introduction
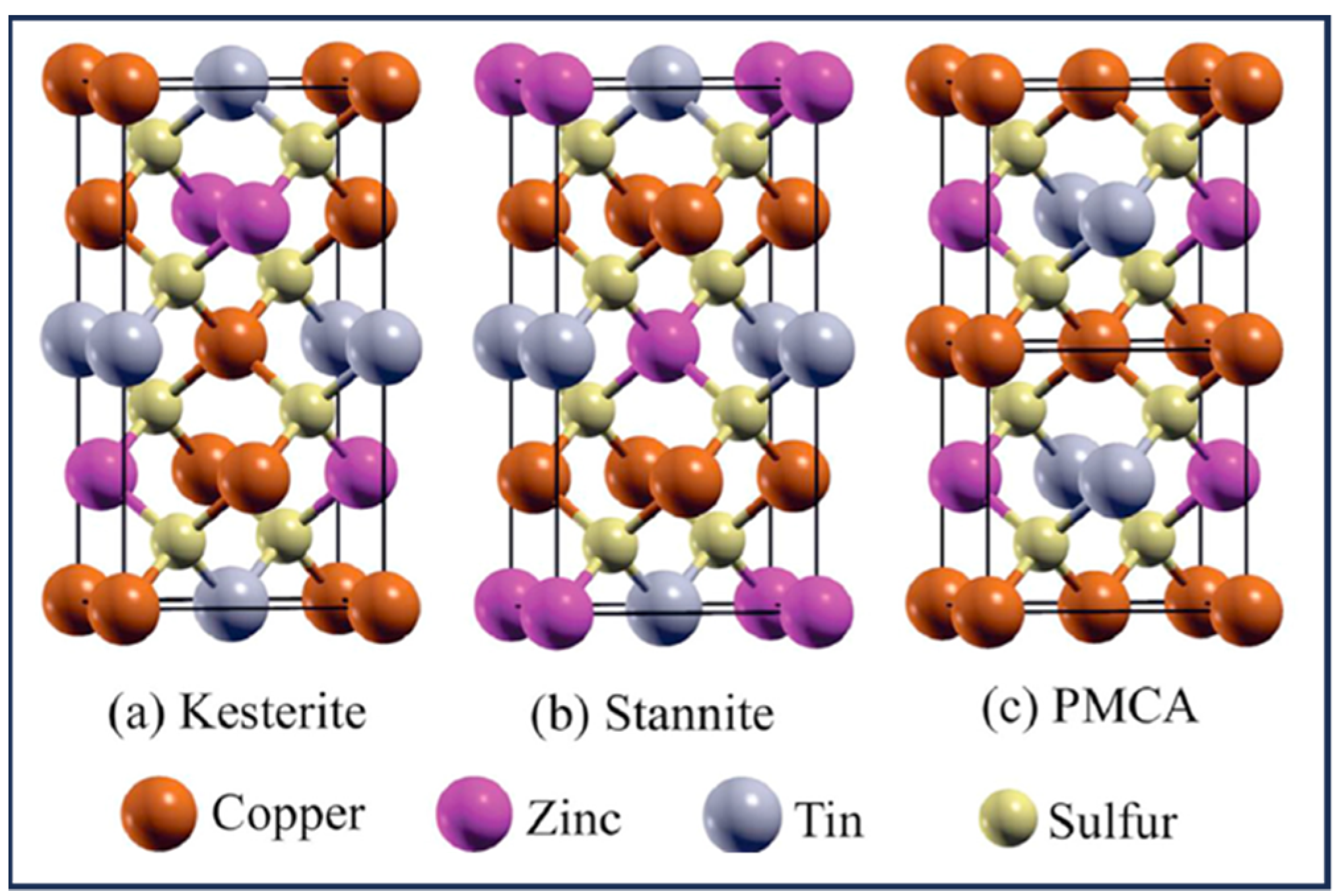
1.1. CZTS/Se Structure and Properties
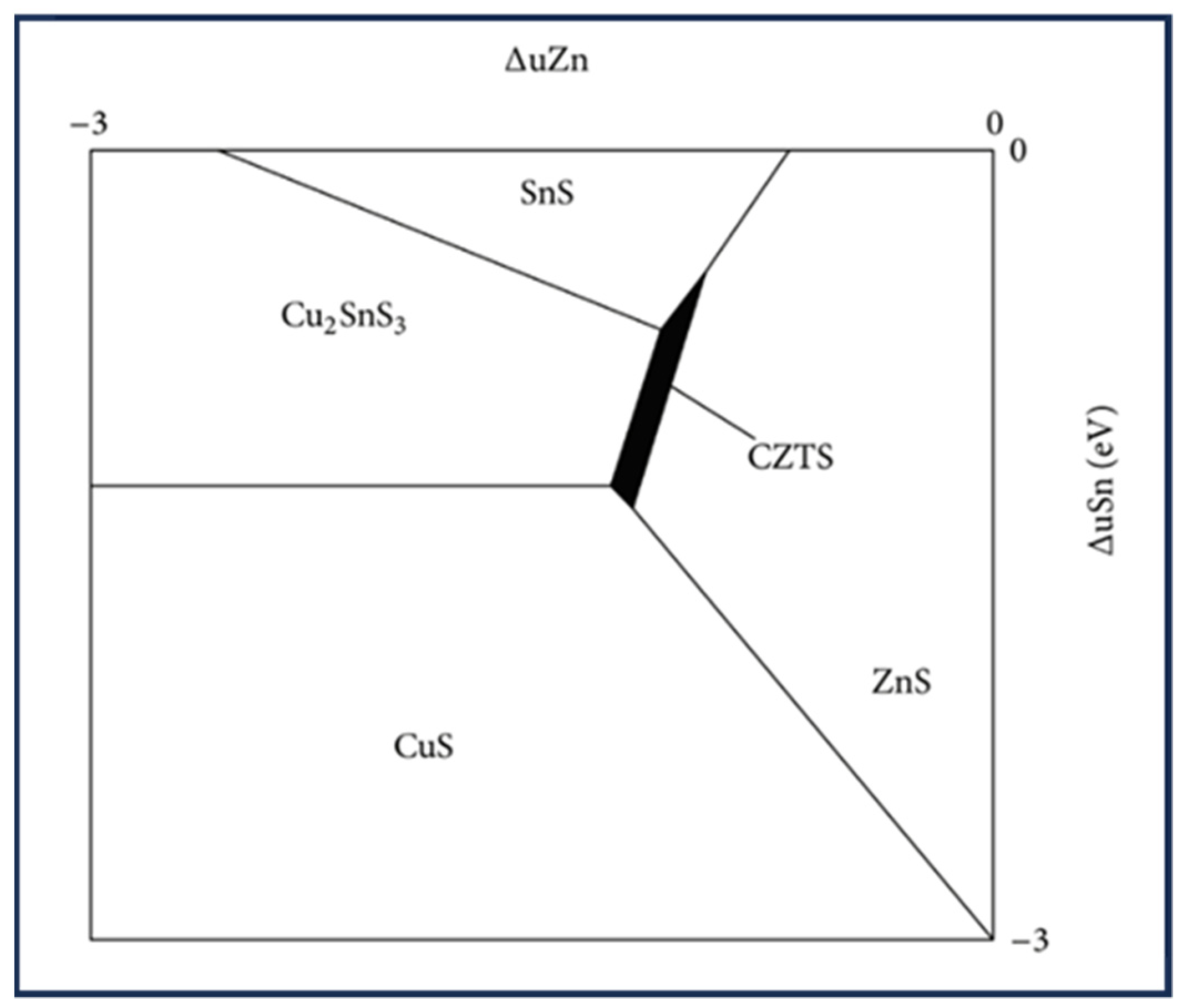
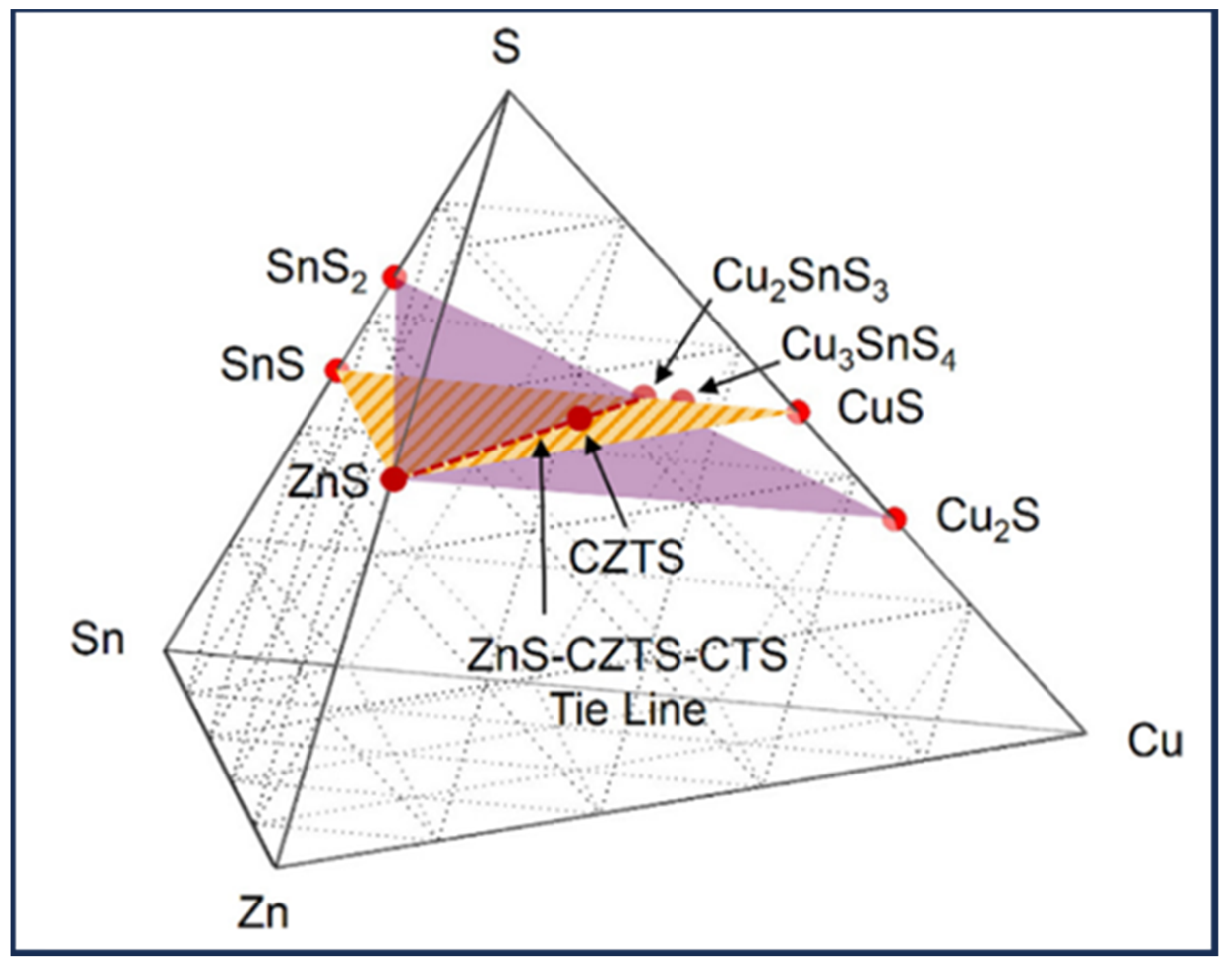
1.2. CZTS Phase Diagram
2. Nanoscale CZTS/Se Material: Synthesis Approaches
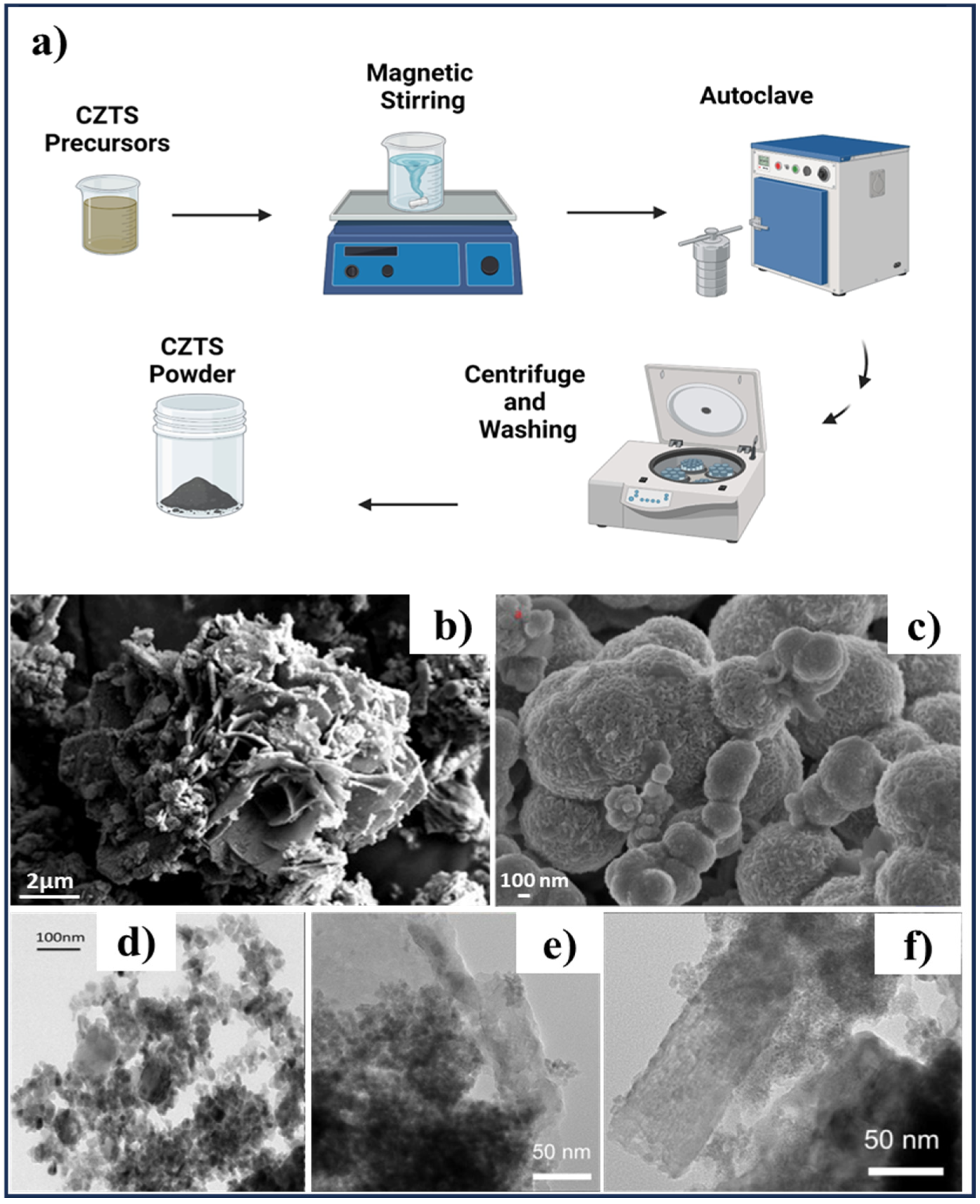
2.1. Hydrothermal Method
2.2. Solvothermal Method
2.3. Sol–Gel Method
2.4. Co-Precipitation Method
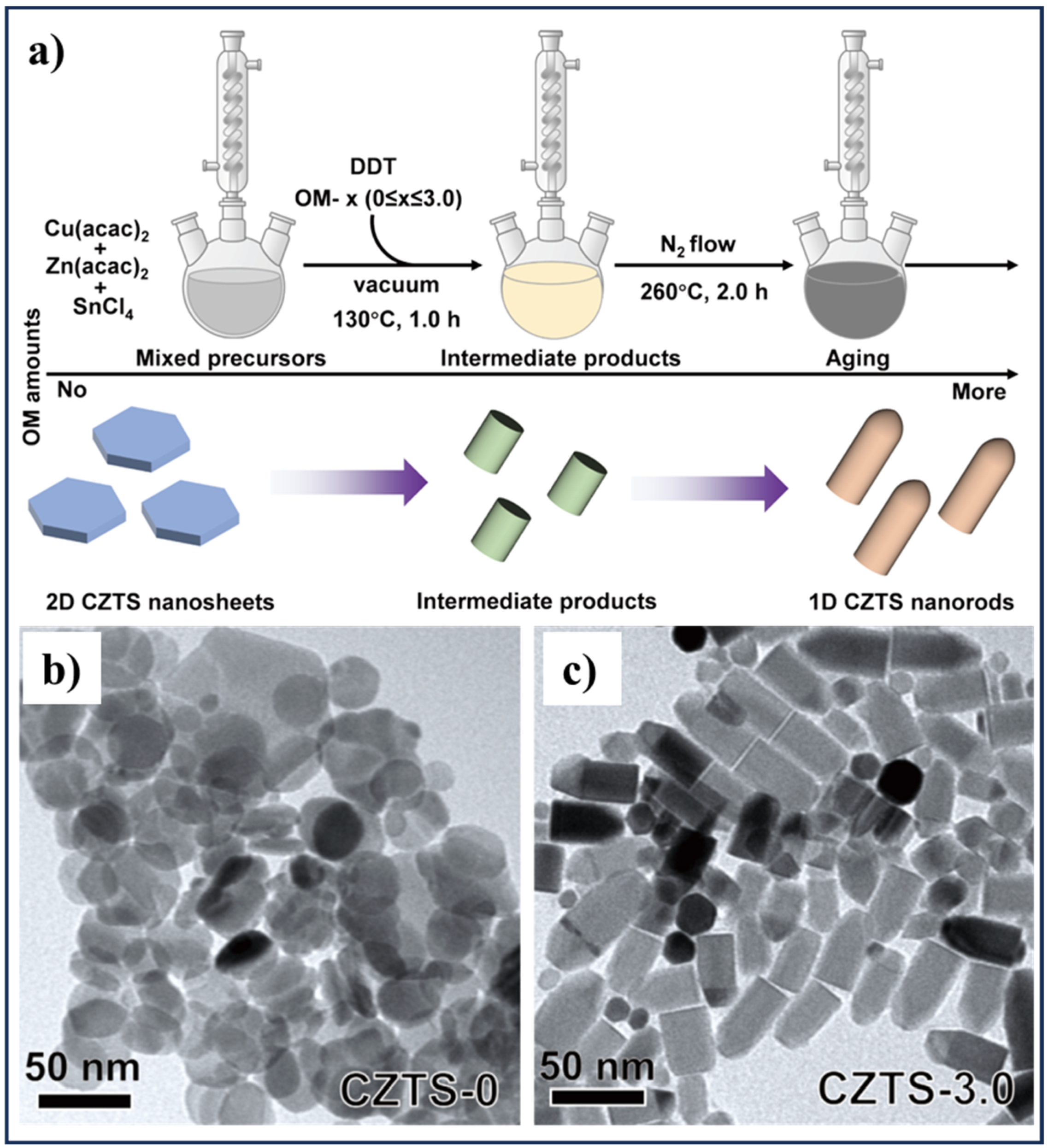
2.5. Hot-Injection and Colloidal Synthesis Methods
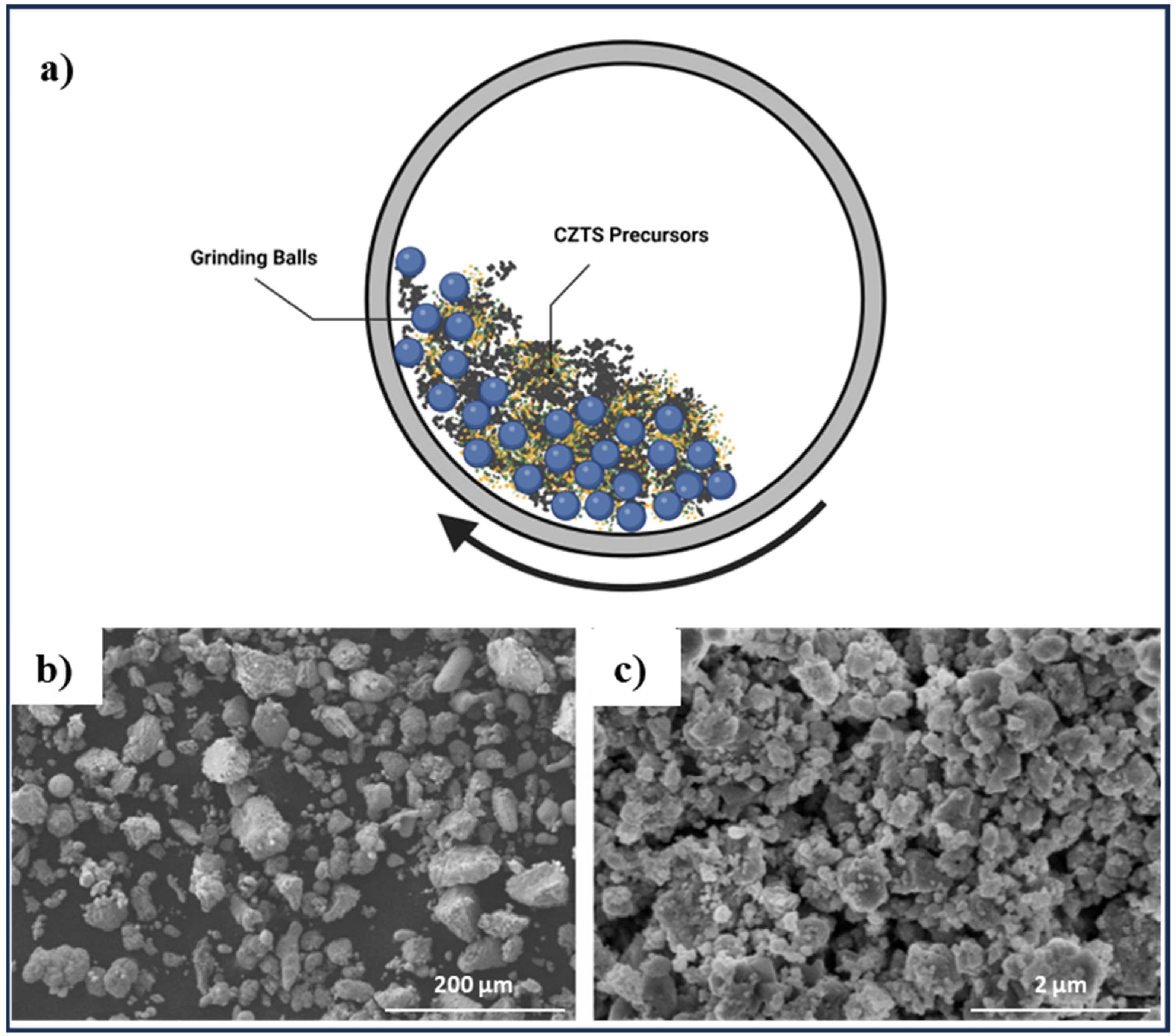
2.6. Solid State, Mechanical Alloying, and Mechanochemical Synthesis Method
3. Thin Film Deposition Techniques of CZTS/Se Nanomaterials
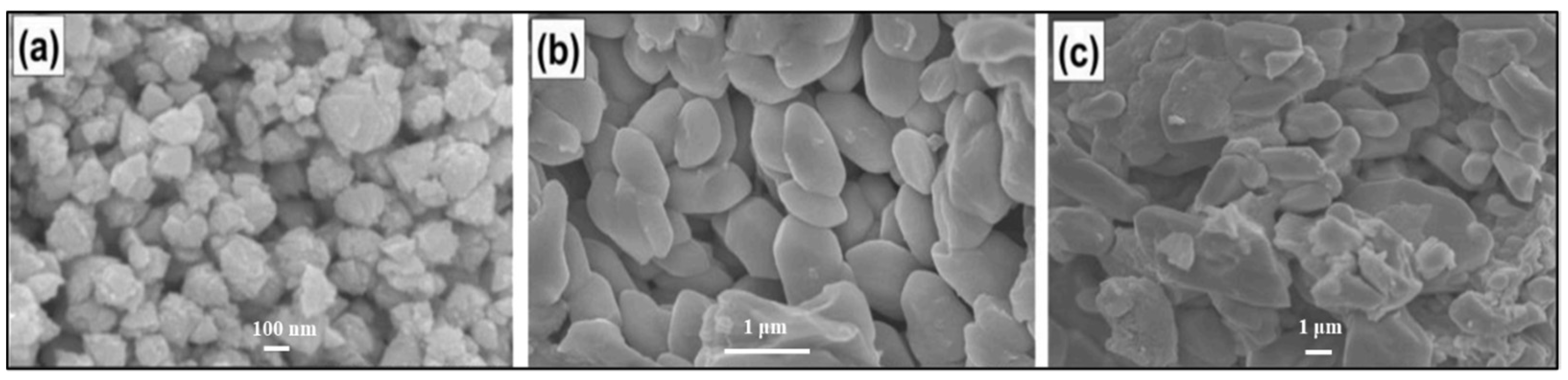
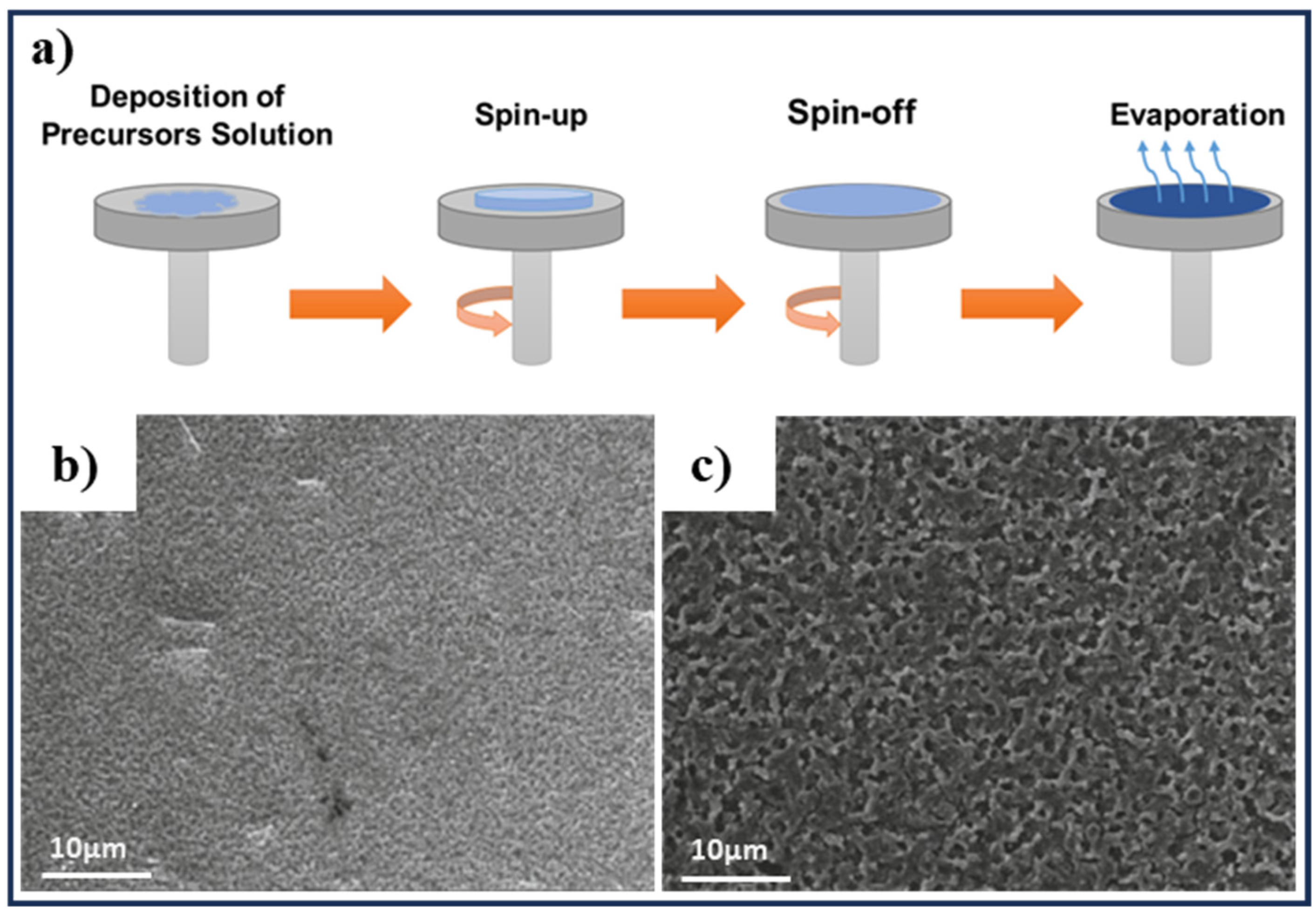
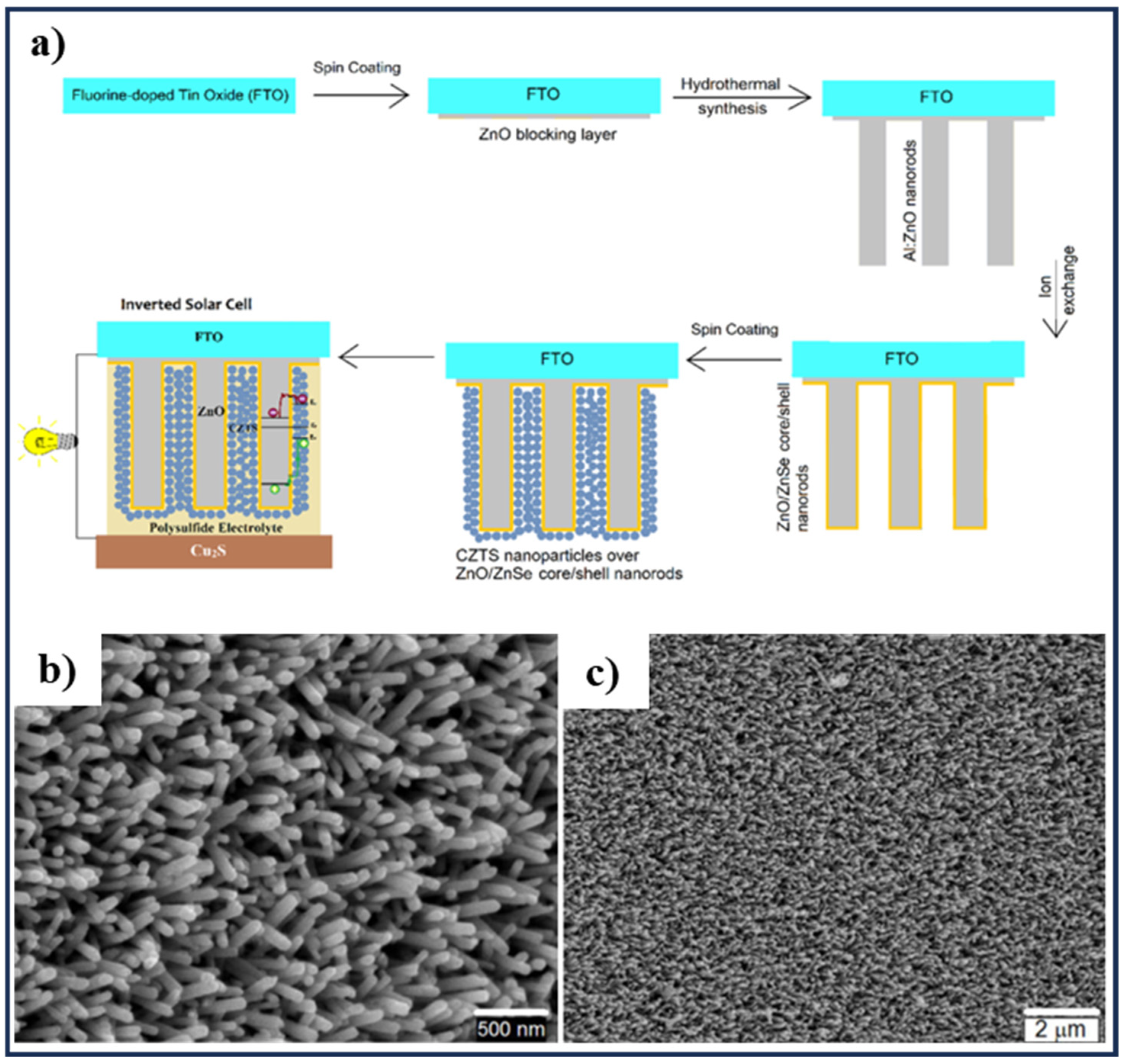
3.1. Spin-Coating Method
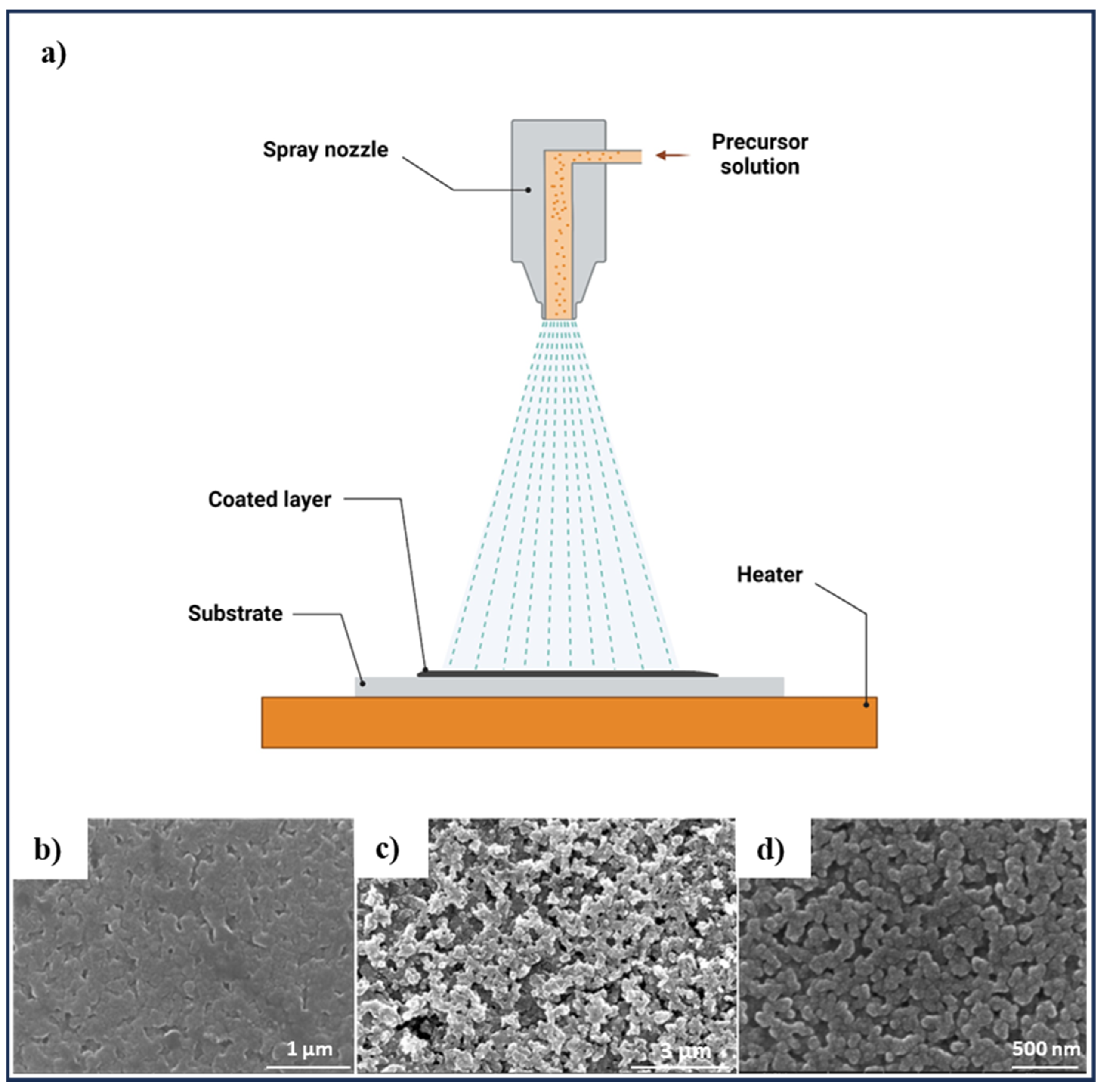
3.2. Spray Pyrolysis Method
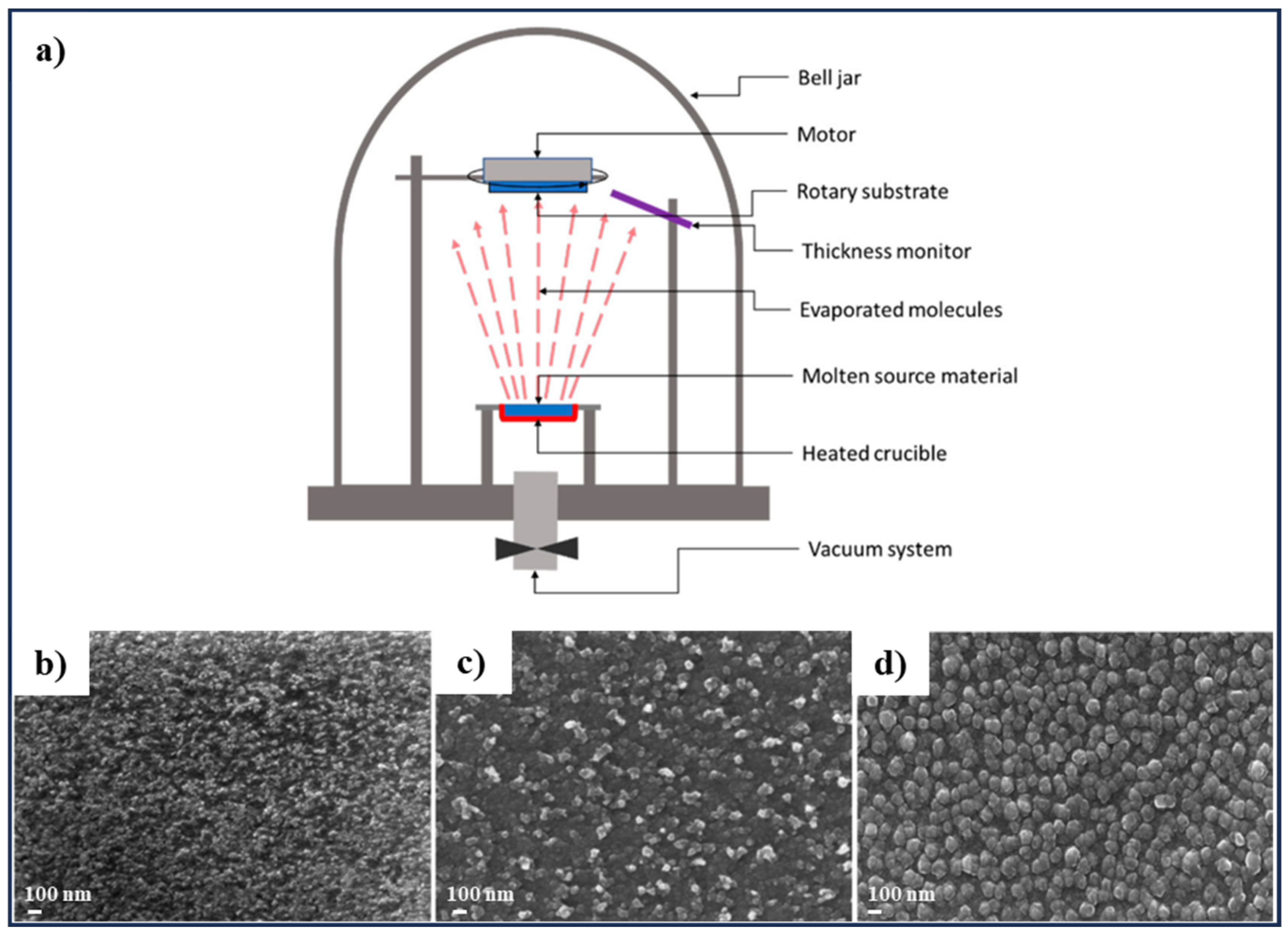
3.3. Thermal Evaporation
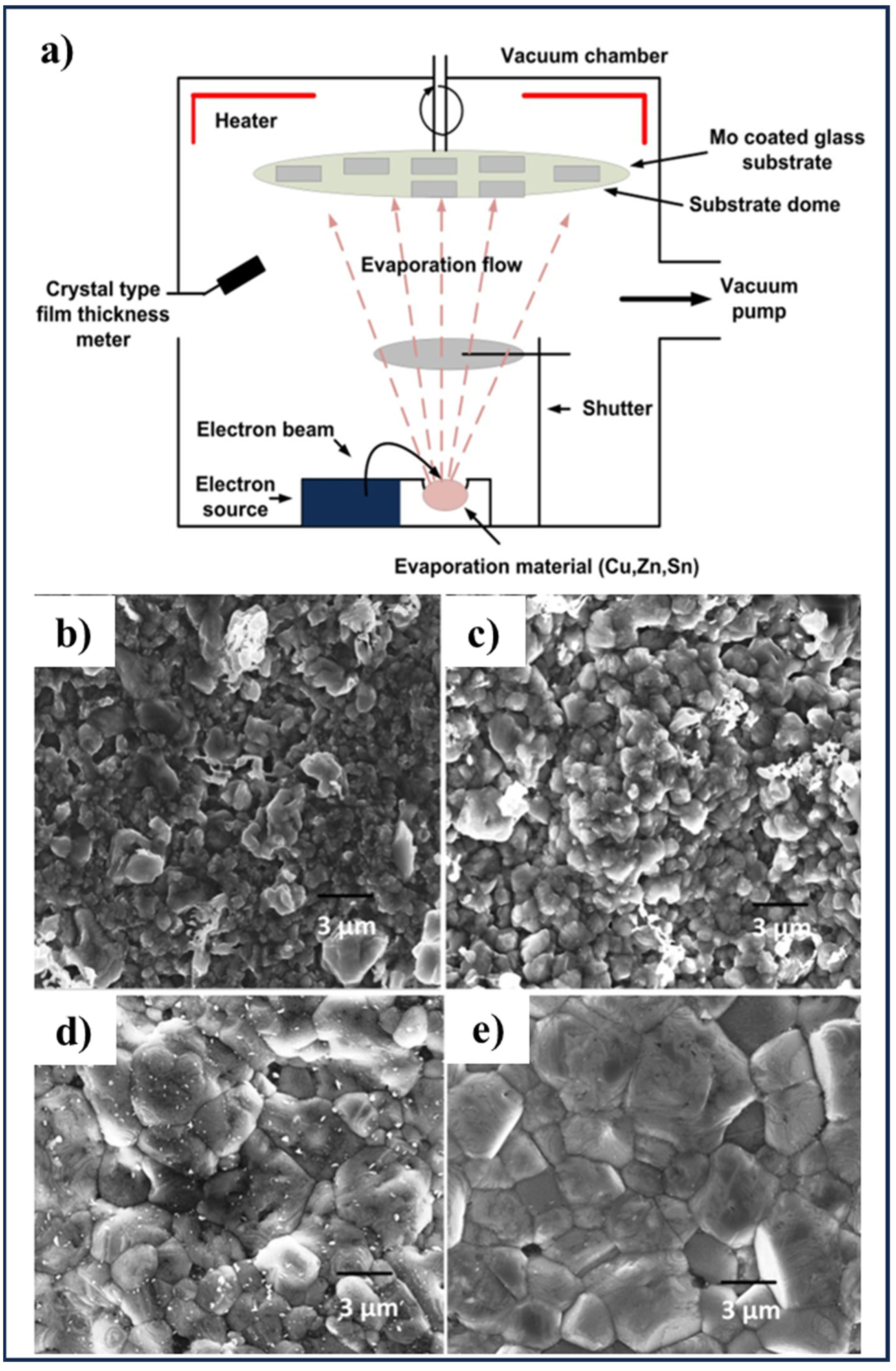
3.4. Electron Beam Evaporation
3.5. Successive Ionic Layer Adsorption and Reaction (SILAR) Method
3.6. Pulsed Laser Deposition
3.7. Electrochemical Deposition Method
4. Addressing Hurdles in Using CZTS/Se Nanomaterials towards Improving Thin Film Solar Cell Performance
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Najm, A.S.; Al-Ghamdi, A.; Amin, M.T.; Al Ghamdi, A.; Moria, H.; Holi, A.M.; Abed, A.M.; Al-Zahrani, A.A.; Sopian, K.; Bais, B.; et al. Towards a promising systematic approach to the synthesis of CZTS solar cells. Sci. Rep. 2023, 13, 15418. [Google Scholar] [CrossRef] [PubMed]
- Olgar, M.A.; Erkan, S.; Zan, R. Dependence of CZTS thin film properties and photovoltaic performance on heating rate and sulfurization time. J. Alloys Compd. 2023, 963, 171283. [Google Scholar] [CrossRef]
- Amrillah, T. Enhancing the value of environment-friendly CZTS compound for next generation photovoltaic device: A review. Sol. Energy 2023, 263, 111982. [Google Scholar] [CrossRef]
- Patil, S.S.; Nadaf, S.N.; Mali, S.S.; Hong, C.K.; Bhosale, P.N. Optoelectronic and Photovoltaic Properties of the Cu2ZnSnS4 Photocathode by a Temperature-Dependent Facile Hydrothermal Route. Ind. Eng. Chem. Res. 2021, 60, 7816–7825. [Google Scholar] [CrossRef]
- Islam, M.F.; Md Yatim, N.; Hashim@Ismail, M.A. A Review of CZTS Thin Film Solar Cell Technology. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 81, 73–87. [Google Scholar] [CrossRef]
- Chtouki, T.; Hachimi, M.A.; Tarbi, A.; Erguig, H. Development of different characterizations of sprayed Cu2ZnSnS4 thin films: A review. Opt. Quantum Electron. 2023, 55, 342. [Google Scholar] [CrossRef]
- Shah, N.; Shah, A.A.; Leung, P.K.; Khan, S.; Sun, K.; Zhu, X.; Liao, Q. A Review of Third Generation Solar Cells. Processes 2023, 11, 1852. [Google Scholar] [CrossRef]
- ElKhamisy, K.; Abdelhamid, H.; El-Rabaie, E.-S.M.; Abdel-Salam, N. A Comprehensive Survey of Silicon Thin-film Solar Cell: Challenges and Novel Trends. Plasmonics 2024, 19, 1–20. [Google Scholar] [CrossRef]
- Di Sabatino, M.; Hendawi, R.; Garcia, A.S. Silicon Solar Cells: Trends, Manufacturing Challenges, and AI Perspectives. Crystals 2024, 14, 167. [Google Scholar] [CrossRef]
- Green, M.A. Silicon solar cells step up. Nat. Energy 2023, 8, 783–784. [Google Scholar] [CrossRef]
- Theelen, M.; Daume, F. Stability of Cu(In,Ga)Se2 solar cells: A literature review. Sol. Energy 2016, 133, 586–627. [Google Scholar] [CrossRef]
- Contreras, M.A.; Mansfield, L.M.; Egaas, B.; Li, J.; Romero, M.; Noufi, R.; Rudiger-Voigt, E.; Mannstadt, W. Wide bandgap Cu(In,Ga)Se2 solar cells with improved energy conversion efficiency. Prog. Photovolt. Res. Appl. 2012, 20, 843–850. [Google Scholar] [CrossRef]
- Nishinaga, J.; Kamikawa, Y.; Sugaya, T.; Ishizuka, S. Comparison of polycrystalline and epitaxial Cu(In, Ga)Se2 solar cells with conversion efficiencies of more than 21%. Sol. Energy Mater. Sol. Cells 2024, 269, 112791. [Google Scholar] [CrossRef]
- Geisthardt, R.M.; Topič, M.; Sites, J.R. Status and Potential of CdTe Solar-Cell Efficiency. IEEE J. Photovolt. 2015, 5, 1217–1221. [Google Scholar] [CrossRef]
- Oni, A.M.; Mohsin, A.S.M.; Rahman, M.M.; Hossain Bhuian, M.B. A comprehensive evaluation of solar cell technologies, associated loss mechanisms, and efficiency enhancement strategies for photovoltaic cells. Energy Rep. 2024, 11, 3345–3366. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Karmakar, A.; Ullah, A.K.M.A.; Manavalan, R.K.; Sakthipandi, K.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. The hybrid halide perovskite: Synthesis strategies, fabrications, and modern applications. Ceram. Int. 2022, 48, 7325–7343. [Google Scholar] [CrossRef]
- Gupta, I.; Dey, A.; Jain, R. Non-toxic solution processed Cu2ZnSn(SSe)4 thin films for photovoltaic Applications: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Khadir, A.; Lahoual, M.; Abdelhafidi, M.K.; Sengouga, N. Design of CZTS/CZTSe tandem solar cells with enhanced performance. Mater. Today Commun. 2023, 37, 107094. [Google Scholar] [CrossRef]
- Song, X.; Ji, X.; Li, M.; Lin, W.; Luo, X.; Zhang, H. A Review on Development Prospect of CZTS Based Thin Film Solar Cells. Int. J. Photoenergy 2014, 2014, 613173. [Google Scholar] [CrossRef]
- Suryawanshi, M.P.; Agawane, G.L.; Bhosale, S.M.; Shin, S.W.; Patil, P.S.; Kim, J.H.; Moholkar, A.V. CZTS based thin film solar cells: A status review. Mater. Technol. 2013, 28, 98–109. [Google Scholar] [CrossRef]
- Yussuf, S.T.; Nwambaekwe, K.C.; Ramoroka, M.E.; Iwuoha, E.I. Photovoltaic efficiencies of microwave and Cu2ZnSnS4 (CZTS) superstrate solar cells. Mater. Today Sustain. 2023, 21, 100287. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Ikram, A.; Zulfequar, M.; Satsangi, V.R. Role and prospects of green quantum dots in photoelectrochemical hydrogen generation: A review. Int. J. Hydrog. Energy 2022, 47, 11472–11491. [Google Scholar] [CrossRef]
- Ikram, A.; Zulfequar, M. Visible light driven CZTS QDs/α-Fe2O3-graphene p–n heterojunction for photoelectrochemical water splitting. Nanotechnology 2023, 34, 315403. [Google Scholar] [CrossRef] [PubMed]
- Sahai, S.; Ikram, A.; Rai, S.; Shrivastav, R.; Dass, S.; Satsangi, V.R. Quantum dots sensitization for photoelectrochemical generation of hydrogen: A review. Renew. Sustain. Energy Rev. 2017, 68, 19–27. [Google Scholar] [CrossRef]
- Yu, K.; Chen, J. Enhancing Solar Cell Efficiencies through 1-D Nanostructures. Nanoscale Res. Lett. 2008, 4, 1. [Google Scholar] [CrossRef]
- Isotta, E.; Pugno, N.M.; Scardi, P. Nanostructured kesterite (Cu2ZnSnS4) for applications in thermoelectric devices. Powder Diffr. 2019, 34, S42–S47. [Google Scholar] [CrossRef]
- Kumar, S.; Ansari, M.Z.; Khare, N. Influence of compactness and formation of metallic secondary phase on the thermoelectric properties of Cu2ZnSnS4 thin films. Thin Solid Film. 2018, 645, 300–304. [Google Scholar] [CrossRef]
- Yang, H.; Jauregui, L.A.; Zhang, G.; Chen, Y.P.; Wu, Y. Nontoxic and abundant copper zinc tin sulfide nanocrystals for potential high-temperature thermoelectric energy harvesting. Nano Lett. 2012, 12, 540–545. [Google Scholar] [CrossRef]
- Long, B.D.; Van Khanh, N.; Binh, D.N.; Hai, N.H. Thermoelectric properties of quaternary chalcogenide Cu2ZnSnS4 synthesised by mechanical alloying. Powder Metall. 2020, 63, 220–226. [Google Scholar] [CrossRef]
- Gour, K.S.; Bhattacharyya, B.; Singh, O.P.; Yadav, A.K.; Husale, S.; Singh, V.N. Nanostructured Cu2ZnSnS4 (CZTS) thin film for self-powered broadband photodetection. J. Alloys Compd. 2018, 735, 285–290. [Google Scholar] [CrossRef]
- Yu, X.; Shavel, A.; An, X.; Luo, Z.; Ibáñez, M.; Cabot, A. Cu2ZnSnS4-Pt and Cu2ZnSnS4-Au Heterostructured Nanoparticles for Photocatalytic Water Splitting and Pollutant Degradation. J. Am. Chem. Soc. 2014, 136, 9236–9239. [Google Scholar] [CrossRef]
- Chang, Z.-X.; Chong, R.-F.; Meng, Y.-N.; Zhou, W.-H.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. High temperature recrystallization of kersterite Cu2ZnSnS4 towards enhanced photocatalytic H2 evolution. Int. J. Hydrog. Energy 2015, 40, 13456–13462. [Google Scholar] [CrossRef]
- Gogoi, G.; Arora, S.; Vinothkumar, N.; De, M.; Qureshi, M. Quaternary semiconductor Cu2ZnSnS4 loaded with MoS2 as a co-catalyst for enhanced photo-catalytic activity. RSC Adv. 2015, 5, 40475–40483. [Google Scholar] [CrossRef]
- Zhou, X.; Gu, X.; Chen, Z.; Wu, Y.; Xu, W.; Bao, J. A novel and sensitive Cu2ZnSnS4 quantum dot–based non–enzymatic glucose sensor. Sens. Actuators B Chem. 2021, 329, 129117. [Google Scholar] [CrossRef]
- Gurav, K.V.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Suryawanshi, M.P.; Agawane, G.L.; Pawar, S.M.; Patil, P.S.; Lee, J.Y.; Lokhande, C.D.; et al. Cu2ZnSnS4 (CZTS)-based room temperature liquefied petroleum gas (LPG) sensor. Sens. Actuators B Chem. 2014, 190, 408–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Sun, K. Cu2ZnSnS4 (CZTS) for Photoelectrochemical CO2 Reduction: Efficiency, Selectivity, and Stability. Nanomaterials 2023, 13, 2762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Liu, M.; Su, J.; Shen, S. Towards efficient solar-to-hydrogen conversion: Fundamentals and recent progress in copper-based chalcogenide photocathodes. Nanophotonics 2016, 5, 524–547. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Venugopal, D.; Ramachandran, K.; Ramesh, R. Synthesis of 2D-CZTS nanoplate as photocathode material for efficient PEC water splitting. J. Mater. Sci. Mater. Electron. 2022, 33, 8493–8503. [Google Scholar] [CrossRef]
- Wang, J.; Yu, N.; Zhang, Y.; Zhu, Y.; Fu, L.; Zhang, P.; Gao, L.; Wu, Y. Synthesis and performance of Cu2ZnSnS4 semiconductor as photocathode for solar water splitting. J. Alloys Compd. 2016, 688, 923–932. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Feng, K.; Huang, D.; Wang, K.; Li, Y.; Jiang, F. Kesterite Cu2ZnSnS4 thin-film solar water-splitting photovoltaics for solar seawater desalination. Cell Rep. Phys. Sci. 2021, 2, 100468. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Current Advances in Quantum-Dots-Based Photoelectrochemical Immunoassays. Chem. Asian. J. 2017, 12, 2780–2789. [Google Scholar] [CrossRef]
- Ceylan, S.; Küçükosman, R.; Yurt, F.; Özel, D.; Öztürk, İ.; Demir, D.; Ocakoglu, K. Antimicrobial activity enhancement of PVA/chitosan films with the additive of CZTS quantum dots. Polym. Bull. 2023, 80, 11273–11293. [Google Scholar] [CrossRef]
- Kumar, R.S.; Maddirevula, S.; Easwaran, M.; Dananjaya, S.H.S.; Kim, M.-D. Antibacterial activity of novel Cu2ZnSnS4 nanoparticles against pathogenic strains. RSC Adv. 2015, 5, 106400–106405. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Dizge, N.; Colak, S.; Ozay, Y.; Bilici, Z.; Yalcin, M.S.; Özdemir, S.; Yatmaz, H. Polyethersulfone membranes modified with CZTS nanoparticles for protein and dye separation: Improvement of antifouling and self-cleaning performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126230. [Google Scholar] [CrossRef]
- Colak, S.; Vejselova Sezer, C.; Demirdogen, R.E.; Ince, M.; Emen, F.; Ocakoglu, K.; Kutlu, H.M. Investigation of in vitro Activities of Cu2ZnSnS4 Nanoparticles in Human Non-Small Cell Lung Cancer. Mater. Today Commun. 2021, 27, 102304. [Google Scholar] [CrossRef]
- Tang, T.; Xu, X.; Wang, Z.; Tian, J.; Yang, Y.; Ou, C.; Bao, H.; Liu, T. Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy. Chem. Commun. 2019, 55, 13148–13151. [Google Scholar] [CrossRef]
- Shi, Z.; Attygalle, D.; Jayatissa, A.H. Kesterite-based next generation high performance thin film solar cell: Current progress and future prospects. J. Mater. Sci. Mater. Electron. 2017, 28, 2290–2306. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Crystal and electronic band structure of Cu2ZnSnX4 (X = S and Se) photovoltaic absorbers: First-principles insights. Appl. Phys. Lett. 2009, 94, 041903. [Google Scholar] [CrossRef]
- Khare, A.; Himmetoglu, B.; Johnson, M.; Norris, D.J.; Cococcioni, M.; Aydil, E.S. Calculation of the lattice dynamics and Raman spectra of copper zinc tin chalcogenides and comparison to experiments. J. Appl. Phys. 2012, 111, 083707. [Google Scholar] [CrossRef]
- Schorr, S.; Hoebler, H.-J.; Tovar, M. A neutron diffraction study of the stannite-kesterite solid solution series. Eur. J. Mineral. 2007, 19, 65–73. [Google Scholar] [CrossRef]
- Schorr, S. The crystal structure of kesterite type compounds: A neutron and X-ray diffraction study. Sol. Energy Mater. Sol. Cells 2011, 95, 1482–1488. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Cui, C.; Wang, X.; Shao, Z.; Pang, S.; Cui, G. CZTSSe solar cells: Insights into interface engineering. J. Mater. Chem. A 2023, 11, 4836–4849. [Google Scholar] [CrossRef]
- Boerasu, I.; Vasile, B.S. Current Status of the Open-Circuit Voltage of Kesterite CZTS Absorber Layers for Photovoltaic Applications-Part I, a Review. Materials 2022, 15, 8427. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sánchez, Y.; López-Marino, S.; Espíndola-Rodríguez, M.; Neuschitzer, M.; Sylla, D.; Fairbrother, A.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Impact of Sn(S,Se) Secondary Phases in Cu2ZnSn(S,Se)4 Solar Cells: A Chemical Route for Their Selective Removal and Absorber Surface Passivation. ACS Appl. Mater. Interfaces 2014, 6, 12744–12751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS–Se solar cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Olekseyuk, I.D.; Dudchak, I.V.; Piskach, L.V. Phase equilibria in the Cu2S–ZnS–SnS2 system. J. Alloys Compd. 2004, 368, 135–143. [Google Scholar] [CrossRef]
- Dudchak, I.V.; Piskach, L.V. Phase equilibria in the Cu2SnSe3–SnSe2–ZnSe system. J. Alloys Compd. 2003, 351, 145–150. [Google Scholar] [CrossRef]
- Nagoya, A.; Asahi, R.; Wahl, R.; Kresse, G. Defect formation and phase stability of Cu2ZnSnS4 photovoltaic material. Phys. Rev. B 2010, 81, 113202. [Google Scholar] [CrossRef]
- Choubrac, L.; Lafond, A.; Guillot-Deudon, C.; Moëlo, Y.; Jobic, S. Structure Flexibility of the Cu2ZnSnS4 Absorber in Low-Cost Photovoltaic Cells: From the Stoichiometric to the Copper-Poor Compounds. Inorg. Chem. 2012, 51, 3346–3348. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.A.; Du, H.; Hlaing Oo, W.M.; Teeter, G.; Scarpulla, M.A. Investigation of combinatorial coevaporated thin film Cu2ZnSnS4 (II): Beneficial cation arrangement in Cu-rich growth. J. Appl. Phys. 2014, 115, 173503. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Koh, S.C.L.; Reaney, I.M.; Acquaye, A.; Schileo, G.; Mustapha, K.B.; Greenough, R. Perovskite solar cells: An integrated hybrid lifecycle assessment and review in comparison with other photovoltaic technologies. Renew. Sustain. Energy Rev. 2017, 80, 1321–1344. [Google Scholar] [CrossRef]
- Maalouf, A.; Okoroafor, T.; Jehl, Z.; Babu, V.; Resalati, S. A comprehensive review on life cycle assessment of commercial and emerging thin-film solar cell systems. Renew. Sustain. Energy Rev. 2023, 186, 113652. [Google Scholar] [CrossRef]
- Kowsar, A.; Farhad, S.F.U.; Rahaman, M.; Islam, M.S.; Imam, A.Y.; Debnath, S.C.; Sultana, M.; Hoque, M.A.; Sharmin, A.; Mahmood, Z.H. Progress in major thin-film solar cells: Growth technologies, layer materials and efficiencies. Int. J. Renew. Energy Res. 2019, 9, 579–597. [Google Scholar]
- Camara, S.M.; Wang, L.; Zhang, X. Easy hydrothermal preparation of Cu2ZnSnS4 (CZTS) nanoparticles for solar cell application. Nanotechnology 2013, 24, 495401. [Google Scholar] [CrossRef] [PubMed]
- Vanalakar, S.A.; Agwane, G.L.; Gang, M.G.; Patil, P.S.; Kim, J.H.; Kim, J.Y. A mild hydrothermal route to synthesis of CZTS nanoparticle inks for solar cell applications. Phys. Status Solidi C 2015, 12, 500–503. [Google Scholar] [CrossRef]
- Akshaya, C.V.; Dilraj, N.; Arjun, G.; Parvathi, E.; Deepak, N.K. A comparative study on the hydrothermal and solvothermal synthesis of CZTS quantum dots. In Materials Today: Proceedings; Elsever: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Sheebha, I.; Sebastian, A.; Vidhya, B. An insight on the growth mechanism of Cu2ZnSnS4 via hydrothermal route. J. Alloys Compd. 2023, 968, 172064. [Google Scholar] [CrossRef]
- Henriquez, R.; Nogales, P.S.; Moreno, P.G.; Cartagena, E.M.; Bongiorno, P.L.; Navarrete-Astorga, E.; Dalchiele, E.A. One-Step Hydrothermal Synthesis of Cu(2)ZnSnS(4) Nanoparticles as an Efficient Visible Light Photocatalyst for the Degradation of Congo Red Azo Dye. Nanomaterials 2023, 13, 1731. [Google Scholar] [CrossRef]
- Cristóbal-García, J.D.; Paraguay-Delgado, F.; Herrera-Pérez, G.M.; Fuentes-Cobas, L.E.; Pantoja-Espinoza, J.C. Study of the thermal decomposition of Cu2ZnSnS4 (CZTS) in different atmospheres: Effect of annealing on its structural and optical properties. J. Mater. Sci. Mater. Electron. 2023, 34, 2013. [Google Scholar] [CrossRef]
- Liu, K.; Ji, N.; Shi, L.; Liu, H. The Phase and Morphology of Cu2ZnSnSe4 Nanopowders by Hydrothermal Method. J. Nanomater. 2014, 2014, 910639. [Google Scholar] [CrossRef]
- Yan, X.; Hu, X.; Komarneni, S. Solvothermal synthesis of CZTS nanoparticles in ethanol: Preparation and characterization. J. Korean Phys. Soc. 2015, 66, 1511–1515. [Google Scholar] [CrossRef]
- Patro, B.; Vijaylakshmi, S.; Sharma, P. Rapid microwave-assisted solvothermal synthesis of Cu2ZnSnS4 (CZTS) nanocrystals for low-cost thin film photovoltaic: Investigation of synthesis parameters and morphology control. J. Mater. Sci. Mater. Electron. 2017, 29, 3370–3380. [Google Scholar] [CrossRef]
- Ahmad, R.; Saddiqi, N.-u.-H.; Wu, M.; Prato, M.; Spiecker, E.; Peukert, W.; Distaso, M. Effect of the Counteranion on the Formation Pathway of Cu2ZnSnS4 (CZTS) Nanoparticles under Solvothermal Conditions. Inorg. Chem. 2020, 59, 1973–1984. [Google Scholar] [CrossRef]
- Wei, A.; Yan, Z.; Zhao, Y.; Zhuang, M.; Liu, J. Solvothermal synthesis of Cu2ZnSnS4 nanocrystalline thin films for application of solar cells. Int. J. Hydrog. Energy 2015, 40, 797–805. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.R.; Hu, J.Q. Solvothermal Synthesis and Characterization of Quaternary Cu2ZnSnSe4 Nanocrystals. Adv. Mater. Res. 2011, 347–353, 848–851. [Google Scholar] [CrossRef]
- Xia, D.; Zheng, Y.; Lei, P.; Zhao, X. Characterization of Cu2ZnSnS4 Thin Films Prepared by Solution-based Deposition Techniques. Phys. Procedia 2013, 48, 228–234. [Google Scholar] [CrossRef][Green Version]
- Khushaim, M.; Alamri, S.; Kattan, N.; Jaber, A.; Alamri, S. Study of kesterite Cu2ZnSnS4 (CZTS) thin films deposited by spray technique for photovoltaic applications. J. Taibah Univ. Sci. 2021, 15, 329–339. [Google Scholar] [CrossRef]
- Orletskyi, I.G.; Solovan, M.M.; Brus, V.V.; Pinna, F.; Cicero, G.; Maryanchuk, P.D.; Maistruk, E.V.; Ilashchuk, M.I.; Boichuk, T.I.; Tresso, E. Structural, optical and electrical properties of Cu2ZnSnS4 films prepared from a non-toxic DMSO-based sol-gel and synthesized in low vacuum. J. Phys. Chem. Solids 2017, 100, 154–160. [Google Scholar] [CrossRef]
- Li, C.; Ha, E.; Wong, W.-L.; Li, C.; Ho, K.-P.; Wong, K.-Y. A facile arrested precipitation method for synthesis of pure wurtzite Cu2ZnSnS4 nanocrystals using thiourea as a sulfur source. Mater. Res. Bull. 2012, 47, 3201–3205. [Google Scholar] [CrossRef]
- Pal, K.; Maurya, D.K.; Chaudhary, P.; Thapa, K.B.; Yadav, B.C. Co-precipitation Synthesis with a Variation of the Sulphur Composition of Kesterite Phase Cu2ZnSnS4 (CZSS) without Annealing Process. J. Phys. Sci. 2021, 32, 27–39. [Google Scholar] [CrossRef]
- Semalti, P.; Sharma, V.; Sharma, S.N. A novel method of water remediation of organic pollutants and industrial wastes by solution- route processed CZTS nanocrystals. J. Mater. 2021, 7, 904–919. [Google Scholar] [CrossRef]
- Méndez-López, A.; Morales-Acevedo, A.; Acosta-Silva, Y.J.; Ortega-López, M. Synthesis and Characterization of Colloidal CZTS Nanocrystals by a Hot-Injection Method. J. Nanomater. 2016, 2016, 7486094. [Google Scholar] [CrossRef]
- Mirbagheri, N.; Engberg, S.; Crovetto, A.; Simonsen, S.B.; Hansen, O.; Lam, Y.M.; Schou, J. Synthesis of ligand-free CZTS nanoparticles via a facile hot injection route. Nanotechnology 2016, 27, 185603. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chawla, P.; Sharma, S.N.; Singh, D.; Vijayan, N. Efficient colloidal route to pure phase kesterite Cu2ZnSnS4 (CZTS) nanocrystals with controlled shape and structure. Superlattices Microstruct. 2018, 119, 59–71. [Google Scholar] [CrossRef]
- Jain, S.; Singh, A.; Gupta, G.; Vijayan, N.; Sharma, S.N. Precursor ratio optimizations for the synthesis of colloidal CZTS nanoparticles for photocatalytic degradation of malachite green. J. Phys. Chem. Solids 2018, 122, 8–18. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Chen, J.; Lin, O.; Yin, Z.; Yang, C.; Tang, A. From kesterite 2D nanosheets to wurtzite 1D nanorods: Controllable synthesis of Cu-Zn-Sn-S and their application in electrocatalytic hydrogen evolution. J. Semicond. 2023, 44, 122701. [Google Scholar] [CrossRef]
- Prabhu, S.; Pandey, N.; Punetha, D.; Chakrabarti, S. A comparative study of Cu2ZnSn(SxSe1−x)4 nanoparticles for solar cells: From chemical synthesis to devices. Mater. Today Commun. 2023, 37, 106905. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H. Cu2ZnSnS4 synthesized through a green and economic process. J. Alloys Compd. 2011, 509, 9627–9630. [Google Scholar] [CrossRef]
- Pareek, D.; Balasubramaniam, K.R.; Sharma, P. Synthesis and characterization of bulk Cu2ZnSnX4 (X: S, Se) via thermodynamically supported mechano-chemical process. Mater. Charact. 2015, 103, 42–49. [Google Scholar] [CrossRef]
- Sahu, M.; Reddy, V.R.M.; Kim, B.; Patro, B.; Park, C.; Kim, W.K.; Sharma, P. Fabrication of Cu(2)ZnSnS(4) Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications. Materials 2022, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Mokurala, K.; Mallick, S.; Bhargava, P. Low Temperature Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Nanoparticle by Solution Based Solid State Reaction Method. Energy Procedia 2014, 57, 73–78. [Google Scholar] [CrossRef]
- Angel Agnes, J.; Beauno, S. Mechanosynthesis of semiconductor nanocrystalline Cu2ZnSnSe4 for solar cells. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Li, A.D.; Liu, W.C. 4-Optical properties of ferroelectric nanocrystal/polymer composites. In Physical Properties and Applications of Polymer Nanocomposites; Tjong, S.C., Mai, Y.W., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 108–158. [Google Scholar]
- Choudhury, S.; Paul, S.; Goswami, S.; Deb, K. Chapter 2-Methods for nanoparticle synthesis and drug delivery. In Advances in Nanotechnology-Based Drug Delivery Systems; Das Talukdar, A., Dey Sarker, S., Patra, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 21–44. [Google Scholar]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. 2-Synthesis, design, and morphology of metal oxide nanostructures. In Metal Oxide Nanostructures; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–57. [Google Scholar]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Mirkovic, T.; Scholes, G.D. 5.06-Nanocrystal Synthesis. In Comprehensive Nanoscience and Technology; Andrews, D.L., Scholes, G.D., Wiederrecht, G.P., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 153–201. [Google Scholar]
- Sakka, S. History of the Sol–Gel Chemistry and Technology. In Handbook of Sol-Gel Science and Technology; Springer: Berlin, Germany, 2016; pp. 1–27. [Google Scholar]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Sen Gupta, A.K.; Farhad, S.F.U.; Habib, M.S.; Hossan, M.R.; Hossain, K.; Das, N.K.; Quamruzzaman, M.; Matin, M.A.; Amin, N. Characterizations of extrinsically doped CZTS thin films for solar cell absorbers fabricated by sol-gel spin coating method. Appl. Surf. Sci. Adv. 2023, 13, 100352. [Google Scholar] [CrossRef]
- Ritchie, C.; Chesman, A.S.R.; Styles, M.; Jasieniak, J.J.; Mulvaney, P. Aqueous Synthesis of High-Quality Cu(2)ZnSnS(4) Nanocrystals and Their Thermal Annealing Characteristics. Langmuir 2018, 34, 1655–1665. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, J.; Cai, W.; Xie, Z.; Yang, Y. Fabrication of Cu2ZnSnS4 thin films by microwave assisted sol-gel method. Superlattices Microstruct. 2019, 126, 83–88. [Google Scholar] [CrossRef]
- Huang, X.; Lv, L.; Hu, Y.; Lou, Z.; Hou, Y.; Teng, F. Enhanced performance in inverted polymer solar cells employing microwave-annealed sol-gel ZnO as electron transport layers. Org. Electron. 2017, 42, 107–114. [Google Scholar] [CrossRef]
- Qotso, S.; Mbule, P.; Mothudi, B. Microwave-assisted sol-gel synthesis of P3HT-ZnO: Sm3+, Yb3+: Study of structure, morphology, optical and electrical conductivity for organic solar cells application. Mater. Chem. Phys. 2023, 294, 127023. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent Advances in Synthesis and Applications of MFe(2)O(4) (M = Co, Cu, Mn, Ni, Zn) Nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 2002, 115, 8706–8715. [Google Scholar] [CrossRef]
- Ghorpade, U.; Suryawanshi, M.; Shin, S.W.; Gurav, K.; Patil, P.; Pawar, S.; Hong, C.W.; Kim, J.H.; Kolekar, S. Towards environmentally benign approaches for the synthesis of CZTSSe nanocrystals by a hot injection method: A status review. Chem. Commun. 2014, 50, 11258–11273. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, M.; Liu, M.; Selopal, G.S.; Navarro-Pardo, F.; Wang, Z.M.; Stansfield, B.; Zhao, H.; Lai, C.-Y.; Radu, D.R.; Rosei, F. Synthesis of highly efficient Cu2ZnSnSxSe4−x (CZTSSe) nanosheet electrocatalyst for dye-sensitized solar cells. Electrochim. Acta 2020, 340, 135954. [Google Scholar] [CrossRef]
- Cao, Y.; Denny, M.S., Jr.; Caspar, J.V.; Farneth, W.E.; Guo, Q.; Ionkin, A.S.; Johnson, L.K.; Lu, M.; Malajovich, I.; Radu, D.; et al. High-Efficiency Solution-Processed Cu2ZnSn(S,Se)4 Thin-Film Solar Cells Prepared from Binary and Ternary Nanoparticles. J. Am. Chem. Soc. 2012, 134, 15644–15647. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, S.; Sun, C.; Wu, H. Facile synthesis of Cu2ZnSnS4 powders by mechanical alloying and annealing. Mater. Lett. 2016, 169, 176–179. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying: Nanotechnology, Materials Science and Powder Metallurgy; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Xu, D.; Zhang, Y. Fabrication of Cu2ZnSnS4 screen printed layers for solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 2042–2045. [Google Scholar] [CrossRef]
- Ahmoum, H.; Chelvanathan, P.; Su'ait, M.S.; Boughrara, M.; Li, G.; Al-Waeli, A.H.A.; Sopian, K.; Kerouad, M.; Amin, N. Impact of preheating environment on microstructural and optoelectronic properties of Cu2ZnSnS4 (CZTS) thin films deposited by spin-coating. Superlattices Microstruct. 2020, 140, 106452. [Google Scholar] [CrossRef]
- Majula, L.; Mlyuka, N.R.; Samiji, M.E.; Bryce, R.S.; Kim, D.Y.; Kim, S.H.; Lee, H.J.; Choi, H.J. Spin-coated kesterite CZTS thin films for photovoltaic applications. J. Korean Phys. Soc. 2015, 67, 1078–1081. [Google Scholar] [CrossRef]
- Agawane, G.L.; Kamble, A.S.; Vanalakar, S.A.; Shin, S.W.; Gang, M.G.; Yun, J.H.; Gwak, J.; Moholkar, A.V.; Kim, J.H. Fabrication of 3.01% power conversion efficient high-quality CZTS thin film solar cells by a green and simple sol–gel technique. Mater. Lett. 2015, 158, 58–61. [Google Scholar] [CrossRef]
- Özdal, T.; Chtouki, T.; Kavak, H.; Figa, V.; Guichaoua, D.; Erguig, H.; Mysliwiec, J.; Sahraoui, B. Effect of Annealing Temperature on Morphology and Optoelectronics Properties of Spin-Coated CZTS Thin Films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 89–99. [Google Scholar] [CrossRef]
- Ziti, A.; Hartiti, B.; Labrim, H.; Doubi, Y.; Joël Tchognia Nkuissi, H.; Nouria, Y.; Fadili, S.; Batan, A.; Tahri, M.; Ridah, A.; et al. Investigation of CZTS absorber layer deposited by spin coating technique for photovoltaic applications. Mater. Today Proc. 2022, 53, 355–360. [Google Scholar] [CrossRef]
- Aftab Akram, M.; Javed, S.; Islam, M.; Mujahid, M.; Safdar, A. Arrays of CZTS sensitized ZnO/ZnS and ZnO/ZnSe core/shell nanorods for liquid junction nanowire solar cells. Sol. Energy Mater. Sol. Cells 2016, 146, 121–128. [Google Scholar] [CrossRef]
- Nakayama, N.; Ito, K. Sprayed films of stannite Cu2ZnSnS4. Appl. Surf. Sci. 1996, 92, 171–175. [Google Scholar] [CrossRef]
- Kishore Kumar, Y.B.; Suresh Babu, G.; Uday Bhaskar, P.; Sundara Raja, V. Preparation and characterization of spray-deposited Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 2009, 93, 1230–1237. [Google Scholar] [CrossRef]
- Kamoun, N.; Bouzouita, H.; Rezig, B. Fabrication and characterization of Cu2ZnSnS4 thin films deposited by spray pyrolysis technique. Thin Solid Film. 2007, 515, 5949–5952. [Google Scholar] [CrossRef]
- Jeganath, K.; Murari, M.S.; Raviprakash, Y. Identification of structural inhomogeneity on spray pyrolyzed Cu2ZnSnS4 thin film using micro-Raman spectroscopy. Phys. Lett. A 2022, 448, 128331. [Google Scholar] [CrossRef]
- Sripan, C.; Madhavan, V.E.; Viswanath, A.K.; Ganesan, R. Sulfurization and annealing effects on thermally evaporated CZTS films. Mater. Lett. 2017, 189, 110–113. [Google Scholar] [CrossRef]
- Khemiri, N.; Chamekh, S.; Kanzari, M. Properties of thermally evaporated CZTS thin films and numerical simulation of earth abundant and non toxic CZTS/Zn(S,O) based solar cells. Sol. Energy 2020, 207, 496–502. [Google Scholar] [CrossRef]
- Sripan, C.; Alagarasan, D.; Varadharajaperumal, S.; Ganesan, R.; Naik, R. Influence of solvent on solution processed Cu2ZnSnS4 nanocrystals and annealing induced changes in the optical, structural properties of CZTS film. Curr. Appl. Phys. 2020, 20, 925–930. [Google Scholar] [CrossRef]
- Tiwari, K.J.; Chetty, R.; Mallik, R.C.; Malar, P. Solid state synthesis and e-beam evaporation growth of Cu2ZnSnSe4 for solar energy absorber applications. Sol. Energy 2017, 153, 173–180. [Google Scholar] [CrossRef]
- Azim-Araghi, M.E.; Safaie, N. Structural, optical and electrical properties of Cu2ZnSnS4 thin film deposited by electron beam evaporation method. Optik 2022, 258, 168936. [Google Scholar] [CrossRef]
- Mkawi, E.M.; Al-Hadeethi, Y.; Shalaan, E.; Bekyarova, E. Substrate temperature effect during the deposition of (Cu/Sn/Cu/Zn) stacked precursor CZTS thin film deposited by electron-beam evaporation. J. Mater. Sci. Mater. Electron. 2018, 29, 20476–20484. [Google Scholar] [CrossRef]
- Mali, S.; Shinde, P.; Betty, C.A.; Bhosale, P.; Oh, Y.; Patil, P. Synthesis and characterization of Cu2ZnSnS4 thin films by SILAR method. J. Phys. Chem. Solids 2012, 73, 735–740. [Google Scholar] [CrossRef]
- Krishnan, A.; Rishad Ali, K.; Vishnu, G.; Kannan, P. Towards phase pure CZTS thin films by SILAR method with augmented Zn adsorption for photovoltaic applications. Mater. Renew. Sustain. Energy 2019, 8, 16. [Google Scholar] [CrossRef]
- Murugan, A.; Siva, V.; Shameem, A.S.; Bahadur, S.A. Optimization of adsorption and reaction time of SILAR deposited Cu2ZnSnS4 thin films: Structural, optical and electrochemical performance. J. Alloys Compd. 2021, 856, 158055. [Google Scholar] [CrossRef]
- Ganesh Kumar, K.; Balaji Bhargav, P.; Gnana Prakash, D.; Kaushik, R.; Reon Mathew, E.; Shriram, M.K.; Veerathangam, K. Investigations on SILAR coated CZTS thin films for solar cells applications. Phase Transit. 2021, 94, 556–566. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Shin, S.W.; Agawane, G.L.; Suryawanshi, M.P.; Gurav, K.V.; Patil, P.S.; Kim, J.H. Effect of post-annealing atmosphere on the grain-size and surface morphological properties of pulsed laser deposited CZTS thin films. Ceram. Int. 2014, 40 Pt B, 15097–15103. [Google Scholar] [CrossRef]
- Adhi Wibowo, R.; Soo Lee, E.; Munir, B.; Ho Kim, K. Pulsed laser deposition of quaternary Cu2ZnSnSe4 thin films. Phys. Status Solidi 2007, 204, 3373–3379. [Google Scholar] [CrossRef]
- Lee, S.M.; Mohanty, B.C.; Jo, Y.H.; Yeon, D.H.; Cho, Y.S. Phase development, microstructure and optical properties of Cu2ZnSnSe4 thin films modified with Pb and Ti. Surf. Coat. Technol. 2013, 231, 389–393. [Google Scholar] [CrossRef]
- Farinella, M.; Inguanta, R.; Spanò, T.; Livreri, P.; Piazza, S.; Sunseri, C. Electrochemical Deposition of CZTS Thin Films on Flexible Substrate. Energy Procedia 2014, 44, 105–110. [Google Scholar] [CrossRef]
- Ziti, A.; Hartiti, B.; Labrim, H.; Fadili, S.; Batan, A.; Ridah, A.; Thevenin, P. Growth and Characterization of CZTS Thin Films Synthetized by Electrodeposition Method for Photovoltaic Applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 948, 012025. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Q.; He, X.; Ding, J.; Zhang, Y.; Han, J.; Liu, J.; Liao, C.; Mei, J.; Lau, W. Electrodeposited CZTS solar cells from Reline electrolyte. Green Chem. 2014, 16, 3841–3845. [Google Scholar] [CrossRef]
- Urazov, K.; Dergacheva, M.; Tameev, A.; Gribkova, O.; Mit’, K. Electrodeposited polyaniline/Cu2ZnSnSe4 heterojunction. J. Solid State Electrochem. 2021, 25, 237–245. [Google Scholar] [CrossRef]
- Mustafa, H.A.M.; Jameel, D.A. Modeling and the main stages of spin coating process: A review. J. Appl. Sci. Technol. Trends 2021, 2, 91–95. [Google Scholar] [CrossRef]
- Swami, S.K.; Kumar, A.; Dutta, V. Deposition of Kesterite Cu2ZnSnS4 (CZTS) Thin Films by Spin Coating Technique for Solar Cell Application. Energy Procedia 2013, 33, 198–202. [Google Scholar] [CrossRef]
- Tanaka, K.; Oonuki, M.; Moritake, N.; Uchiki, H. Cu2ZnSnS4 thin film solar cells prepared by non-vacuum processing. Sol. Energy Mater. Sol. Cells 2009, 93, 583–587. [Google Scholar] [CrossRef]
- Tyona, M.D. A theoritical study on spin coating technique. Adv. Mater. Res. 2013, 2, 195–208. [Google Scholar] [CrossRef]
- Salehabadi, A.; Enhessari, M.; Ahmad, M.I.; Ismail, N.; Gupta, B.D. Chapter 8-Fabrication of sensors. In Metal Chalcogenide Biosensors; Salehabadi, A., Enhessari, M., Ahmad, M.I., Ismail, N., Gupta, B.D., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 143–174. [Google Scholar]
- Kafle, B.P. Chapter 6-Introduction to nanomaterials and application of UV–Visible spectroscopy for their characterization. In Chemical Analysis and Material Characterization by Spectrophotometry; Kafle, B.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 147–198. [Google Scholar]
- Jain, S.; Semalti, P.; Singh, V.N.; Sharma, S. Chemical Route Synthesis and Properties of CZTS Nanocrystals for Sustainable Photovoltaics; CRC Press: New York, NY, USA, 2020; pp. 195–216. [Google Scholar]
- Bashir, A.; Awan, T.I.; Tehseen, A.; Tahir, M.B.; Ijaz, M. Chapter 3-Interfaces and surfaces. In Chemistry of Nanomaterials; Awan, T.I., Bashir, A., Tehseen, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–87. [Google Scholar]
- Wasa, K.; Kitabatake, M.; Adachi, H. 2-Thin Film Processes. In Thin Film Materials Technology; Wasa, K., Kitabatake, M., Adachi, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2004; pp. 17–69. [Google Scholar]
- Goh, K.H.; Haseeb, A.S.M.A.; Wong, Y.H. Samarium Oxide and Samarium Oxynitride Thin Film as Alternative Gate Oxide on Silicon Substrate. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Vanalakar, S.A.; Agawane, G.L.; Shin, S.W.; Suryawanshi, M.P.; Gurav, K.V.; Jeon, K.S.; Patil, P.S.; Jeong, C.W.; Kim, J.Y.; Kim, J.H. A review on pulsed laser deposited CZTS thin films for solar cell applications. J. Alloys Compd. 2015, 619, 109–121. [Google Scholar] [CrossRef]
- Anil, S.; Venkatesan, J.; Shim, M.S.; Chalisserry, E.P.; Kim, S.K. 4-Bone response to calcium phosphate coatings for dental implants. In Bone Response to Dental Implant Materials; Piattelli, A., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 65–88. [Google Scholar]
- Escoubas, L.; Simon, J.J.; Le Rouzo, J.; Bermudez, V. 16-Innovative approaches in thin film photovoltaic cells. In Optical Thin Films and Coatings; Piegari, A., Flory, F., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 596–630. [Google Scholar]
- Prakash, S.; Yeom, J. Chapter 4-Advanced Fabrication Methods and Techniques. In Nanofluidics and Microfluidics; Prakash, S., Yeom, J., Eds.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 87–170. [Google Scholar]
- Feng, L.; Sun, X.; Yao, S.; Liu, C.; Xing, W.; Zhang, J. 3-Electrocatalysts and Catalyst Layers for Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–132. [Google Scholar]
- Li, J.; Ma, T.; Wei, M.; Liu, W.; Jiang, G.; Zhu, C. The Cu2ZnSnSe4 thin films solar cells synthesized by electrodeposition route. Appl. Surf. Sci. 2012, 258, 6261–6265. [Google Scholar] [CrossRef]
- Zhou, H.; Hsu, W.-C.; Duan, H.-S.; Bob, B.; Yang, W.; Song, T.-B.; Hsu, C.-J.; Yang, Y. CZTS nanocrystals: A promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 2013, 6, 2822–2838. [Google Scholar] [CrossRef]
- Repins, I.L.; Moutinho, H.; Choi, S.G.; Kanevce, A.; Kuciauskas, D.; Dippo, P.; Beall, C.L.; Carapella, J.; DeHart, C.; Huang, B.; et al. Indications of short minority-carrier lifetime in kesterite solar cells. J. Appl. Phys. 2013, 114, 084507. [Google Scholar] [CrossRef]
- Courel, M.; Vigil-Galán, O.; Jiménez-Olarte, D.; Espíndola-Rodríguez, M.; Saucedo, E. Trap and recombination centers study in sprayed Cu2ZnSnS4 thin films. J. Appl. Phys. 2014, 116, 134503. [Google Scholar] [CrossRef]
- Siebentritt, S. Why are kesterite solar cells not 20% efficient? Thin Solid Film. 2013, 535, 1–4. [Google Scholar] [CrossRef]
- Lund, E.; Scarpulla, M. Modeling Cu2ZnSnS4 (CZTS) solar cells with kesterite and stannite phase variation. Proc. SPIE 2013, 8620, 15. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Selyshchev, O.; Dzhagan, V.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. “Green” Aqueous Synthesis and Advanced Spectral Characterization of Size-Selected Cu2ZnSnS4 Nanocrystal Inks. Sci. Rep. 2018, 8, 13677. [Google Scholar] [CrossRef]
- Baid, M.; Hashmi, A.; Jain, B.; Singh, A.K.; Susan, M.A.B.H.; Aleksandrova, M. A comprehensive review on Cu2ZnSnS4 (CZTS) thin film for solar cell: Forecast issues and future anticipation. Opt. Quantum Electron. 2021, 53, 45. [Google Scholar] [CrossRef]
- Erkan, E.; Chawla, V.; Repins, I.; Scarpulla, M. Interplay between surface preparation and device performance in CZTSSe solar cells: Effects of KCN and NH4OH etching. Sol. Energy Mater. Sol. Cells 2015, 136, 78–85. [Google Scholar] [CrossRef]
- Ritzer, M.; Schönherr, S.; Schöppe, P.; Wisniewski, W.; Giraldo, S.; Gurieva, G.; Johannes, A.; Plass, C.; Ritter, K.; Martinez-Criado, G.; et al. On the Germanium Incorporation in Cu2ZnSnSe4 Kesterite Solar Cells Boosting Their Efficiency. ACS Appl. Energy Mater. 2019, 3, 558–564. [Google Scholar] [CrossRef]
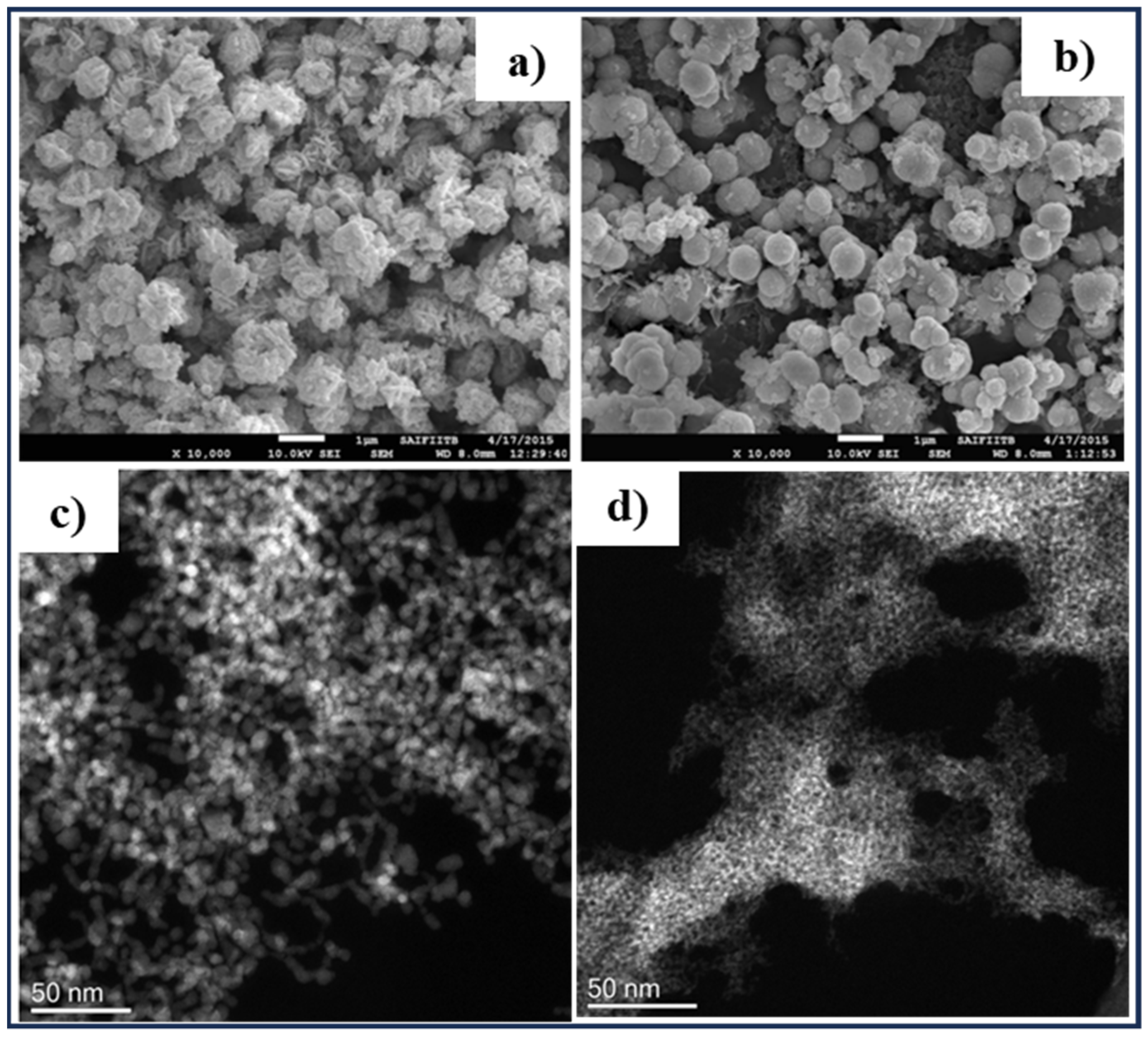
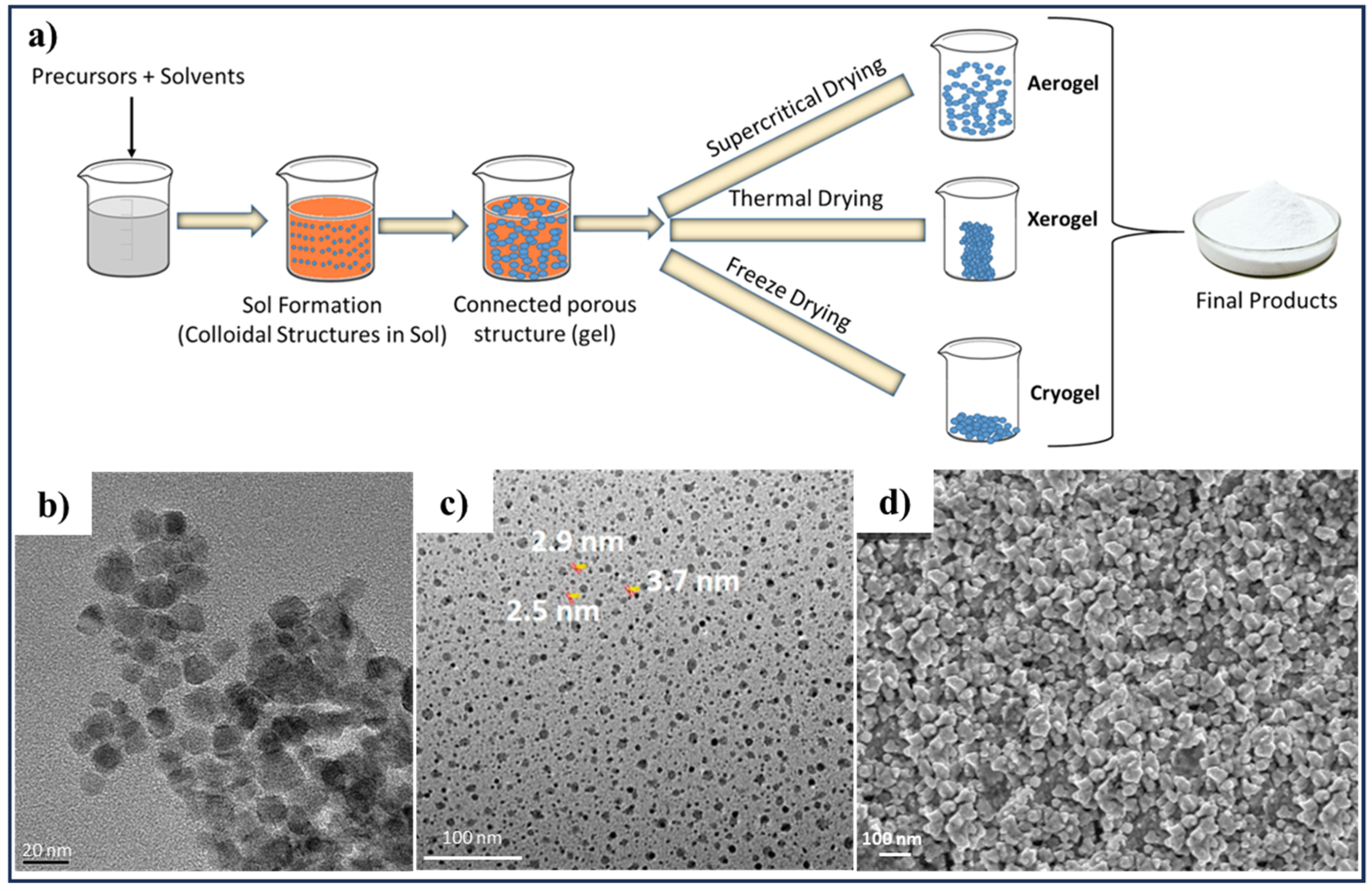

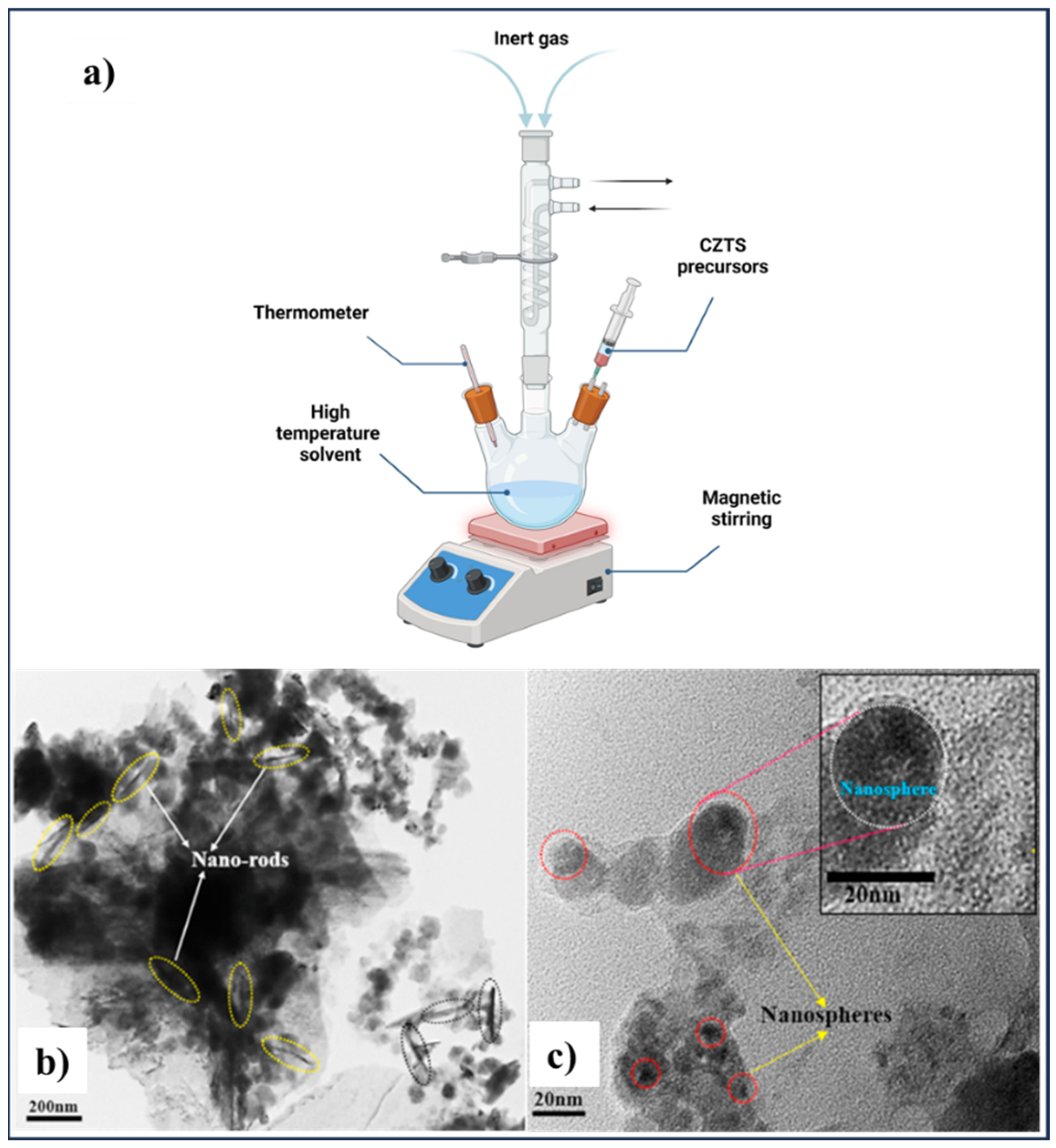
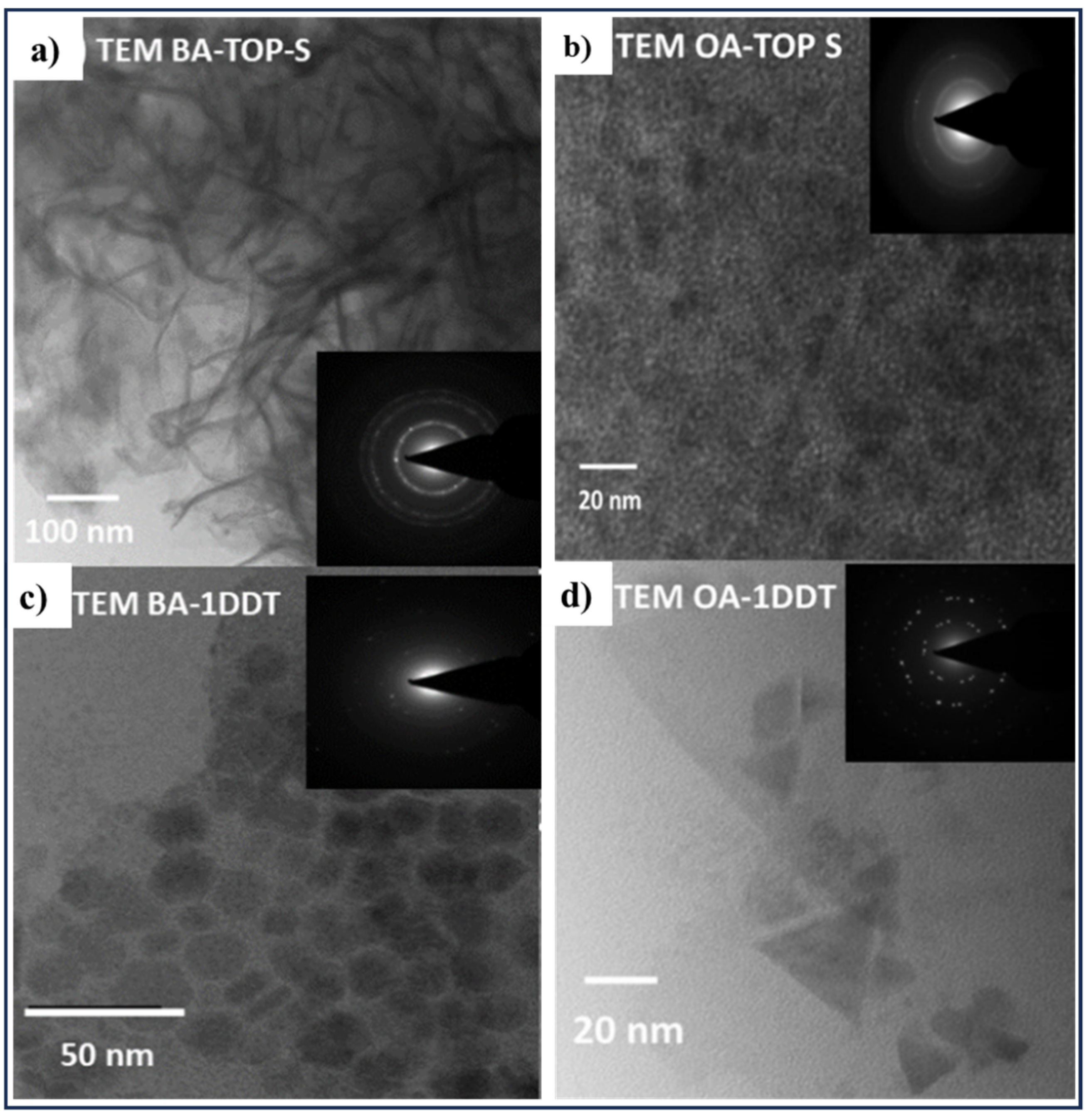

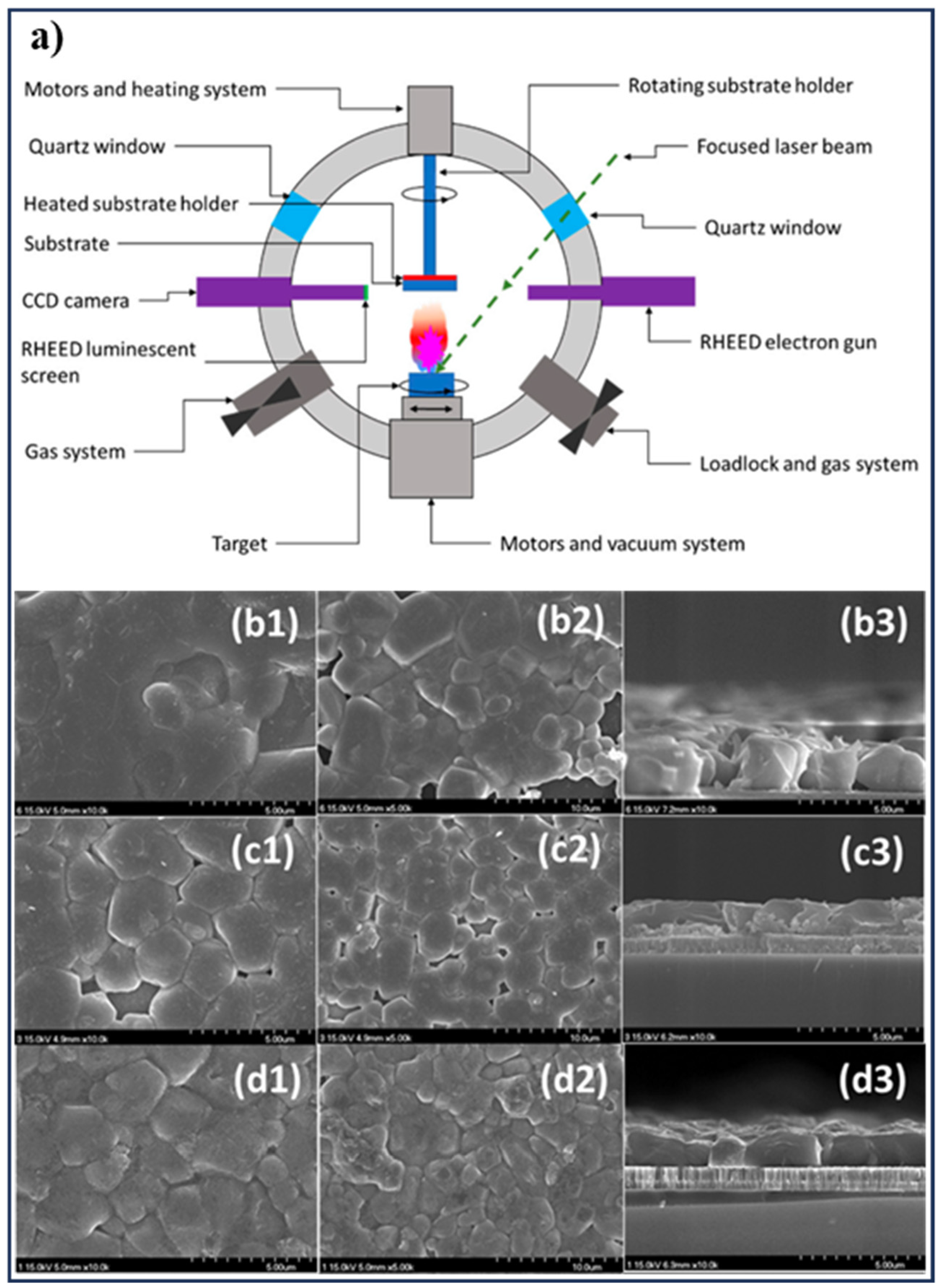
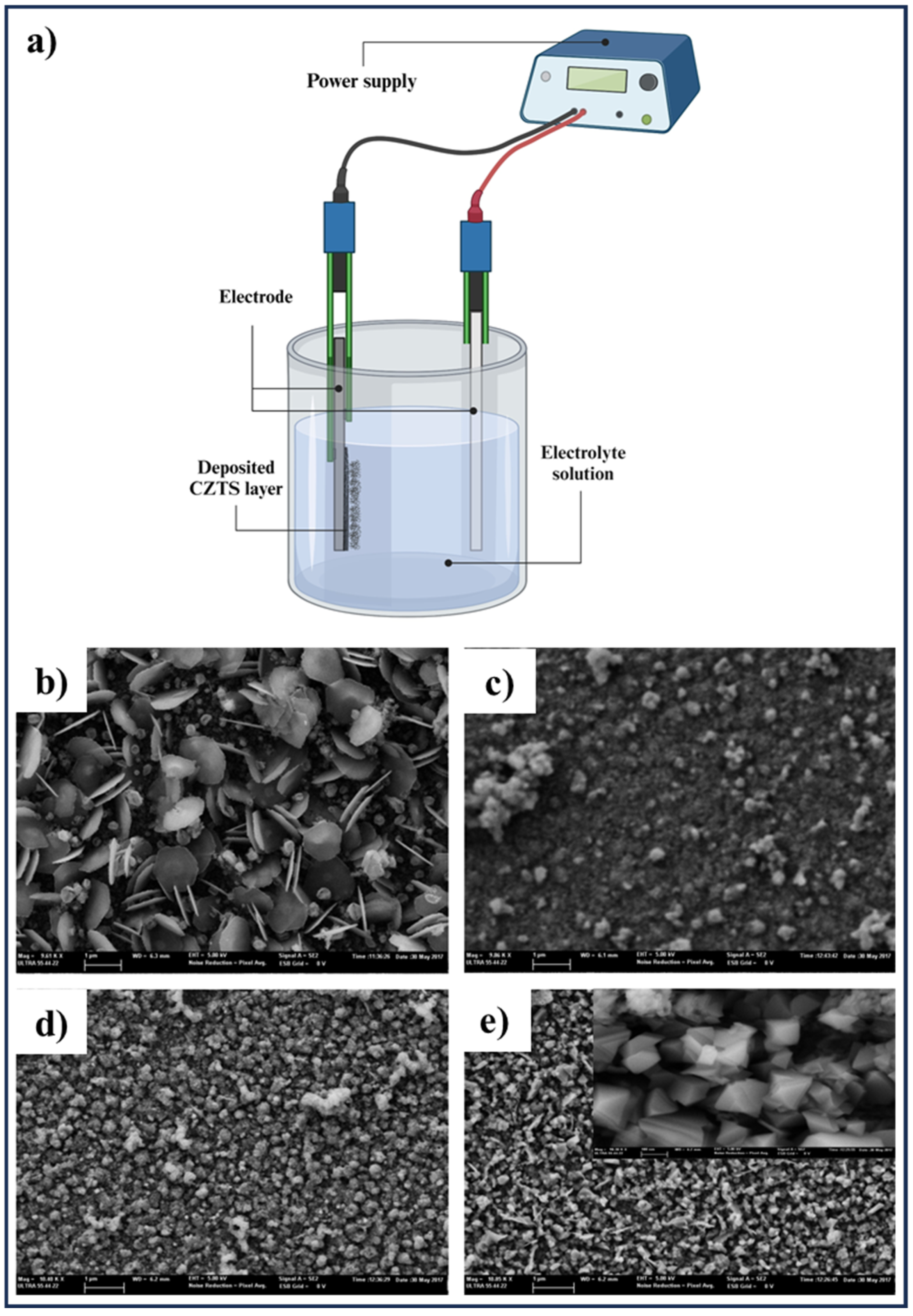
| Methods | Nanoscale CZTS/Se | Precursor Materials | Conditions | Ref. |
|---|---|---|---|---|
| Hydrothermal | CZTS nanoparticles | CuCl2, ZnCl2, SnCl2, and Na2S | At 230 °C for 24 h, different concentration ratios of Na2S as the sulfur source, and CuCl2 as the copper source | [66] |
| CZTS pure kesterite structure nanoparticles with size 20–30 nm | CuCl2, ZnCl2, SnCl2, and Na2S | At 220 °C for 12, 18, and 24 h | [67] | |
| CZTS Quantum Dots with size of 10.74 nm and 12.2 nm | CuCl2, ZnCl2, SnCl2, and thiourea (CH4N2S) | At 230 °C for 24 h, precursors in a molar ratio of 2:1:1:8 (Cu:Zn:Sn:S) | [68] | |
| CZTS hierarchical flower-like structure | CuCl2, ZnCl2, SnCl2, and thiourea (CH4N2S) | At 180 °C for 4, 8, 16 and 20 h | [69] | |
| CZTS nanoparticles with an average size 7 nm–60 nm, rod-shape, and spherical nanocrystal shapes | CuCl2, ZnCl2, SnCl2, and thiourea (CH4N2S) | At 200 °C for 72 h | [70] | |
| CZTS nanoparticles with the domain size between 5 and 6 nm | CuSO4, Zn(NO3)2, SnCl2, thiourea (CH4N2S) | At 200 °C for 1 h | [71] | |
| CZTSe nano-powders with polygon flakes morphology with thickness of about 30~40 nm and 50~200 nm diameters | CuCl2, ZnCl2, SnCl2, and SeO2 | At 160–200 °C for different times (10–90 h) | [72] | |
| Solvothermal | CZTS nanocrystals with the size of 6–9 nm | CuCl2, ZnCl2, SnCl2, and thiourea (CH4N2S) | At 160 °C, 180 °C, and 200 °C for reaction times of 6 to 24 h | [73] |
| CZTS nanocrystals, change of the flower like morphology to spherical type by changing reactants concentration | CuCl, ZnCl2, SnCl4, and thiourea (CH4N2S) | At 140, 160, 180 and 200 °C for 10, 20, 30, and 40 min, | [74] | |
| CZTS nanoparticles, An ellipsoidal to spherical shape growing from a mean size of 7 to 15 nm, respectively, from chlorides precursors, and 2–3 nm particle size from acetate precursors | Sn(IV) acetate, Cu(II) acetate, Zn(II) acetate dehydrate, CuCl2, ZnCl2, SnCl2, CS2 | At 200 °C for 2 and 24 h, At 250 °C for 2, 4, 8, and 24 h, using different metal salt precursors | [75] | |
| CZTS spherical particles containing nanocrystals of 11–12 nm in diameter | CuCl2, ZnCl2, SnCl2, and thiourea (CH4N2S) | At 200 °C for 24 h | [76] | |
| CZTSe dispersed nanocrystals with size of 8–12 nm | CuCl, ZnCl2, SnCl4, and Se powders | At 200 °C for 24 h | [77] | |
| Sol–gel | CZTS crystalline size of about 10–20 | Copper (II) acetylacetone, zinc (II) acetate, tin (II) chloride, and sulfide powder | In argon protection atmosphere | [78] |
| CZTS nanoparticles with sizes ranging between 2 and 4 nm | CuCl2·2H2O, ZnCl2, SnCl2·2H2O, and CH4N2S | At 50–60 °C, without sulfurization and toxic atmosphere | [79] | |
| CZTS films with average crystallites size of 60 nm | CuCl2, ZnCl2, zinc acetate, and thiourea (CH4N2S) | Dissolved in dimethyl sulfoxide at room temperature | [80] | |
| Co-precipitation | CZTS nanocrystals in the range of 8–10 nm | copper (II) acetate, tin (IV) acetate, zinc acetate, and thiourea | Under N2 atmosphere | [81] |
| CZTS crystalline size of 15–17 nm | Cupric (II) chloride, zinc (II) acetate, tin (II) chloride, thiourea ethanol as the sulfur anion source | Water as a solvent without any surfactants, noble gases, and vacuum process. | [82] | |
| Hot-injection and colloidal synthesis | CZTS nanoparticles, size between 2–5 nm, Agglomerated nanorods and nano spherical particles | CuCl2, ZnCl2, SnCl4, and sulfur powder | Using different ligands: Trioctylphosphine oxide, Trioctylphosphine, n-butylamine and Octadecene | [83] |
| CZTS nanocrystals, average size of 4–9 nm | CuCl2, ZnCl, SnCl2, and thioacetamide | Using oleylamine (OLA) as both the solvent and the nanocrystal stabilizer, different process time | [84] | |
| CZTS nanoparticles with an average diameter of 23 ± 11 nm | CuCl2, zinc acetate, SnCl2, pure sulfur | Ligand-free | [85] | |
| CZTS nanoparticles with different morphology from thin plates with hexagonal symmetry to triangular shaped nanoparticles | CuCl2, ZnCl2, SnCl4, Sulfur sources: 1-Dodecanethiol (1-DDT) and Trioctylphosphine-S (TOP-S) | Various capping ligands and sulfur sources (ligands: butylamine (BA) and oleic acid (OA), | [86] | |
| CZTS rod-shape nanoparticles | CuCl2, ZnCl2, SnCl4, and sulfur powder | Different Zn/Sn precursor ratio (Zn:Sn ratio as 1.2:1 2:1 and 4:1) | [87] | |
| CZTS nanocrystals from 2D nanosheets to 1D nanorods | Cu(acac)2, Zn(acac)2, SnCl4, and 1-dodecanethiol (DDT) | One-pot colloidal synthesis assisted by continuous addition of oleylamine (OM) as a surfactant | [88] | |
| CZTS/Se nanoparticles | CuCl2, ZnCl2, SnCl2, sulfur, and selenium powder | Cu2ZnSn (SxSe1-x)4 (x = 0.0, 0.4, 1.0) | [89] | |
| Solid state, Mechanical alloying | CZTS particles in range of 0.07–3.71 mm | Elemental powders of Cu, Zn, Sn, and S | Ball-to-powder weight ratio of 5:1, 50 Hz frequency, 300 rpm revolution speed and 600 rpm rotation speed, milling time of 10, 15, 20, 25, 30, 35 h | [90] |
| CZTS nanoparticles, particle size in the range of 50–60 nm | Elemental powders of Cu, Zn, Sn, and S | Ball-to-powder ratio of 10:1, milling speed 300 rpm, under Ar atmosphere for 16 h | [30] | |
| CZTSe and CZTS nanocrystalline powders, particle size distribution in the range of 133–286 nm | Elemental powders of Cu, Zn, Sn, S, and Se | Ball-to-powder mixture weight ratio of 5:1, 450 rpm for 30 h, 1-Butanol added to the mixture as a wet medium to enhance the efficiency of ball milling and preventing the agglomeration | [91] | |
| CZTS nanocrystalline with grain size between 10 and 20 nm | Elemental powders of Cu, Zn, Sn, and S flakes | Ball-to-powder weight ratio of 100:1, ethanol as lubricant, time duration of 15, 30, 60, 90, and 180 min | [27] | |
| CZTS nano-powder | Elemental Cu, Zn, Sn, and S | 450 rpm for 30 h with butanol | [92] | |
| CZTS equiaxed nanoparticles with approximate size of 10 to 15 nm | Cu(acac)2, Zn(acac)2, SnCl2, and thiourea (CH4N2S) | Milling with ethanol, synthesized in tubular furnace at 250 °C for 1–12 h in argon atmosphere | [93] | |
| CZTSe with crystalline size of 10–2 nm | Elemental Cu, Zn, Sn, and Se | Ball-to-powder ratio of 5:1, various milling times | [94] |
| Deposition Method | CZTS Thin Film Morphologies | Conditions | Ref. |
|---|---|---|---|
| Spin coating | CZTS thin film with different surface morphological characteristics | Preheated in various atmospheres (air, Ar, and N2) before sulfurization at 580 °C | [118] |
| CZTS thin film with various degrees of crystallization | Various compositions, annealing at 500 °C in N2 atmosphere | [119] | |
| CZTS thin film with various surface morphology | Annealing temperature in the range of 525 °C and 575 °C (under H2S gas atmosphere) | [120] | |
| Evolution of the CZTS thin film surface morphology from a non-crystallized, compact, uniform, and dense texture with cracks to an aggregated grains and nanoparticles with non-uniform distribution with porous texture | Annealing temperatures (300, 400, 500 and 550 °C) | [121] | |
| CZTS thin film with different roughness and closely packed surface | Sol–gel spin coating doped by Cd and Mg, and followed by sulfurization | [104] | |
| CZTS thin film with crystallite size of 7.02, 7.74, and 9.09 nm, crystallite number of 2.31, 2.03, and 1.26 × 1018 (m−2) | Annealing in air at 300 °C and duration times of 5, 10 and 20 min | [122] | |
| Core/shell vertically aligned nanorods array | Condition is briefly described in the spin-coating section | [123] | |
| Spray pyrolysis | CZTS thin film with stannite structure | Sprayed at a flow rate of 2.5–3.0 mL·min−1 using a nitrogen gas flow of 3.2 mL·min−1 for 90 min, heated to 280–360 °C | [124] |
| CZTS film with different crystallinity | Substrate temperature (563–723°K), without annealing and post-deposition sulfurization | [125] | |
| CZTS thin film surface with micro-crystallites range from 0.02 to 0.2 μm | Sprayed for 30 min and 60 min on substrates with temperature from 553°K to 633°K | [126] | |
| CZTS thin film with inhomogeneity and the phase segregation | Substrate temperature of 325 °C, sulfurization at 550 °C with elemental sulfur in an Argon atmosphere | [127] | |
| CZTS film with various surface morphology from agglomerations of small grains with some voids to dense surface with the porous structure and significantly clear grains | Substrate temperature of 250, 300, and 350 °C | [79] | |
| Thermal Evaporation | CZTS film with different phase formation and phase purity | Post-deposition annealing at 300, 400, 450, 500, and 550°C for 15 min, sulfurization at 500 and 550 °C for 15 min | [128] |
| CZTS film with different surface morphology from flat and smooth with negligible grains to a homogeneous and compact surface with densely packed grains | Non-heated substrate, post-deposition annealing under different atmospheres: (1) vacuum annealing, and (2) sulfur annealing followed by vacuum annealing | [129] | |
| Polycrystalline CZTS thin film with tetragonal crystal structure | Using CZTS nano-structured materials synthesized by different solvent (water, ethylene diamine, and ethylene) for the deposition process, post deposition annealing at 450 °C in nitrogen atmosphere for 1 h | [130] | |
| Electron Beam Evaporation | CZTSe thin film | Hot pressed near stoichiometric CZTSe as the bulk source, electron beam current varied between few mA to 110 mA without any post deposition annealing | [131] |
| Sandwich device (Al (50 nm)/CZTS (80 nm)/Al (50 nm)) | Deposited with depositing rate of 0.5 nm/S at room temperature | [132] | |
| CZTS film with different surface morphology from rough surface with many pinholes to large grain size surrounded by smaller grains | Deposition of the CZT precursors on pre-heated substrates followed by a sulfurization, deposition rate of ~10 nm s−1, substrate temperatures of 220, 250, 280 and 310 °C | [133] | |
| SILAR | CZTS thin films, surface morphology from non-uniform distribution of agglomerated particles to many larger CZTS particles with uniform average grain size of 100 nm | CuSO4, ZnSO4, SnSO4, and Na2S dissolved in H2O, 10, 20, 30, and 40 number of immersion cycles with immersion time of 30 s | [134] |
| Phase pure CZTS thin flms | Separate bath for Zn2+ ions to avoid formation of Cu3SnS4 (CTS) and Cu2S phases, followed by annealing at elevated temperature | [135] | |
| Homogeneous CZTS films with nano-crystalline agglomerated spherical-like particles, well distributed over the surface | Different dipping times, conducting and nonconducting substrates, followed by annealing at 400 °C | [136] | |
| Compositional pure CZTS thin film | Varius precursor concentration, dipping time and number of cycles (30, 40, and 50 cycles), annealing in Ar atmosphere at 250 °C for 4 h | [137] | |
| PLD | Homogeneous composition CZTS thin film | Annealing at 550 °C in different atmospheres (Ar, N2, and S powder) | [138] |
| Single-phase stannite CZTSe thin film | Substrate temperatures varied from room temperature to 500 °C | [139] | |
| Cu2−xPbxZnSnSe4 (x = 0, 0.06 and 0.18) and Cu2ZnSn1−yTiySe4 (y = 0, 0.1. 0.3 and 1.0) thin films | Modified target with Pb and Ti, selenization carried out at 450 °C for 30 min | [140] | |
| Electrochemical Deposition method | CZTS thin film | Different standard electrochemical potentials, under N2 atmosphere, different bath composition | [141] |
| CZTS thin film with different grain formation and crystallization | Sulfurization in N2 + H2S atmosphere at different temperatures of 350, 375, 400, and 425 °C for 10 min | [142] | |
| Uniform and compact CZTS thin film | Deep eutectic solvent (named Reline) as a green electrolyte, in a mixture of H2S and N2 at 550 °C for 1 h | [143] | |
| CZTSe thin film consist of nanocrystals with size of 25–50 nm | At a constant potential of 0.6 V for 30 min at room temperature | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, S.; Kaur, N.; Radu, D.R. Nanoscale Cu2ZnSnSxSe(4−x) (CZTS/Se) for Sustainable Solutions in Renewable Energy, Sensing, and Nanomedicine. Crystals 2024, 14, 479. https://doi.org/10.3390/cryst14050479
Mohammadi S, Kaur N, Radu DR. Nanoscale Cu2ZnSnSxSe(4−x) (CZTS/Se) for Sustainable Solutions in Renewable Energy, Sensing, and Nanomedicine. Crystals. 2024; 14(5):479. https://doi.org/10.3390/cryst14050479
Chicago/Turabian StyleMohammadi, Sayedmahdi, Navdeep Kaur, and Daniela R. Radu. 2024. "Nanoscale Cu2ZnSnSxSe(4−x) (CZTS/Se) for Sustainable Solutions in Renewable Energy, Sensing, and Nanomedicine" Crystals 14, no. 5: 479. https://doi.org/10.3390/cryst14050479
APA StyleMohammadi, S., Kaur, N., & Radu, D. R. (2024). Nanoscale Cu2ZnSnSxSe(4−x) (CZTS/Se) for Sustainable Solutions in Renewable Energy, Sensing, and Nanomedicine. Crystals, 14(5), 479. https://doi.org/10.3390/cryst14050479









