Synthesis and Band Gap Characterization of High-Entropy Ceramic Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Liu, J.X.; Tu, T.Z.; Wang, W.; Zhang, G.J. High-Entropy Oxides for Catalysis: A Diamond in the Rough. Chem. Eng. J. 2023, 451, 138659. [Google Scholar] [CrossRef]
- Aamlid, S.S.; Oudah, M.; Rottler, J.; Hallas, A.M. Understanding the Role of Entropy in High Entropy Oxides. J. Am. Chem. Soc. 2023, 145, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Reece, M.J. Review of High Entropy Ceramics: Design, Synthesis, Structure and Properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Y.; Xu, Y.; Zhang, H.; Shao, Z.; Wang, Z.; Chen, H. Novel High-Entropy Oxides for Energy Storage and Conversion: From Fundamentals to Practical Applications. Green Energy Environ. 2023, 8, 1341–1357. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High Entropy Oxides for Reversible Energy Storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [PubMed]
- Schweidler, S.; Tang, Y.; Lin, L.; Karkera, G.; Alsawaf, A.; Bernadet, L.; Breitung, B.; Hahn, H.; Fichtner, M.; Tarancón, A.; et al. Synthesis of Perovskite-Type High-Entropy Oxides as Potential Candidates for Oxygen Evolution. Front. Energy Res. 2022, 10, 983979. [Google Scholar] [CrossRef]
- Salian, A.; Mandal, S. Review on the Deposition, Structure and Properties of High Entropy Oxide Films: Current and Future Perspectives. Bull. Mater. Sci. 2022, 45, 49. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.Z.; Zhang, Z.G.; Kuramoto, K.; Zhang, H.; Jia, Y. A New Class of Spinel High-Entropy Oxides with Controllable Magnetic Properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Fracchia, M.; Manzoli, M.; Anselmi-Tamburini, U.; Ghigna, P. A New Eight-Cation Inverse High Entropy Spinel with Large Configurational Entropy in Both Tetrahedral and Octahedral Sites: Synthesis and Cation Distribution by X-ray Absorption Spectroscopy. Scr. Mater. 2020, 188, 26–31. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and Microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 High Entropy Oxide Characterized by Spinel Structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef] [PubMed]
- Lizeth Katherine, T.N.; Vendula, B.; Jaroslav, K.; Jaroslav, C. Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels. Gels 2023, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Panchal, D.; Sharma, A.; Pal, S. Engineered MoS2 Nanostructures for Improved Photocatalytic Applications in Water Treatment. Mater. Today Sustain. 2023, 21, 100264. [Google Scholar] [CrossRef]
- Sánchez-Albores, R.M.; Reyes-Vallejo, O.; Ríos-Valdovinos, E.; Fernández-Madrigal, A.; Pola-Albores, F. Analysis and characterization of BiVO4/FeOOH and BiVO4/α-Fe2O3 nanostructures photoanodes for photoelectrochemical water splitting. J. Mater. Sci. Mater. Electron. 2023, 34, 1001. [Google Scholar] [CrossRef]
- Nundy, S.; Tatar, D.; Kojčinović, J.; Ullah, H.; Ghosh, A.; Mallick, T.K.; Meinusch, R.; Smarsly, B.M.; Tahir, A.A.; Djerdj, I. Bandgap Engineering in Novel Fluorite-Type Rare Earth High-Entropy Oxides (RE-HEOs) with Computational and Experimental Validation for Photocatalytic Water Splitting Applications. Adv. Sustain. Syst. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-Entropy Ceramics: Review of Principles, Production and Applications. Mater. Sci. Eng. R. Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Sedegov, A.; Vorotilo, S.; Tsybulin, V.; Kuskov, K.; Moscovskikh, D. Synthesis and Study of High-Entropy Ceramics Based on the Carbides of Refractory Metals. IOP Conf. Ser. Mater. Sci. Eng. 2019, 558, 012043. [Google Scholar] [CrossRef]
- Edalati, P.; Wang, Q.; Razavi-Khosroshahi, H.; Fuji, M.; Ishihara, T.; Edalati, K. Photocatalytic Hydrogen Evolution on a High-Entropy Oxide. J. Mater. Chem. A 2020, 8, 3814–3821. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Zhu, C.; Liu, S.; Liu, H.; Zhang, Y.; Yue, Y. Optical bandgap and luminescence in Er3+ doped oxyfluoro-germanate glass-ceramics. J. Non-Cryst. Solids 2021, 555, 120533. [Google Scholar] [CrossRef]
- Allaham, M.M.; Dallaev, R.; Burda, D.; Sobola, D.; Nebojsa, A.; Knápek, A.; Mousa, M.S.; Kolařík, V. Energy gap measurements based on enhanced absorption coefficient calculation from transmittance and reflectance raw data. Phys. Scr. 2024, 99, 025952. [Google Scholar] [CrossRef]

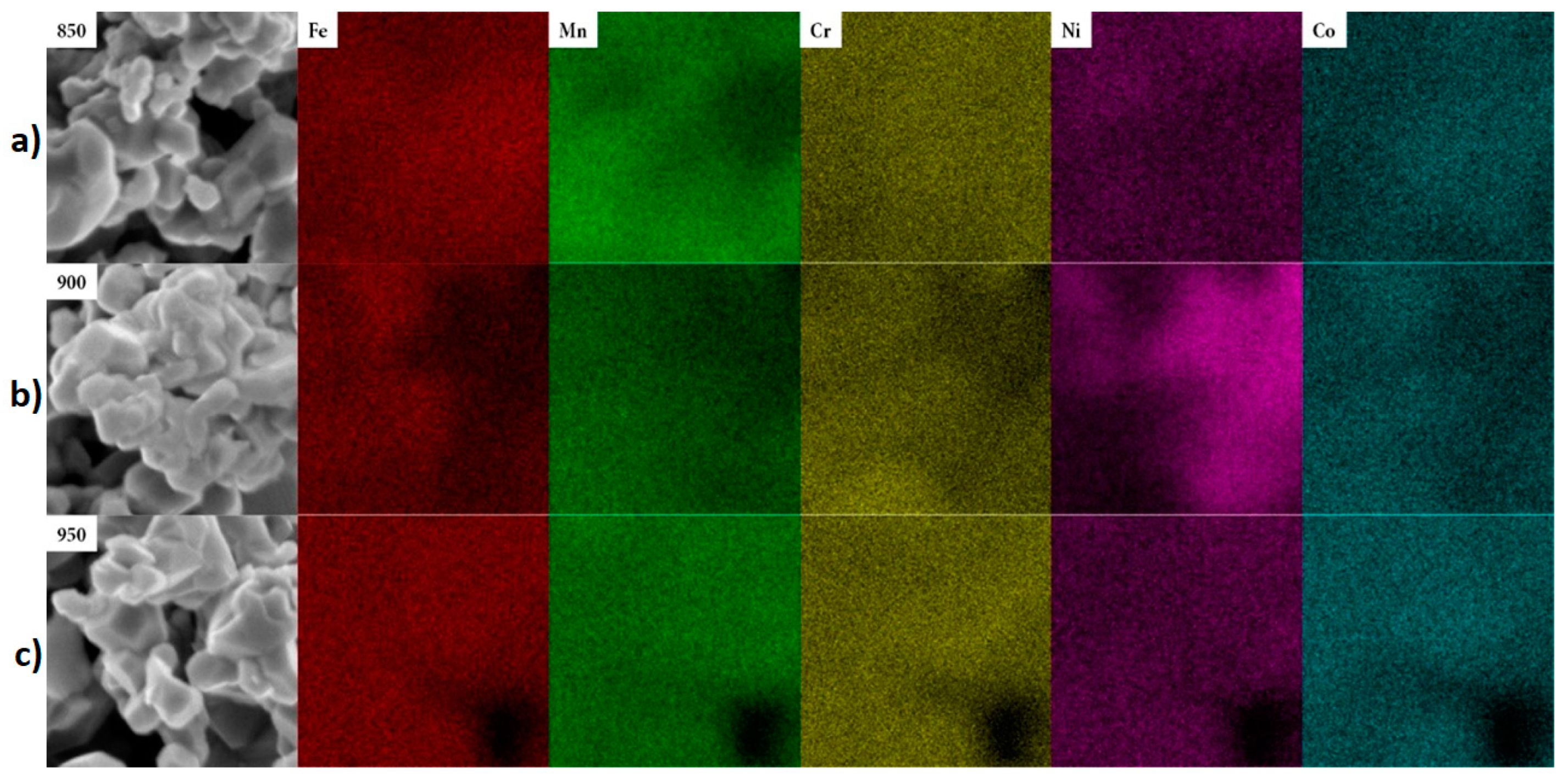
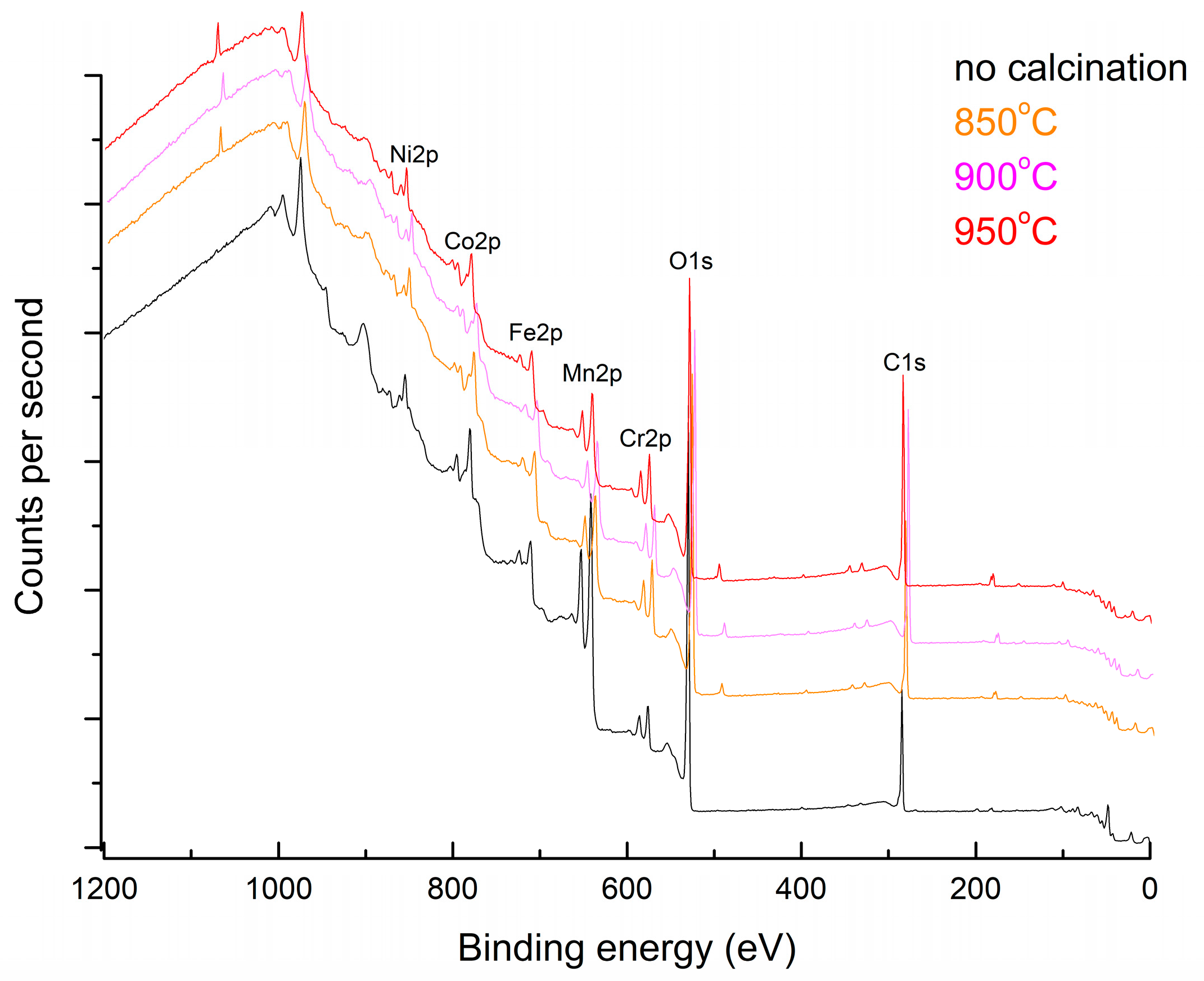
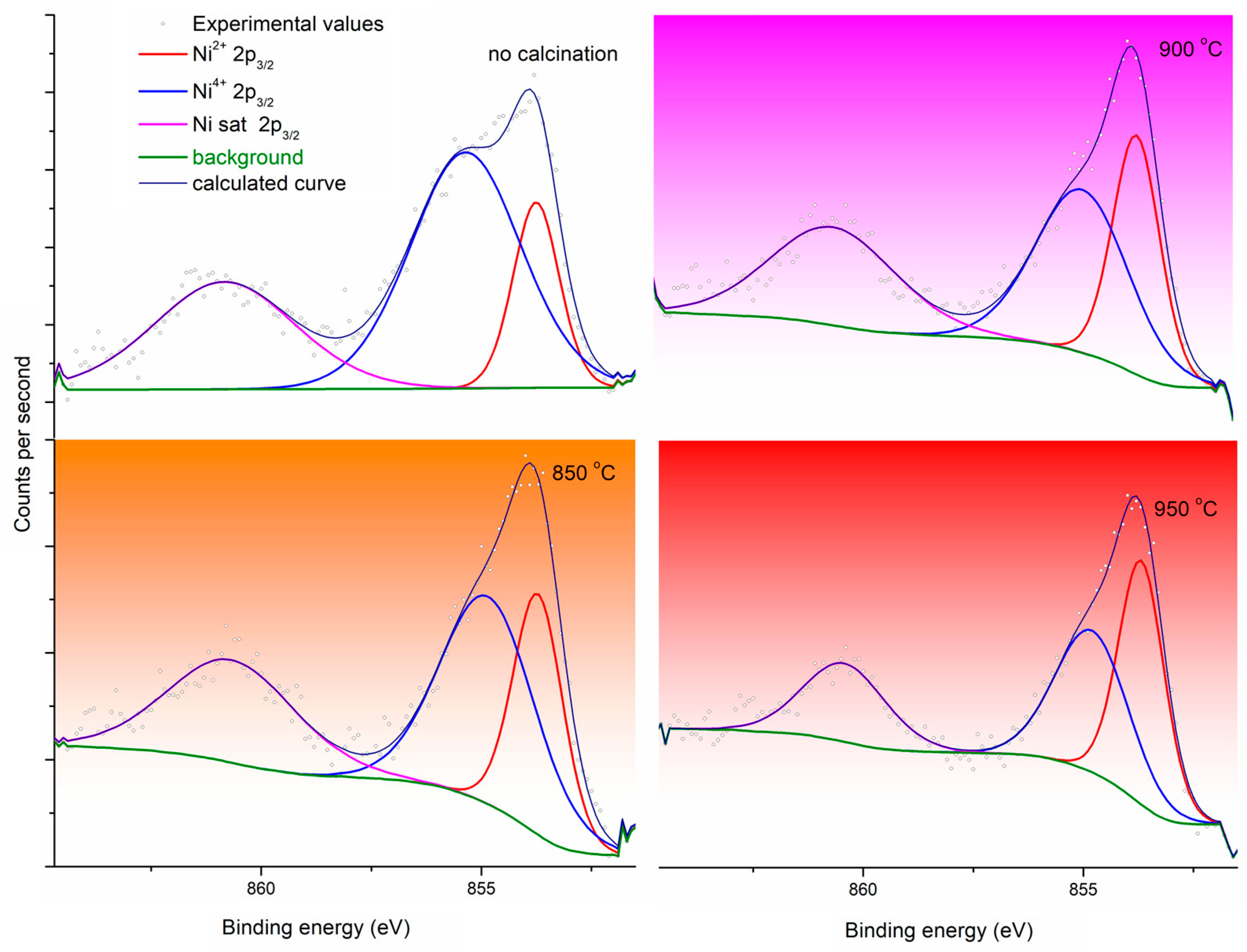
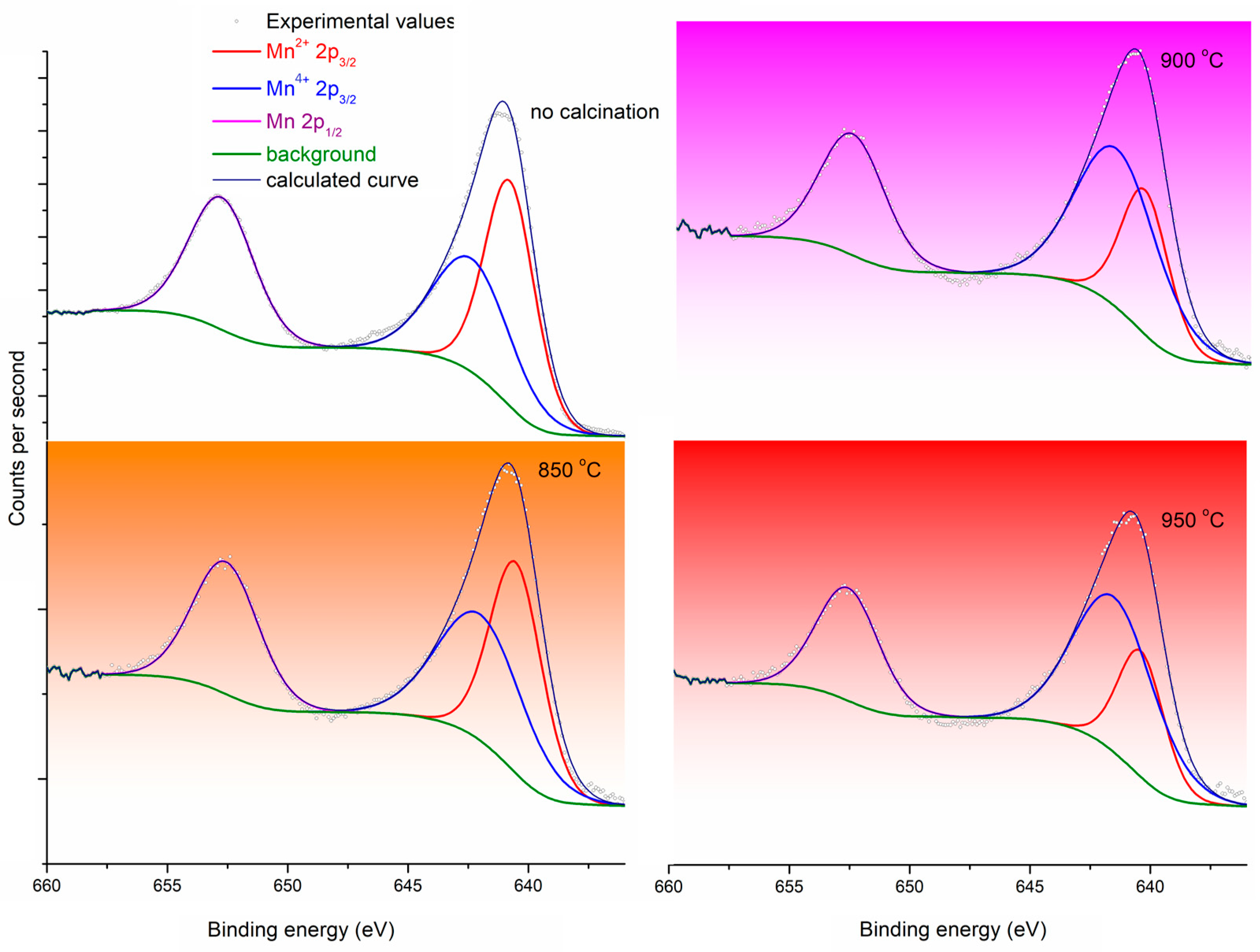
| Peak Name | At%, No Calcination | At%, 850 °C | At%, 900 °C | At%, 950 °C |
|---|---|---|---|---|
| O1s | 65 | 75.17 | 83.05 | 78.92 |
| Ni2p | 5.06 | 1.37 | 1.75 | 1.8 |
| Mn2p | 15.56 | 6.86 | 1.12 | 3.41 |
| Cr2p | 3.80 | 4.67 | 3.74 | 3.64 |
| Co2p | 6.23 | 4.65 | 6.07 | 6.58 |
| Fe2p | 4.35 | 7.27 | 4.26 | 5.63 |
| Peak Name | %, No Calcination | %, 850 °C | %, 900 °C | %, 950 °C |
|---|---|---|---|---|
| Ni2+ 2p3/2 | 17.18 | 26.78 | 29.08 | 39.82 |
| Ni3+ 2p3/2 | 52.40 | 43.92 | 39.11 | 37.00 |
| Ni sat 2p3/2 | 30.42 | 29.30 | 31.80 | 23.17 |
| Peak Name | %, No Calcination | %, 850 °C | %, 900 °C | %, 950 °C |
|---|---|---|---|---|
| Mn2+ 2p3/2 | 38.94 | 35.88 | 24.77 | 22.80 |
| Mn4+ 2p3/2 | 31.29 | 35.58 | 46.88 | 49.70 |
| Mn 2p3/2 | 29.78 | 28.55 | 28.35 | 27.50 |
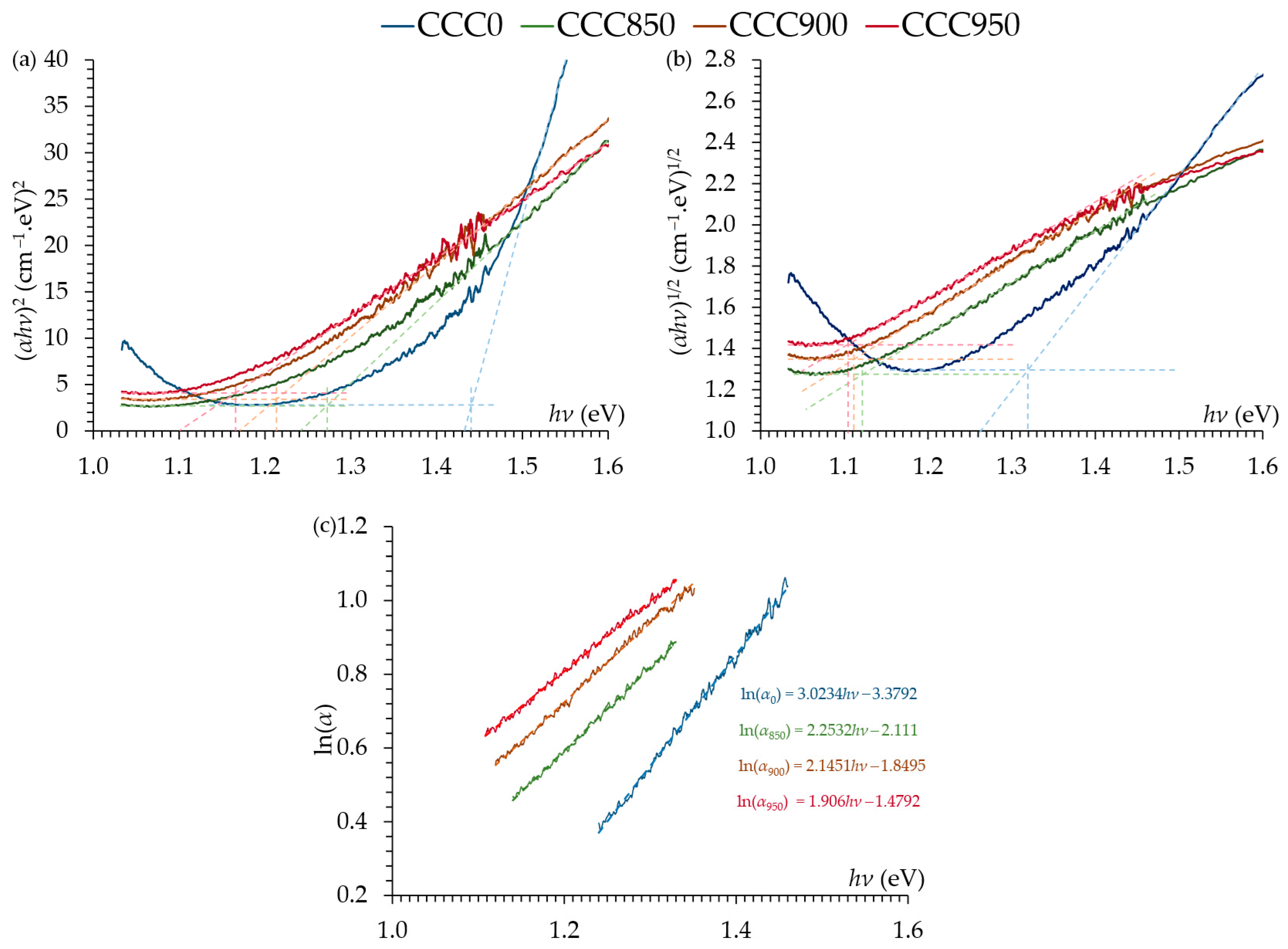
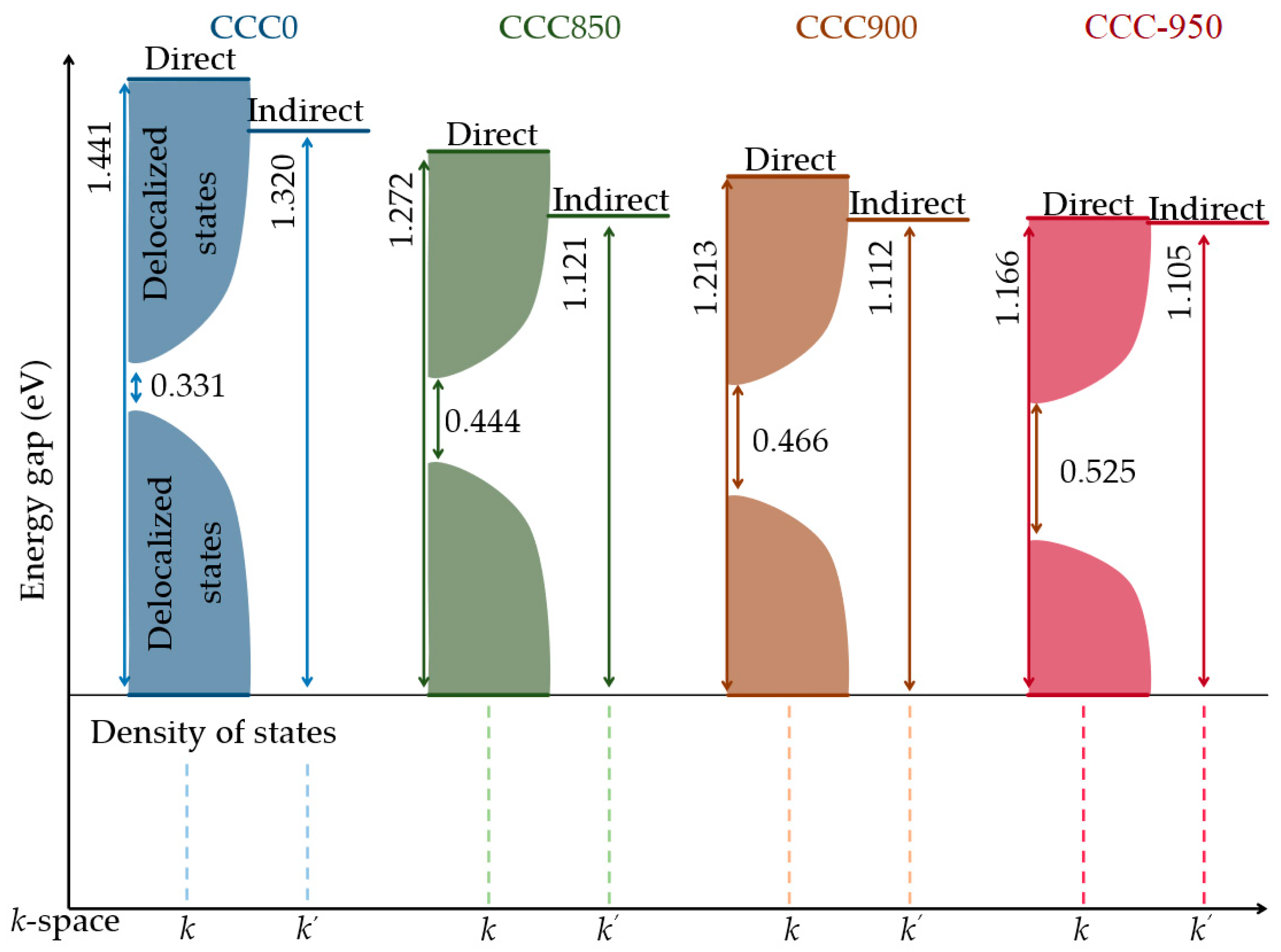
| Sample | (eV) | (eV) | (eV) | |
|---|---|---|---|---|
| CCC0 | 1.441 | 1.320 | 0.331 | 12.88 |
| CCC850 | 1.272 | 1.121 | 0.444 | 17.28 |
| CCC900 | 1.213 | 1.112 | 0.466 | 18.13 |
| CCC950 | 1.166 | 1.105 | 0.525 | 20.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallaev, R.; Spusta, T.; Allaham, M.M.; Spotz, Z.; Sobola, D. Synthesis and Band Gap Characterization of High-Entropy Ceramic Powders. Crystals 2024, 14, 295. https://doi.org/10.3390/cryst14040295
Dallaev R, Spusta T, Allaham MM, Spotz Z, Sobola D. Synthesis and Band Gap Characterization of High-Entropy Ceramic Powders. Crystals. 2024; 14(4):295. https://doi.org/10.3390/cryst14040295
Chicago/Turabian StyleDallaev, Rashid, Tomáš Spusta, Mohammad M. Allaham, Zdenek Spotz, and Dinara Sobola. 2024. "Synthesis and Band Gap Characterization of High-Entropy Ceramic Powders" Crystals 14, no. 4: 295. https://doi.org/10.3390/cryst14040295
APA StyleDallaev, R., Spusta, T., Allaham, M. M., Spotz, Z., & Sobola, D. (2024). Synthesis and Band Gap Characterization of High-Entropy Ceramic Powders. Crystals, 14(4), 295. https://doi.org/10.3390/cryst14040295






