Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material
Abstract
1. Introduction
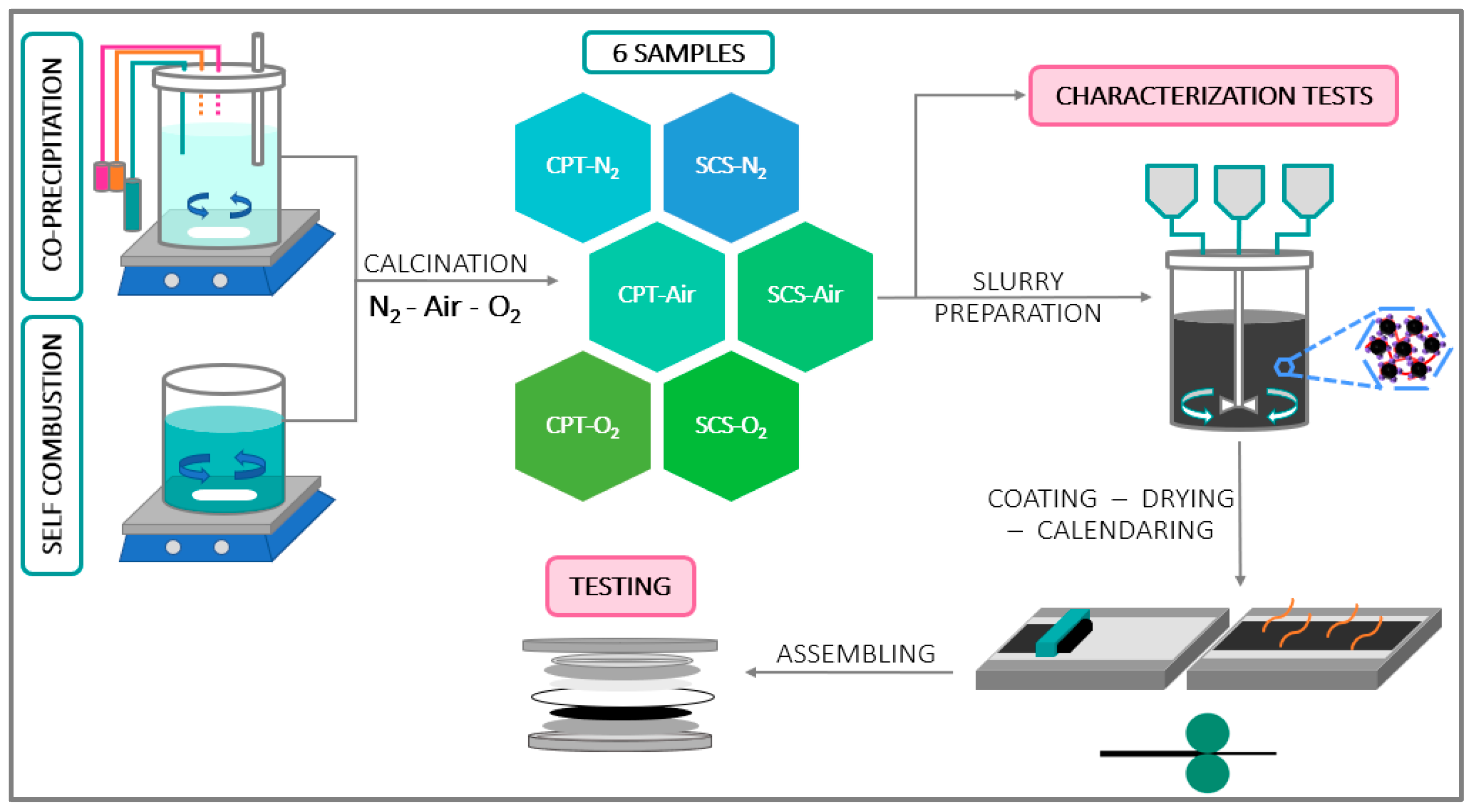
2. Materials and Methods
2.1. SCS Approach
2.2. CPT Approach
2.3. Calcination
2.4. Slurry Preparation—Coating—Calendaring—Drying
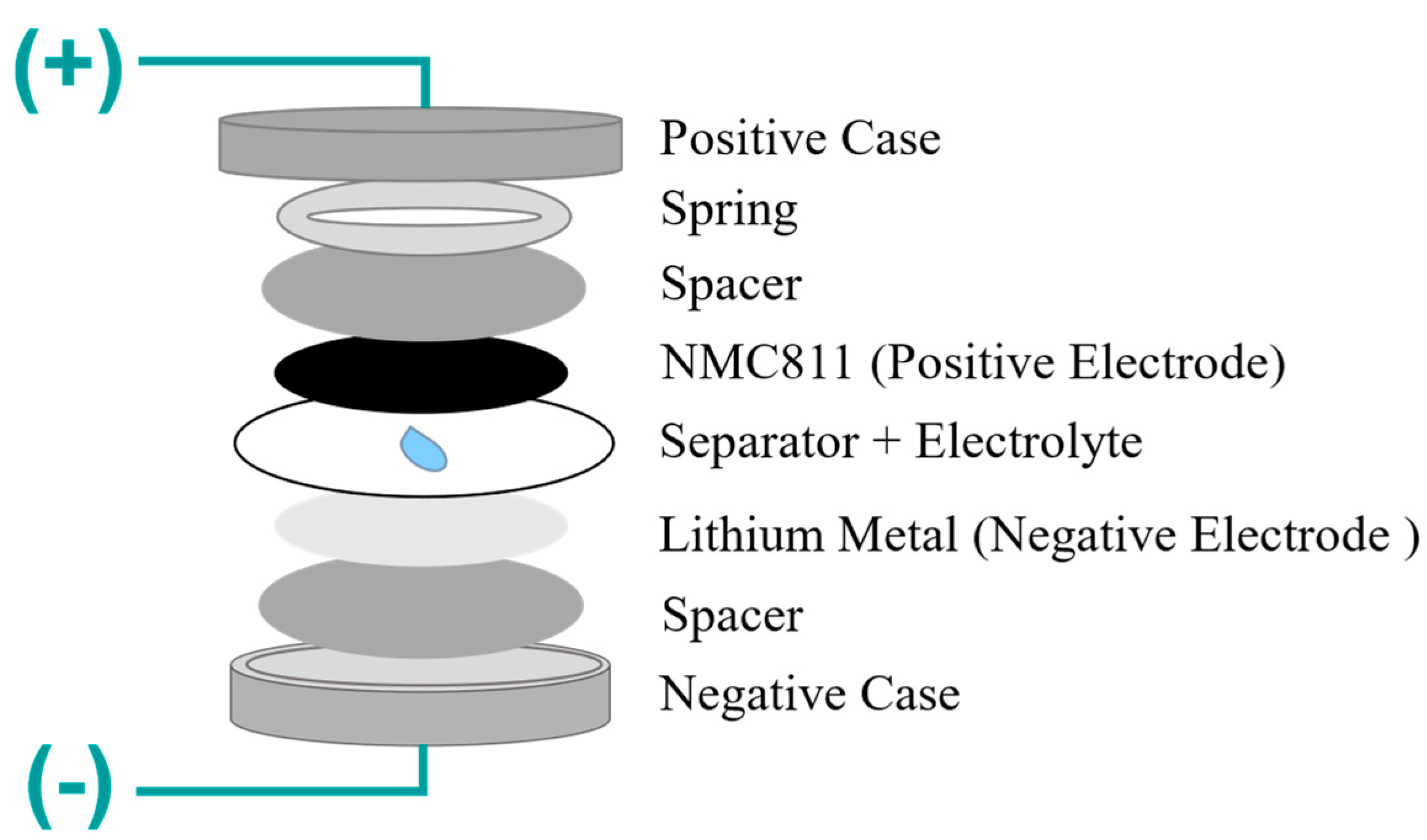
2.5. Assembling Process
2.6. XRD
2.7. SEM and EDS
2.8. ICP
2.9. PSD
2.10. Cycling and C-Rate Performance
3. Results and Discussions
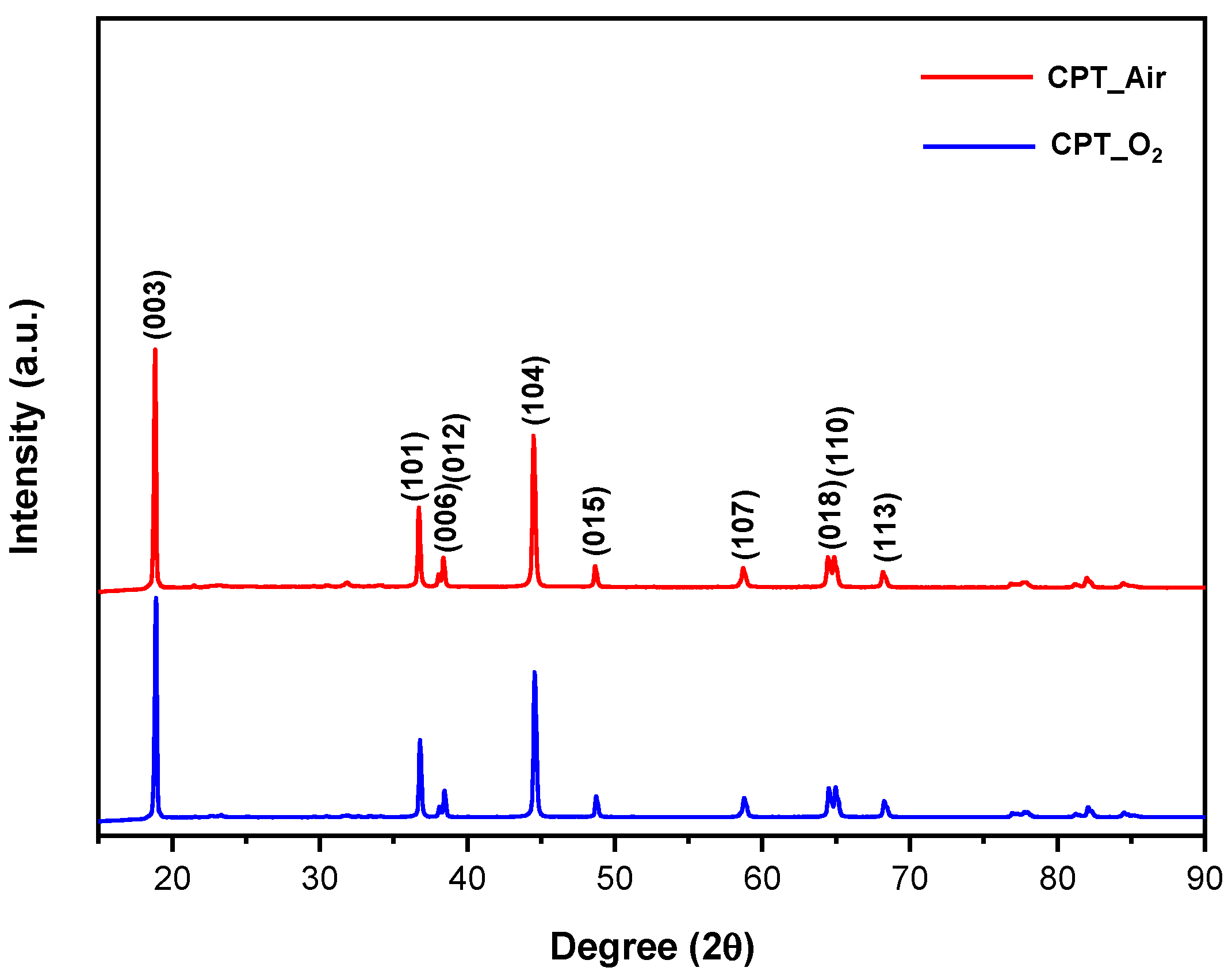
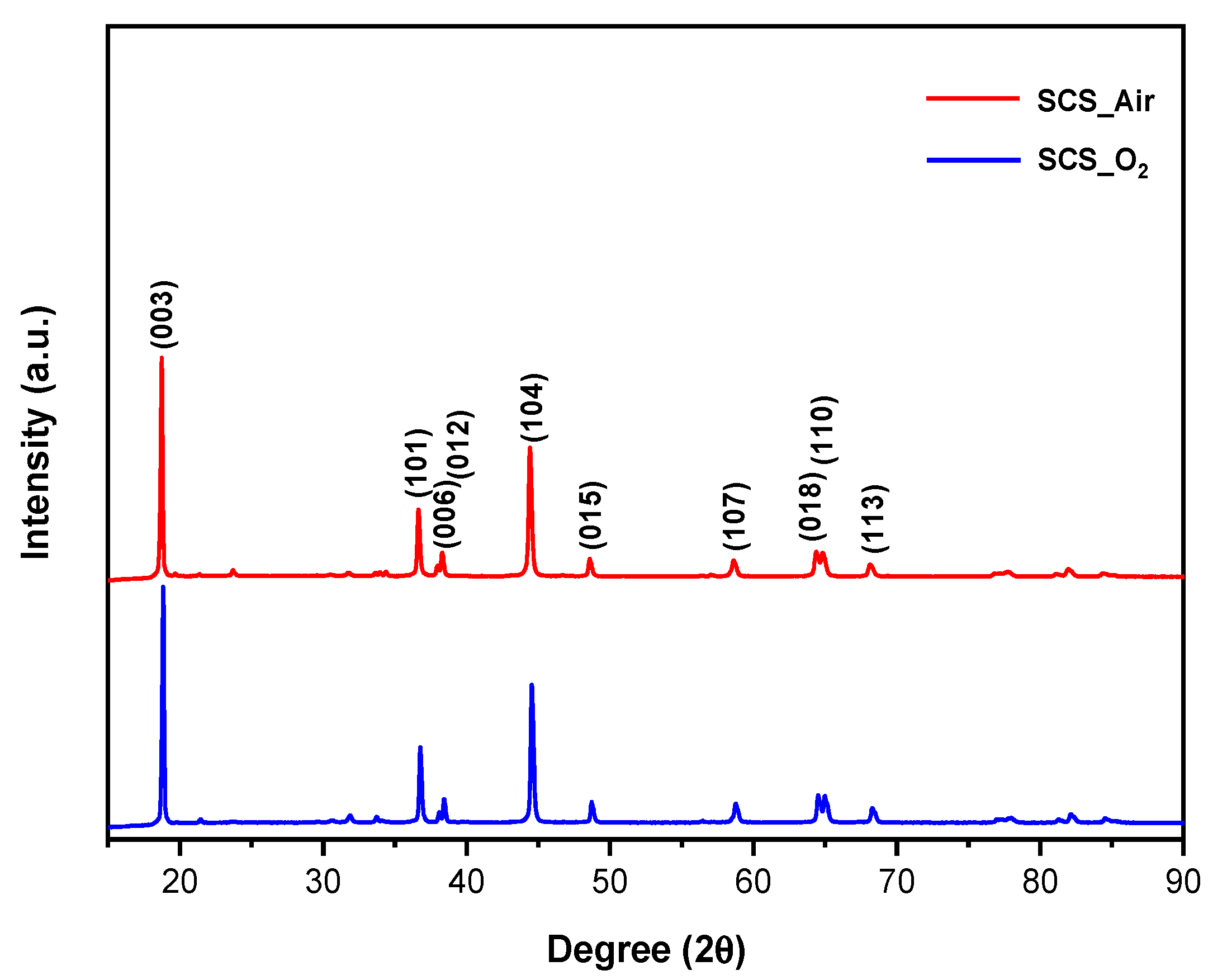
3.1. XRD
3.2. SEM and EDS Maps
3.3. ICP
3.4. PSD
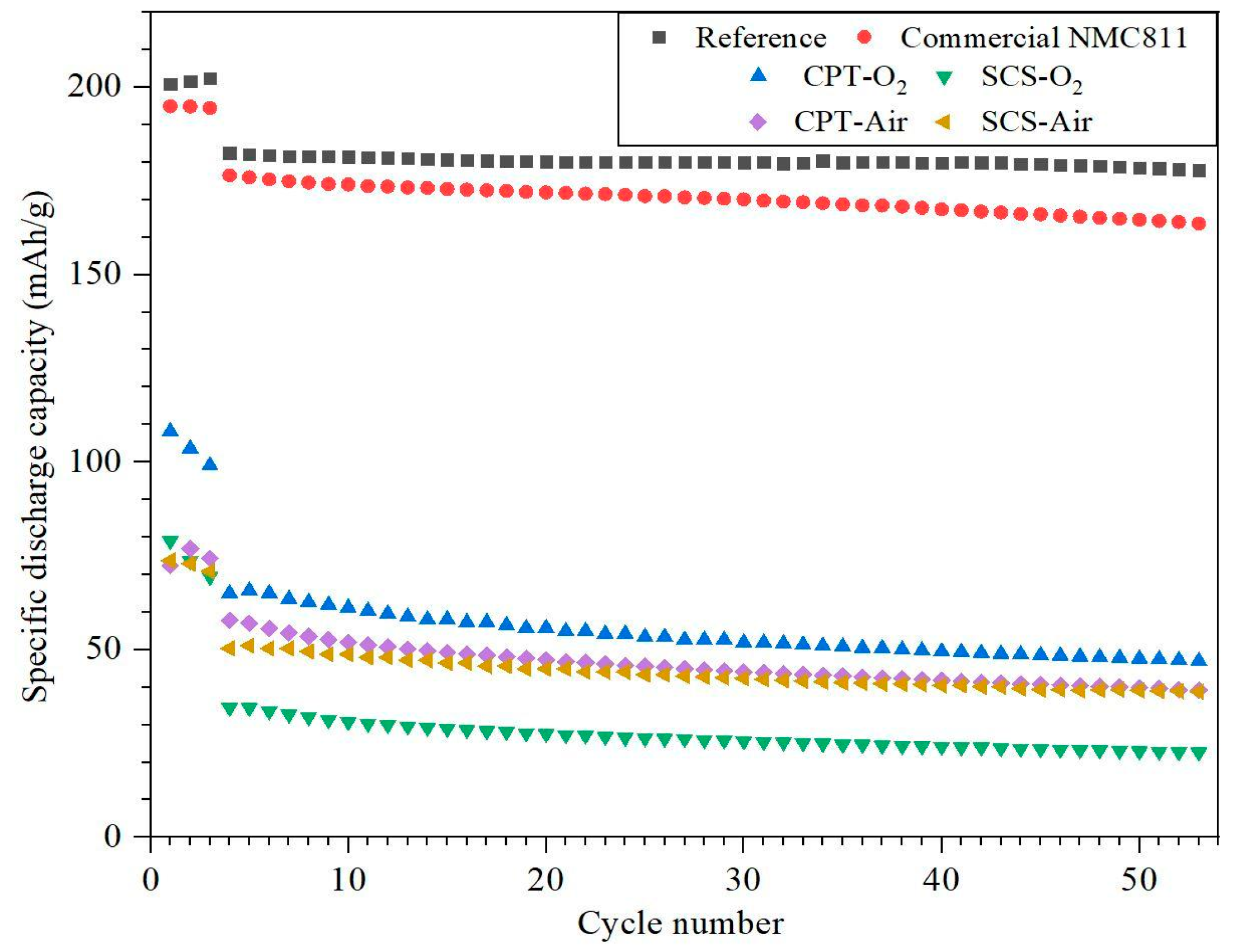
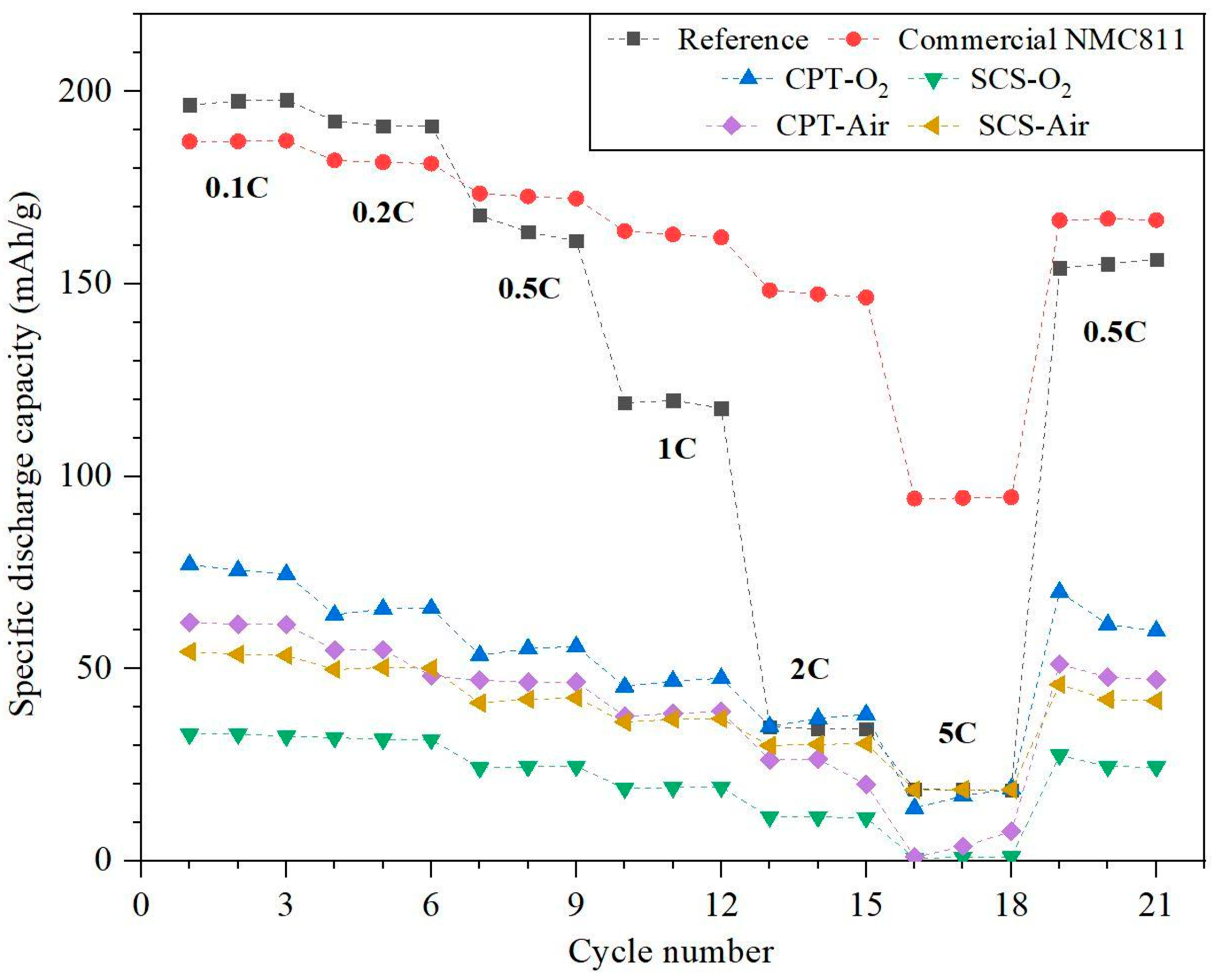
3.5. Cycling and C-Rate Performance Test
4. Conclusions
- The SEM and PSD characterization tests showed that NMC811 synthesized through the CPT approach displayed a finer morphology, with particle sizes similar to those of commercial NMC811.
- As evidence of the XRD analysis, the samples which were treated under the nitrogen atmosphere are composed of a mixture of not layered Mn, Co, and Ni oxides. Therefore, nitrogen-treated samples are not able to intercalate lithium ions and they do not show any charge/discharge capacity during the cycling performance test.
- While the electrochemical performance of coin cells can be influenced by various factors including the electrolyte, anode material, and assembling process, it is noteworthy that CPT-O2 exhibited the highest discharge capacity and capacity retention compared to all other samples, which is correlated to the morphology of the cathode particles.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Lee, J.; Park, S.; Song, J.; Kim, J. High-voltage cathode materials by combustion-based preparative approaches for Li-ion batteries application. J. Power Sources 2020, 472, 228368. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, H.; Zi, Z.; Zhang, L.; Xu, X. Core-shell and concentration-gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries. J. Mater. Chem. A Mater. 2017, 5, 4254–4279. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Peralta, D.; Salomon, J.; Colin, J.F.; Boulineau, A.; Fabre, F.; Bourbon, C.; Amestoy, B.; Gutel, E.; Bloch, D.; Patoux, S. Submicronic LiNi1/3Mn1/3Co1/3O2 synthesized by co-precipitation for lithium ion batteries—Tailoring a classic process for enhanced energy and power density. J. Power Sources 2018, 396, 527–532. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Stone, W.; Weber, R.; Hy, S.; Dahn, J.R. Synthesis of Single Crystal LiNi0.5Mn0.3Co0.2O2 for Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3529–A3537. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, X.; Dahn, J.R. Synthesis of Single Crystal LiNi0.6Mn0.2Co0.2O2 with Enhanced Electrochemical Performance for Lithium Ion Batteries. J. Electrochem. Soc. 2018, 165, A1038–A1045. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Kong, X.; Fedorovskaya, E.; Kallio, T. Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J. Power Sources 2022, 540, 231633. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Röser, S.; Börner, M.; Winter, M. Do Increased Ni Contents in LiNixMnyCozO2 (NMC) Electrodes Decrease Structural and Thermal Stability of Li Ion Batteries? A Thorough Look by Consideration of the Li+ Extraction Ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Malik, M.; Chan, K.H.; Azimi, G. Review on the synthesis of LiNixMnyCo1−x−yO2 (NMC) cathodes for lithium-ion batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- Leifer, N.; Penki, T.; Nanda, R.; Grinblat, J.; Luski, S.; Aurbach, D.; Goobes, G. Linking structure to performance of Li1.2Mn0.54Ni0.13Co0.13O2 (Li and Mn rich NMC) cathode materials synthesized by different methods. Phys. Chem. Chem. Phys. 2020, 22, 9098–9109. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research Progress of Single-Crystal Nickel-Rich Cathode Materials for Lithium Ion Batteries. Small Methods 2021, 5, 2100234. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Koenig, G.M. A review on synthesis and engineering of crystal precursors produced: Via coprecipitation for multicomponent lithium-ion battery cathode materials. CrystEngComm 2020, 22, 1514–1530. [Google Scholar] [CrossRef]
- Cho, J. LiNi0.74Co0.26−xMgxO2 cathode material for a Li-ion cell. Chem. Mater. 2000, 12, 3089–3094. [Google Scholar] [CrossRef]
- Ying, J.; Wan, C.; Jiang, C.; Li, Y. Preparation and characterization of high-density spherical LiNi0.8Co0.2O2 cathode material for lithium secondary batteries. J. Power Sources 2001, 99, 78–84. [Google Scholar] [CrossRef]
- Patil, K.C.; Aruna, S.T.; Mimani, T. Combustion synthesis: An update. Curr. Opin. Solid State Mater. Sci. 2002, 6, 507–512. [Google Scholar] [CrossRef]
- Mugumya, J.H.; Rasche, M.L.; Rafferty, R.F.; Patel, A.; Mallick, S.; Mou, M.; Bobb, J.A.; Gupta, R.B.; Jiang, M. Synthesis and Theoretical Modeling of Suitable Co-precipitation Conditions for Producing NMC111 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 12261–12270. [Google Scholar] [CrossRef]
- Hua, W.; Wang, K.; Knapp, M.; Schwarz, B.; Wang, S.; Liu, H.; Lai, J.; Müller, M.; Schökel, A.; Missyul, A.; et al. Chemical and Structural Evolution during the Synthesis of Layered Li(Ni,Co,Mn)O2 Oxides. Chem. Mater. 2020, 32, 4984–4997. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Estevez, L.; Wang, C.; Zhang, J.G. Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries. Nano Energy 2018, 49, 538–548. [Google Scholar] [CrossRef]
- Bockholt, H.; Haselrieder, W.; Kwade, A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. Powder Technol. 2016, 297, 266–274. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.-T. The Influence of Polyvinylidene Fluoride (PVDF) Binder Properties on LiNi0.33Mn0.33Co0.33O2 (NMC) Electrodes Made by a Dry-Powder-Coating Process. J. Electrochem. Soc. 2019, 166, A2151–A2157. [Google Scholar] [CrossRef]
- Primo, E.N.; Chouchane, M.; Touzin, M.; Vazquez, P.; Franco, A.A. Understanding the calendering processability of Li(Ni0.33Mn0.33Co0.33)O2-based cathodes. J. Power Sources 2021, 488, 229361. [Google Scholar] [CrossRef]
- Available online: https://www.icdd.com/ (accessed on 20 January 2024).
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, L.; Wang, F.; Wu, K.; Zhao, L.; Li, M.R.; Zhang, H.L.; Wu, Q.; Wang, J. Effects of precursor, synthesis time and synthesis temperature on the physical and electrochemical properties of Li(Ni1−x−yCoxMny)O2 cathode materials. J. Power Sources 2014, 248, 180–189. [Google Scholar] [CrossRef]
- Liu, S.; Dang, Z.; Liu, D.; Zhang, C.; Huang, T.; Yu, A. Comparative studies of zirconium doping and coating on LiNi0.6Co0.2Mn0.2O2 cathode material at elevated temperatures. J. Power Sources 2018, 396, 288–296. [Google Scholar] [CrossRef]
- Li, J.; Liang, G.; Zheng, W.; Zhang, S.; Davey, K.; Pang, W.K.; Guo, Z. Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review. Nano Mater. Sci. 2022, 5, 404–420. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Zhang, J. Surface-Coated LiNi0.8Co0.1Mn0.1O2 (NCM811) Cathode Materials by Al2O3, ZrO2, and Li2O-2B2O3 Thin-Layers for Improving the Performance of Lithium Ion Batteries. Front. Mater. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Jouanneau, S.; Eberman, K.W.; Krause, L.J.; Dahn, J.R. Synthesis, Characterization, and Electrochemical Behavior of Improved Li[NixCo1−2xMnx]O2 (0.1 ≤ x ≤ 0.5). J. Electrochem. Soc. 2003, 150, A1637–A1642. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Du, Z.; Daniel, C.; Dunlop, A.R.; Polzin, B.J.; Jansen, A.N.; Krumdick, G.K.; Wood, D.L. Impact of secondary particle size and two-layer architectures on the high-rate performance of thick electrodes in lithium-ion battery pouch cells. J. Power Sources 2021, 515, 230429. [Google Scholar] [CrossRef]
- Pişkin, B.; Uygur, C.S.; Aydınol, M.K. Morphology effect on electrochemical properties of doped (W and Mo) 622NMC, 111NMC, and 226NMC cathode materials. Int. J. Hydrogen Energy 2020, 45, 7874–7880. [Google Scholar] [CrossRef]

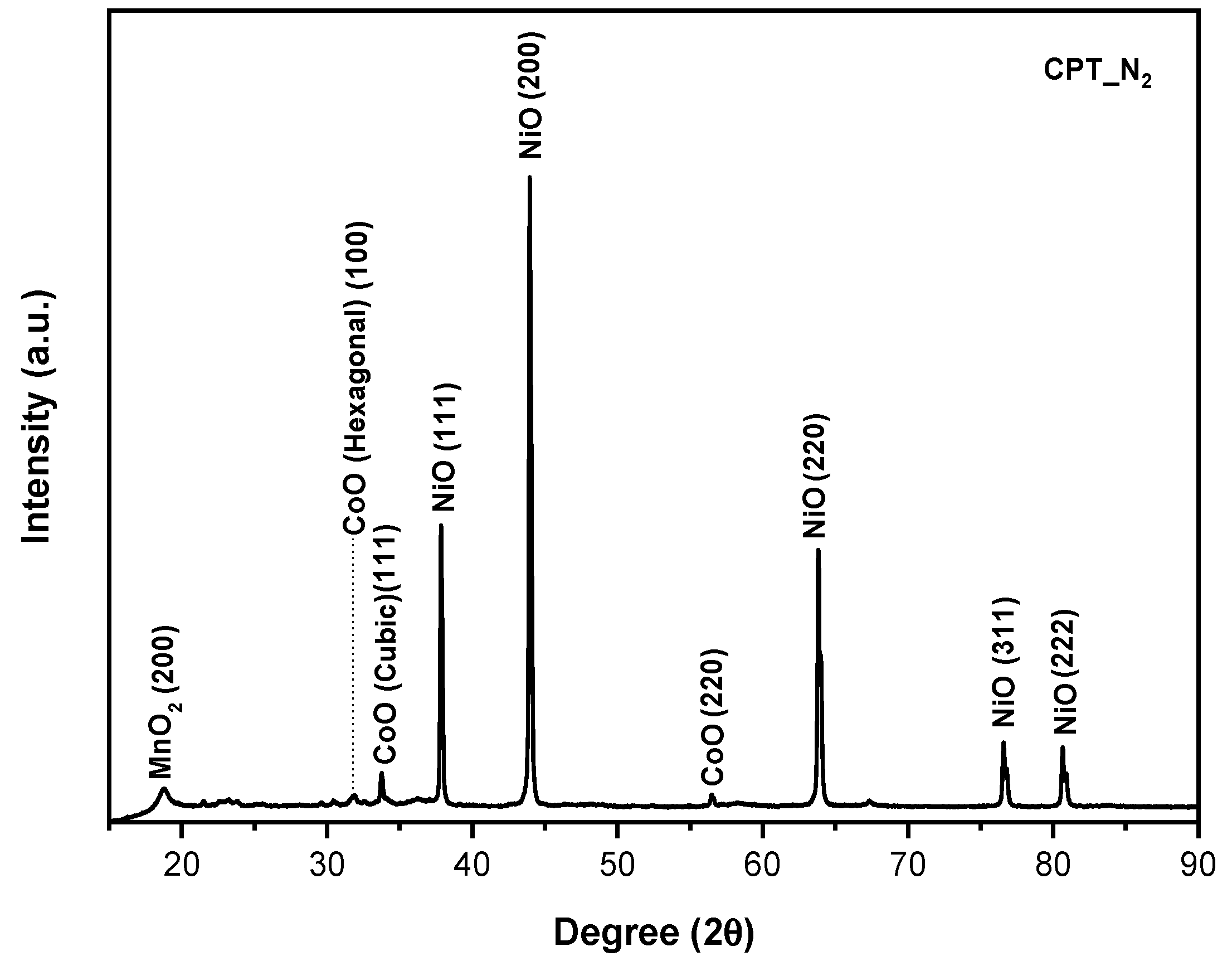
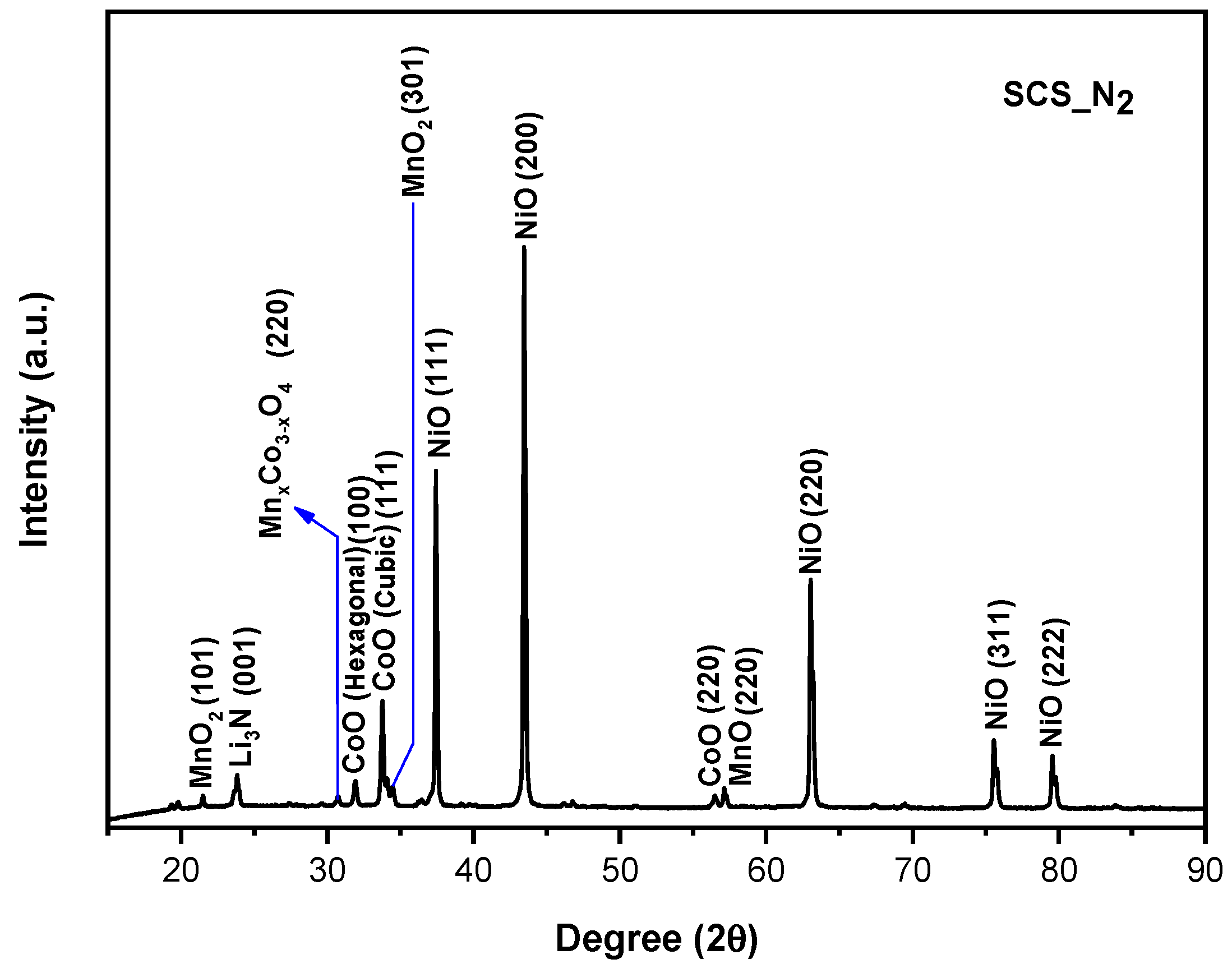

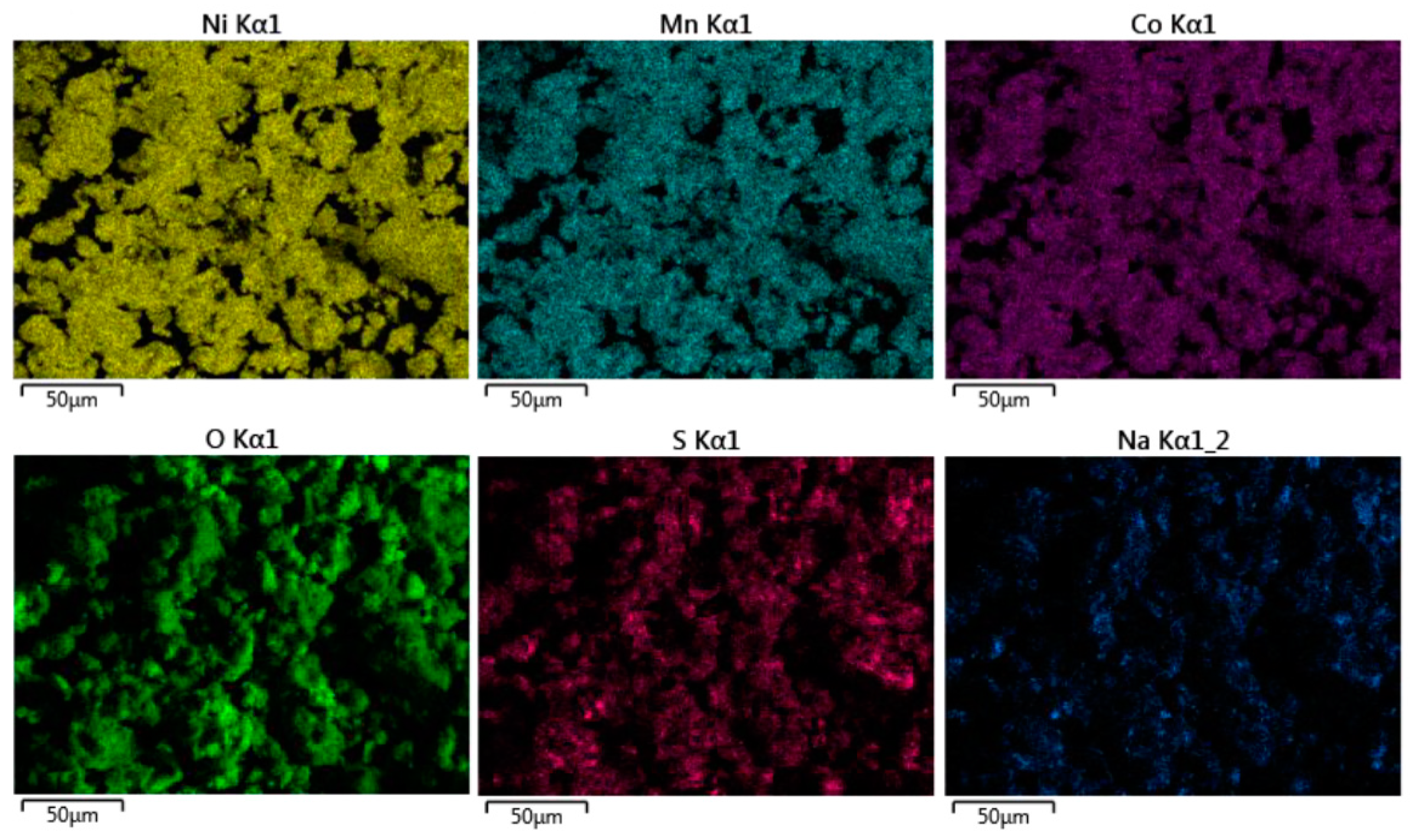
| Sample | a (Å) | c (Å) | I(003)/I(104) | OLi3b | ONi3a | Rexp | Rwp |
|---|---|---|---|---|---|---|---|
| CPT-O2 | 2.685 | 13.274 | 1.58 | 0.056 | 0.072 | 0.77 | 7.54 |
| CPT-Air | 2.685 | 13.284 | 1.52 | 0.017 | 0.076 | 0.73 | 6.81 |
| SCS-O2 | 2.684 | 13.273 | 1.76 | 0.051 | 0.072 | 0.73 | 7.70 |
| SCS-Air | 2.690 | 13.337 | 1.72 | 0.041 | 0.075 | 0.72 | 7.78 |
| Sample | NiO | c-CoO | h-CoO | MnO2 | MnO | MnxCo3−xO4 | Li3N | Rexp | Rwp |
|---|---|---|---|---|---|---|---|---|---|
| CPT-N2 | 89.7 | 0.1 | 4.8 | 5.4 | - | - | - | 0.77 | 10.09 |
| SCS-N2 | 69.6 | 1.7 | 5.3 | 2.1 | 5.1 | 0.5 | 15.7 | 0.76 | 7.48 |
| Sample ID | Nominal Stoichiometric Ratio of Li:TMs | ICP Result in Stoichiometric Ratio of Li:TMs |
|---|---|---|
| CPT-O2 | 1.15:1.00 | 1.07:1.00 |
| CPT-Air | 1.15:1.00 | 1.10:1.00 |
| CPT-N2 | 1.15:1.00 | 0.96:1.00 |
| SCS-O2 | 2.00:1.00 | 1.54:1.00 |
| SCS-Air | 2.00:1.00 | 1.41:1.00 |
| SCS-N2 | 2.00:1.00 | 1.29:1.00 |
| Sample ID | D10 (µm) | D50 (µm) | D90 (µm) |
|---|---|---|---|
| CPT-Air | 3.08 | 13.57 | 32.53 |
| CPT-O2 | 3.46 | 14.19 | 28.25 |
| CPT-N2 | 9.18 | 20.81 | 37.99 |
| SCS-Air | 9.35 | 18.99 | 33.02 |
| SCS-O2 | 12.99 | 25.05 | 42.29 |
| SCS-N2 | 10.46 | 25.05 | 44.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiozzo, A.; Ghaseminezhad, K.; Mazzucco, A.; Giuliano, M.; Rocca, R.; Dotoli, M.; Nicol, G.; Nervi, C.; Baricco, M.; Sgroi, M.F. Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material. Crystals 2024, 14, 137. https://doi.org/10.3390/cryst14020137
Tiozzo A, Ghaseminezhad K, Mazzucco A, Giuliano M, Rocca R, Dotoli M, Nicol G, Nervi C, Baricco M, Sgroi MF. Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material. Crystals. 2024; 14(2):137. https://doi.org/10.3390/cryst14020137
Chicago/Turabian StyleTiozzo, Arianna, Keyhan Ghaseminezhad, Asya Mazzucco, Mattia Giuliano, Riccardo Rocca, Matteo Dotoli, Giovanna Nicol, Carlo Nervi, Marcello Baricco, and Mauro Francesco Sgroi. 2024. "Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material" Crystals 14, no. 2: 137. https://doi.org/10.3390/cryst14020137
APA StyleTiozzo, A., Ghaseminezhad, K., Mazzucco, A., Giuliano, M., Rocca, R., Dotoli, M., Nicol, G., Nervi, C., Baricco, M., & Sgroi, M. F. (2024). Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material. Crystals, 14(2), 137. https://doi.org/10.3390/cryst14020137








