Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
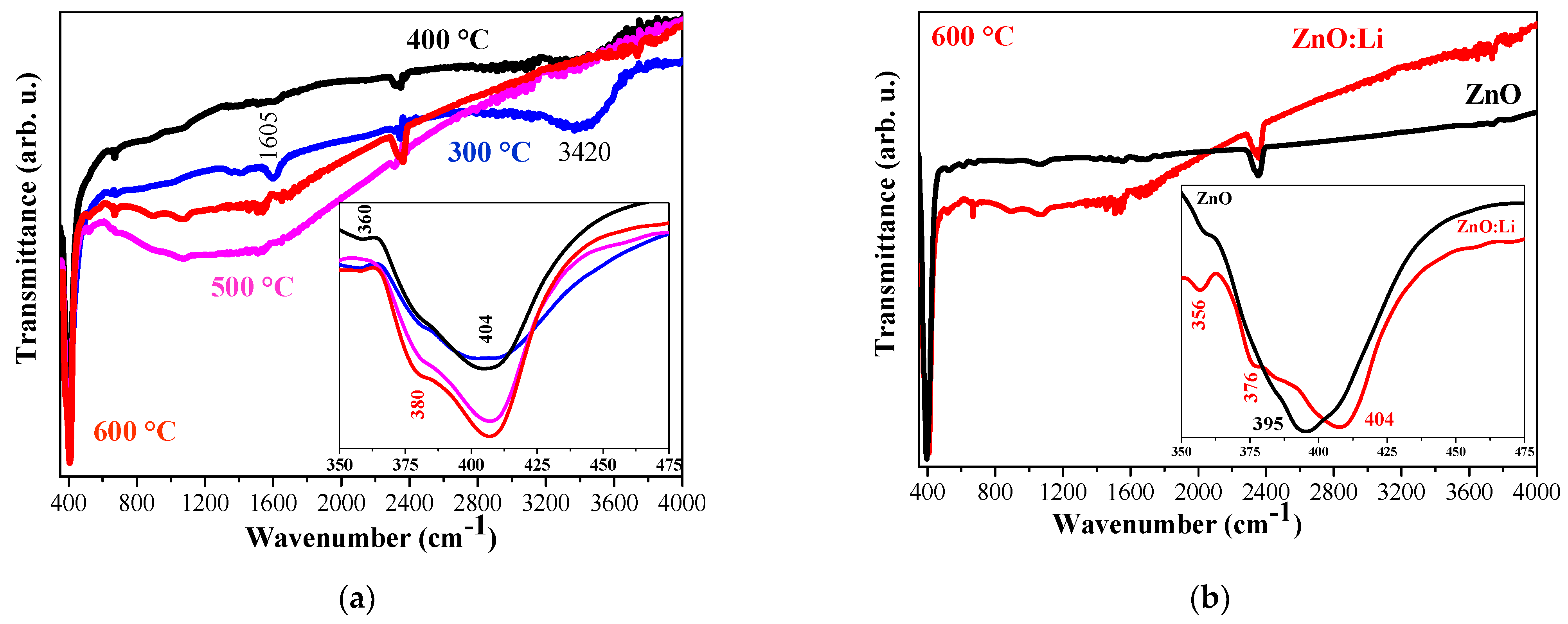
3.1. FTIR Investigation
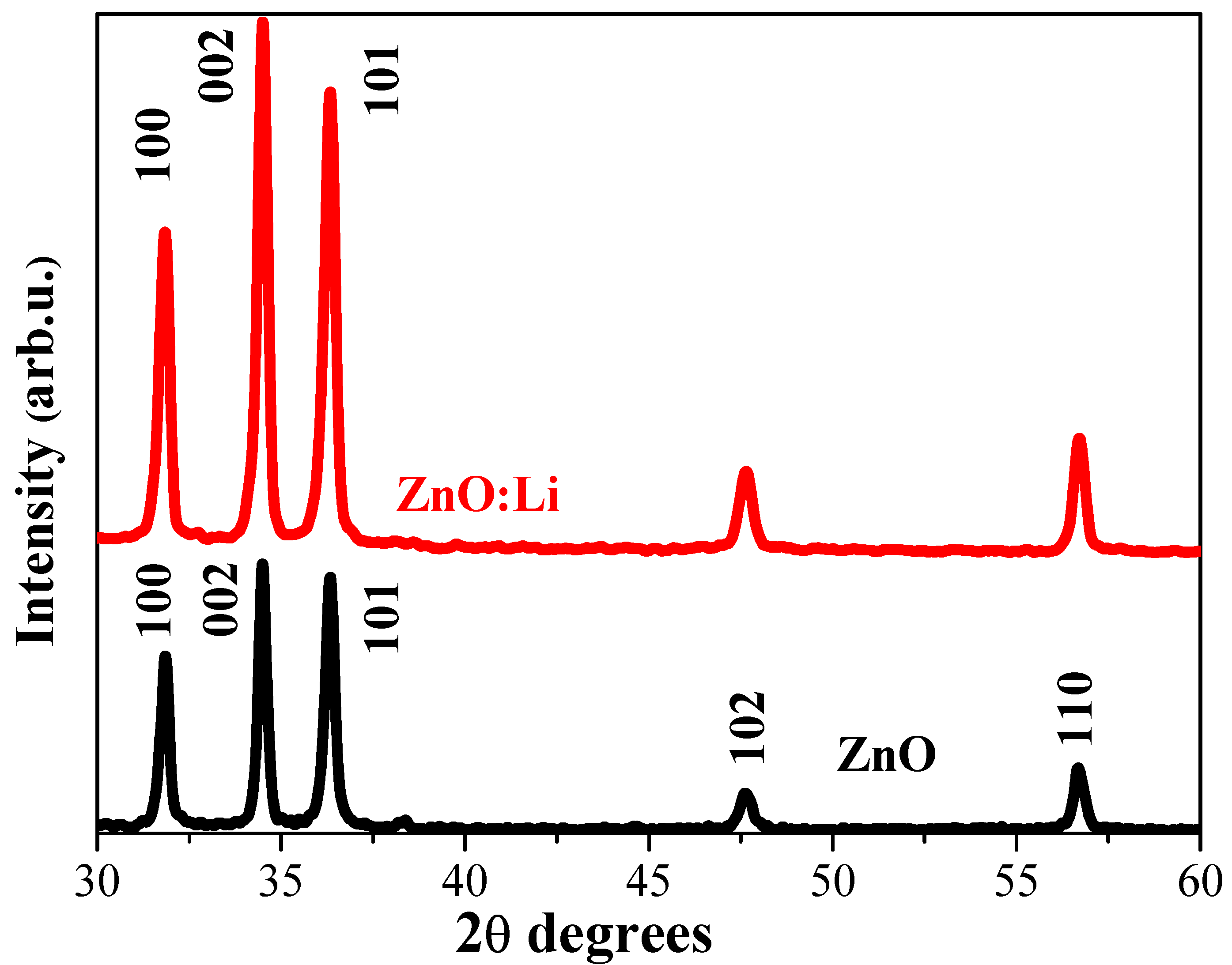
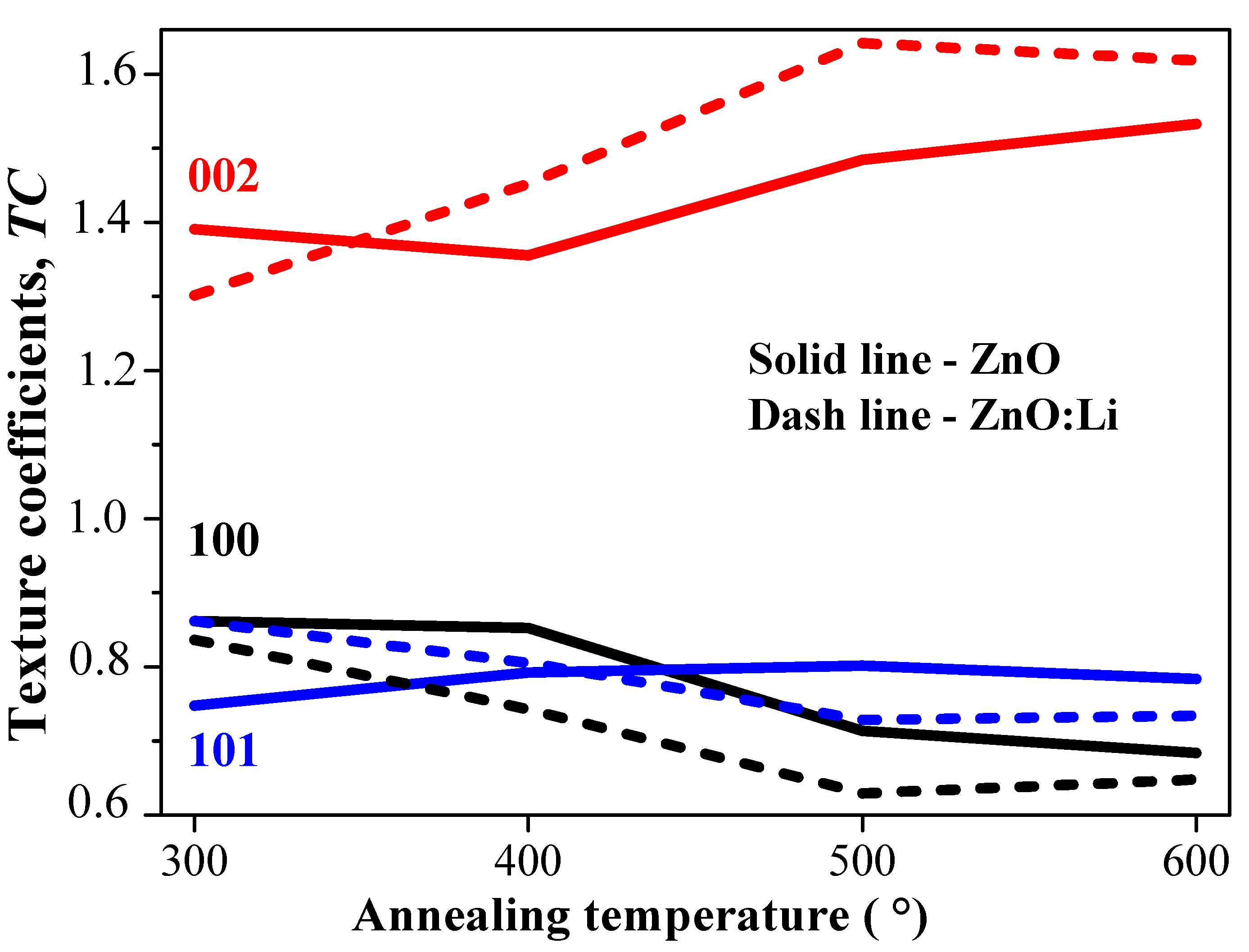
3.2. XRD Structural Study
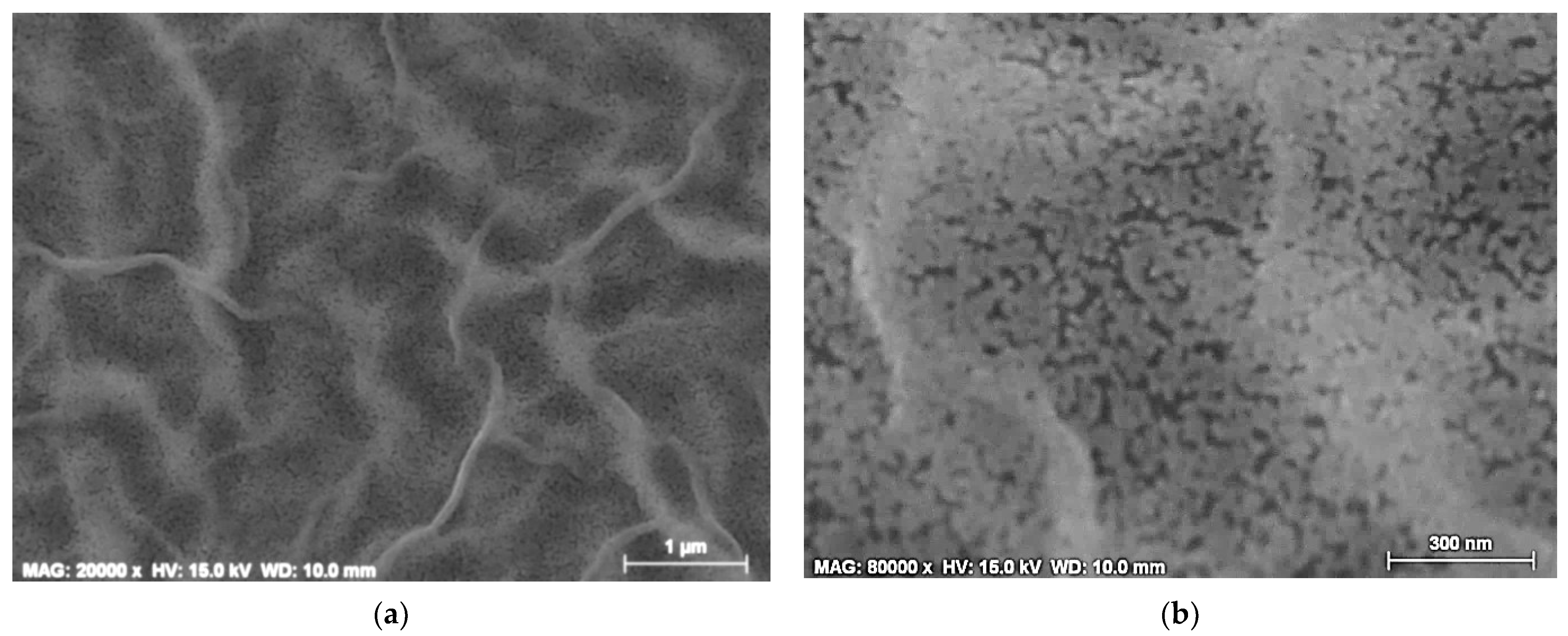
3.3. Film Surface Morphology
3.4. Optical Characterization
3.5. Electrical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morales, C.; Leinen, D.; del Campo, A.; Ares, J.R.; Sanchez, C.; Flege, J.I.; Gutierrez, A.; Prieto, P.; Soriano, L. Growth and characterization of ZnO thin films at low temperatures: From room temperature to −120 °C. J. Alloys Comp. 2021, 884, 161056. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, C.; Anwand, W.; Wagner, A.; Butterling, M.; Rahman, M.A.; Phillips, M.R.; Ton-That, C.; Younas, M.; Su, S.; et al. Vacancy cluster in ZnO films grown by pulsed laser deposition. Sci. Rep. 2019, 9, 3534. [Google Scholar] [CrossRef] [PubMed]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Muchuweni, E.; Sathiaraj, T.S.; Nyakotyo, H. Synthesis and characterization of zinc oxide thin films for optoelectronic applications. Heliyon 2017, 3, e00285. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Pandey, K.; Dutta, J.; Brahma, S.; Rao, B.; Liu, C.P. Review on ZnO-based piezotronics and piezoelectric nanogenerators: Aspects of piezopotential and screening effect. J. Phys. Mater. 2021, 4, 044011. [Google Scholar] [CrossRef]
- Khatibani, A.B. Characterization and Ethanol Sensing Performance of Sol-Gel Derived Pure and Doped Zinc Oxide Thin Films. J. Electron. Mater. 2019, 48, 3784–3793. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2010, 10, 42838–42859. [Google Scholar] [CrossRef]
- Hála, M.; Fujii, S.; Redinger, A.; Inoue, Y.; Rey, G.; Thevenin, M.; Deprédurand, V.; Weiss, T.P.; Bertram, T.; Siebentritt, S. Highly conductive ZnO films with high near infrared transparency. Prog. Photovolt. Res. Appl. 2015, 23, 1630–1641. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Sathasivam, S.; Carmalt, C.J. n-Type conducting P doped ZnO thin films via chemical vapor deposition. RSC Adv. 2020, 10, 34527–34533. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kumar, V.; Swart, H.C.; Purohit, L.P. P-type conductivity in doped and codoped ZnO thin films synthesized by RF magnetron sputtering. J. Modern Opt. 2015, 62, 1368–1373. [Google Scholar] [CrossRef]
- Mia, M.N.H.; Mia, M.F.; Pervez, M.F.; Khalid Hossain, M.K.; Rahman, M.R.; Uddin, M.J.; Al Mashud, M.A.; Ghosh, H.K.; Hoq, M. Influence of Mg content on tailoring optical bandgap of Mg-doped ZnO thin film prepared by sol-gel method. Results Phys. 2017, 7, 2683–2691. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, J.P.; Sánchez-Vicente, C. The Effect of Rare Earths on the Response of Photo UV-Activate ZnO Gas Sensors. Sensors 2022, 22, 8150. [Google Scholar] [CrossRef] [PubMed]
- Punia, K.; Lal, G.; Dolia, S.N.; Kumar, S. Defects and oxygen vacancies tailored structural, optical, photoluminescence and magnetic properties of Li doped ZnO nanohexagons. Ceram. Int. 2020, 46, 12296–12317. [Google Scholar] [CrossRef]
- Lee, W.; Leem, J.-Y. Ultraviolet Photoresponse Properties of Li-Doped ZnO Thin Films Prepared by Sol–Gel Spin-Coating Method. J. Nanosci. Nanotechnol. 2017, 17, 5697–5700. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Yu, A.; Zhang, Y.; Zhai, J.; Wang, Z.L. Flexible Li-doped ZnO piezotronic transistor array for in-plane strain mapping. Nano Energy 2019, 55, 341–347. [Google Scholar] [CrossRef]
- Khosravi, P.; Ebrahimi, S.A.S. Structural, Electrical and Optical Characterization of ZnO:Li Thin Films Prepared by Sol-Gel Spin Coating. J. Ultrafine Grained Nanostruct Mater. 2023, 56, 108–120. [Google Scholar]
- Salah, M.; Azizi, S.; Boukhachem, A.; Khaldi, C.; Amlouk, M.; Lamloumi, J. Effects of lithium doping on: Microstructure, morphology, nanomechanical properties and corrosion behaviour of ZnO thin films grown by spray pyrolysis technique. J. Mater. Sci. Mater. Elect. 2019, 30, 1767–1785. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Chiu, W.-Y. Enhanced Electrical Properties and Stability of P-Type Conduction in ZnO Transparent Semiconductor Thin Films by Co-Doping Ga and N. Coatings 2020, 10, 1069. [Google Scholar] [CrossRef]
- Zagal-Padilla, C.K.; Gamboa, S.A. Role of native defects on the opto-electronic properties of p-type ZnO synthesized during the most straightforward method: Only water. Appl. Phys. A 2023, 129, 183. [Google Scholar] [CrossRef]
- Scajev, P.; Durena, R.; Onufrijevs, P.; Miasojedovas, S.; Malinauskas, T.; Stanionyte, S.; Zarkov, A.; Zukuls, A.; Bite, I.; Smits, K. Morphological and optical property study of Li doped ZnO produced by microwave-assisted solvothermal synthesis. Mater. Sci. Semicond. Process. 2021, 135, 106069. [Google Scholar] [CrossRef]
- Chirakkara, S.; Krupanidhi, S.B. Pulsed laser deposited ZnO/ZnO:Li multilayer for blue light emitting diodes. J. Lumin. 2011, 131, 1649–1654. [Google Scholar] [CrossRef]
- Jin, M.; Li, Z.; Huang, F.; Xia, Y.; Ji, X.; Wang, W. Critical conditions for the formation of p-type ZnO with Li doping. RSC Adv. 2018, 8, 30868–30874. [Google Scholar] [CrossRef] [PubMed]
- Ahmoum, H.; Boughrara, M.; Suait, M.S.; Kerouad, M. Effect of position and concentration of Li on ZnO physical properties: Density functional investigation. Chem. Phys. Lett. 2019, 719, 45–53. [Google Scholar] [CrossRef]
- Xiao, B.; Ye, Z.; Zhang, Y.; Zeng, Y.; Zhu, L.; Zhao, B. Fabrication of p-type Li-doped ZnO films by pulsed laser deposition. Appl. Surf. Sci. 2006, 253, 895–897. [Google Scholar] [CrossRef]
- Bornand, V.; Mezy, A. Morphological and ferroelectric studies of Li-doped ZnO thin films. Mater. Lett. 2013, 107, 357–360. [Google Scholar] [CrossRef]
- Kafadaryan, E.A.; Petrosyan, S.I.; Hayrapetyan, A.G.; Hovsepyan, R.K.; Manukyan, A.L.; Vardanyan, E.S.; Zerrouk, A.F. Infrared 45° reflectometry of Li doped ZnO films. J. Appl. Phys. 2004, 95, 3005–3009. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, B.N.; Lee, S.-B.; Boo, J.-H. Study on the doping effect of Li-doped ZnO film. Thin Solid Films 2008, 516, 5586–5589. [Google Scholar] [CrossRef]
- Gartner, M.; Stroescu, H.; Mitrea, D.; Nicolescu, M. Various Applications of ZnO Thin Films Obtained by Chemical Routes in the Last Decade. Molecules 2023, 28, 4674. [Google Scholar] [CrossRef]
- Fujihara, S.; Sasaki, C.; Kimura, T. Effects of Li and Mg doping on microstructure and properties of sol-gel ZnO thin films. J. Eur. Ceram. Soc. 2001, 21, 2109–2112. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vetruyen, B. Study of ZnO sol–gel films: Effect of annealing. Mater. Lett. 2010, 64, 1147–1149. [Google Scholar] [CrossRef]
- Petrovic, Z.; Ristic, M.; Music, S. Development of ZnO microstructures produced by rapid hydrolysis of zinc acetylacetonate. Ceram. Int. 2014, 40, 10953–10959. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Ramachandran, K. Increasing the Reactive Sites of ZnO Nanoparticles by Li Doping for Ethanol Sensing. Mater. Res. Express 2019, 6, 015024. [Google Scholar] [CrossRef]
- Istrate, A.-I.; Nastase, F.; Mihalache, I.; Comanescu, F.; Gavrila, R.; Tutunaru, O.; Müller, R. Synthesis and characterization of Ca doped ZnO thin films by sol–gel method. J. Sol-Gel Sci. Technol. 2019, 92, 585–597. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef]
- Bilgin, V. Preparation and characterization of ultrasonically sprayed zinc oxide thin films doped with lithium. J. Electronic. Mater. 2009, 38, 1969–1978. [Google Scholar] [CrossRef]
- Hussein, H.M. Photosensitive analysis of spin coated Cu doped ZnO thin film synthesized by hydrothermal method. Results Opt. 2023, 13, 100543. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. J. Theor. Exper. Appl. Phys. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Sobczak, K.; Lojkowski, W. Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals 2018, 8, 179. [Google Scholar] [CrossRef]
- Hsu, H.-P.; Lin, D.-Y.; Lu, C.-Y.; Ko, T.-S.; Chen, H.-Z. Effect of Lithium Doping on Microstructural and Optical Properties of ZnO Nanocrystalline Films Prepared by the Sol-Gel Method. Crystals 2018, 8, 228. [Google Scholar] [CrossRef]
- Hjiri, M.; Aida, M.S.; Lemine, O.M.; El Mir, L. Study of defects in Li-doped ZnO thin films. Mater. Sci. Semicond. Process. 2019, 89, 149. [Google Scholar] [CrossRef]
- Sharmila, B.; Singha, M.K.; Dwivedi, P. Impact of annealing on structural and optical properties of ZnO thin films. Microeloect. J. 2023, 135, 105759. [Google Scholar]
- Khan, M.I.; Neha, T.R.; Billah, M.M. UV-irradiated sol-gel spin coated AZO thin films: Enhanced optoelectronic properties. Heliyon 2022, 8, e08743. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.A.; Afify, A.S. Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique. Materials 2022, 15, 6178. [Google Scholar] [CrossRef] [PubMed]
- Podia, M.; Tripathi, A.K. Structural, optical and luminescence properties of ZnO thin films: Role of hot electrons defining the luminescence mechanisms. J. Lumin. 2022, 252, 119331. [Google Scholar] [CrossRef]
- Salam, S.; Islam, M.; Akram, A. Sol–gel synthesis of intrinsic and aluminum-doped zinc oxide thin films as transparent conducting oxides for thin film solar cell. Thin Solid Films 2013, 529, 242–247. [Google Scholar] [CrossRef]
- Maache, A.; Chergui, A.; Djouadi, D.; Benhaoua, B.; Chelouche, A.; Boudissa, M. Effect of La doping on ZnO thin films physical properties: Correlation between strain and morphology. Optik 2019, 180, 1018–1026. [Google Scholar] [CrossRef]
- Caglar, M.; Caglar, Y.; Aksoy, S.; Ilican, S. Temperature dependence of the optical band gap and electrical conductivity of sol-gel derived undoped and Li-doped ZnO films. Appl. Surf. Sci. 2010, 256, 4966–4971. [Google Scholar] [CrossRef]
- Shohany, B.G.; Zak, A.K. Doped ZnO nanostructures with selected elements—Structural, morphology and optical properties: A review. Ceram. Int. 2020, 46, 5507–5520. [Google Scholar] [CrossRef]
- Salah, M.; Azizi, S.; Boukhachem, A.; Khaldi, C.; Amlouk, M.; Lamloumi, J. Structural, morphological, optical and photodetector properties of sprayed Li-doped ZnO thin films. J. Mater. Sci. 2017, 52, 10439–10454. [Google Scholar] [CrossRef]
- Meziane, K.; El Hichou, A.; El Hamidi, A.; Chhiba, M.; Bourial, A.; Almaggoussi, A. Li concentration dependence of structural properties and optical band gap of Li-doped ZnO films. Appl. Phys. A 2017, 123, 430. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Ali, M.A.; Afifi, G.; Imam, H. Annealing effects on the structural and optical properties of growth ZnO thin films fabricated by pulsed laser deposition (PLD). J. Mater. Sci. Mater. Electron. 2014, 25, 5071–5077. [Google Scholar] [CrossRef]
- EL-Fadl, A.A.; Mohamad, G.A.; El-Moiz, A.B.A.; Rashad, M. Optical constants of Zn1−xLixO films prepared by chemical bath deposition technique. Phys. B Cond. Matt. 2005, 366, 44–54. [Google Scholar] [CrossRef]
- Tezel, F.M.; Kariper, I.A. Structural and Optical Properties of Undoped and Silver, Lithium and Cobalt-doped ZnO thin films. Surf. Rev. Lett. 2020, 27, 1950138. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; El-Said, A.S.; Heller, R. Enhancement of the refractive index of sputtered zinc oxide thin films through doping with Fe2O3. J. Alloys Compd. 2017, 690, 453–460. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Badgujar, A.C.; Yadav, B.S.; Jha, G.K.; Dhage, S.R. Room Temperature Sputtered Aluminum-Doped ZnO Thin Film Transparent Electrode for Application in Solar Cells and for Low-Band-Gap Optoelectronic Devices. ACS Omega 2022, 7, 14203–14210. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, F.; Zhao, N.; Yang, P. Influence of Annealing Temperature on Optical Properties of Sandwiched ZnO/Metal/ZnO Transparent Conductive Thin Films. Micromachines 2022, 13, 296. [Google Scholar] [CrossRef]
- Mahajan, C.M.; Takwale, M.G. Precursor molarity dependent growth rate, microstructural, optical and electrical properties of spray pyrolytically deposited transparent conducting ZnO thin films. Micro Nanostr. 2022, 163, 107131. [Google Scholar] [CrossRef]
- Mallick, A.; Ghosh, S.; Basak, D. Highly conducting and transparent low-E window films with high figure of merit values based on RF sputtered Al and In co-doped ZnO. Mater. Sci. Semicond. Process. 2020, 119, 105240. [Google Scholar] [CrossRef]
- Das, A.; Das, G.; Kabiraj, D.; Basak, D. High conductivity along with high visible light transparency in Al implanted sol-gel ZnO thin film with an elevated figure of merit value as a transparent conducting layer. J. Alloys Compd. 2020, 835, 155221. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chiu, L.C.; Juang, J.Y. High haze Ga and Zr co-doped zinc oxide transparent electrodes for photovoltaic applications. J. Alloys Compd. 2022, 901, 163678. [Google Scholar] [CrossRef]



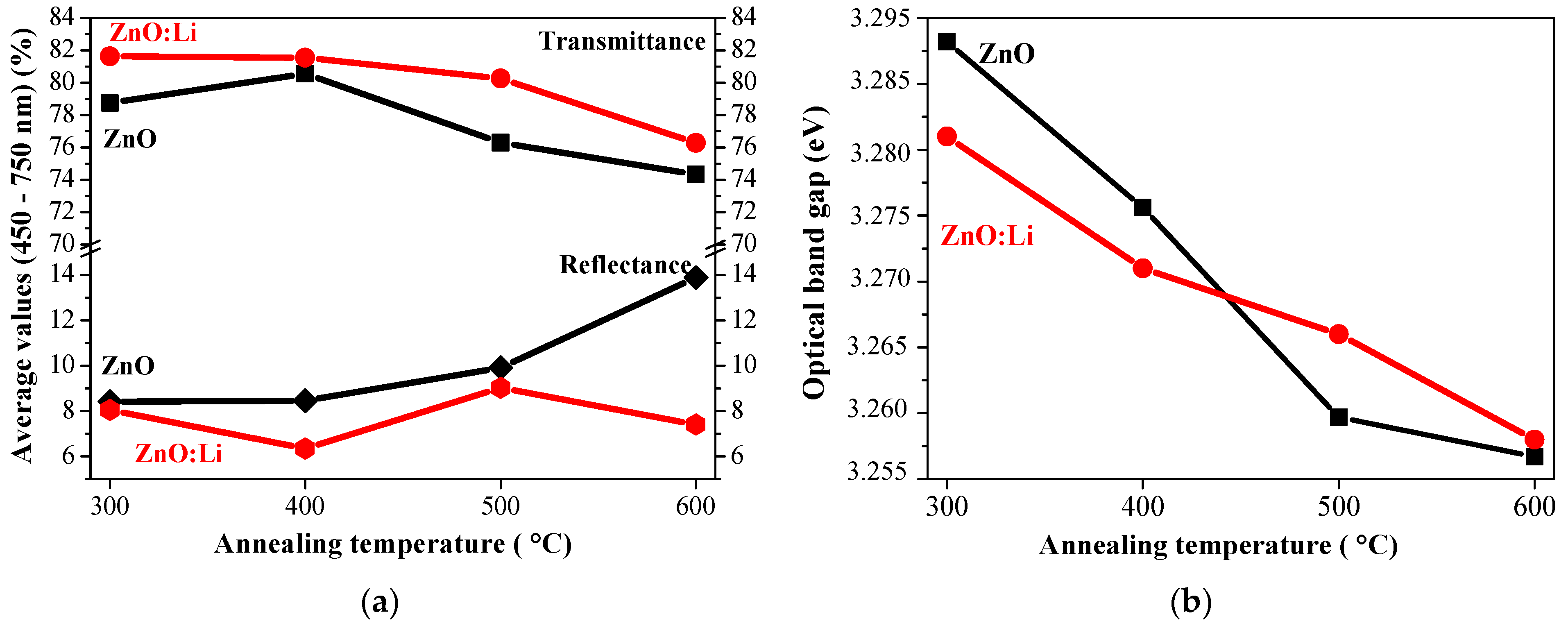
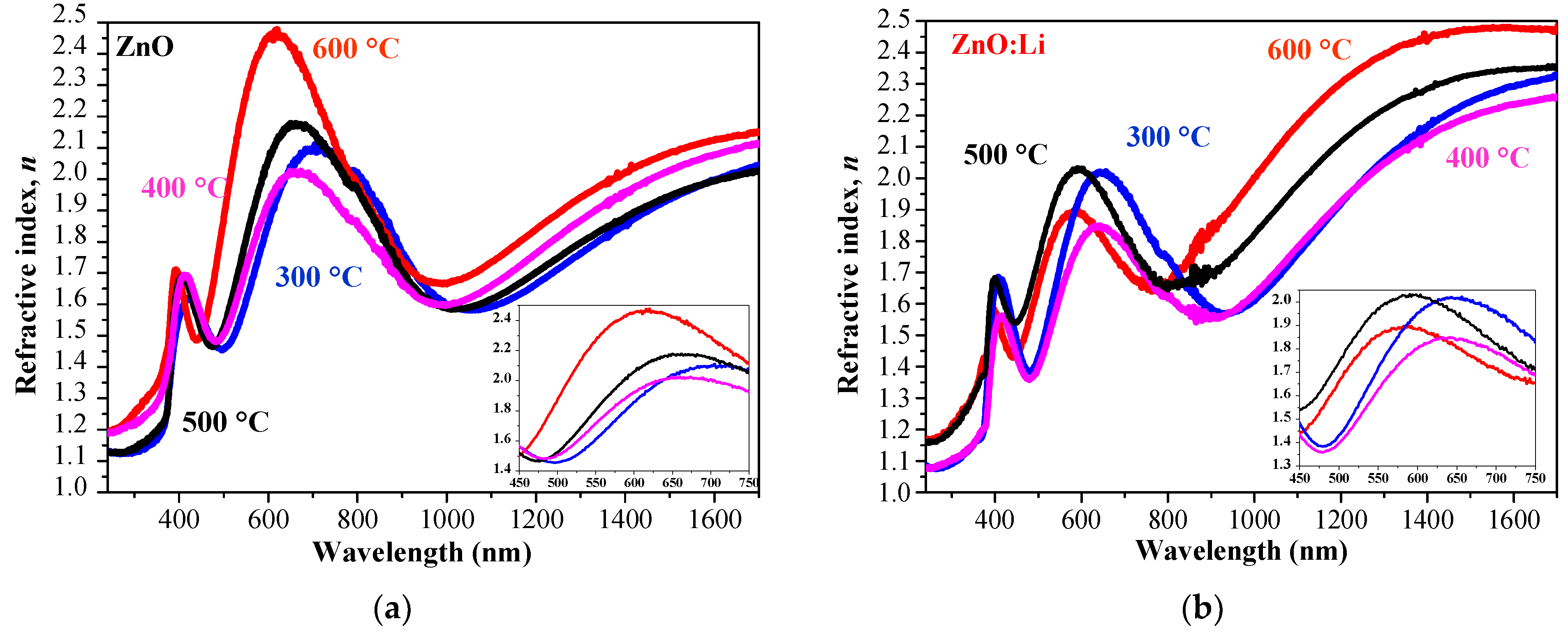
| Tannealing [°C] | Parameter | Undoped ZnO | ZnO:Li |
|---|---|---|---|
| 300 | d [nm] | 11.8 (7) | 11.6 (7) |
| 1/d2 × 10−4 [1/nm2] | 71 | 74 | |
| a [Å] | 3.241 (3) | 3.243 (3) | |
| c [Å] | 5.199 (6) | 5.201 (6) | |
| c/a ratio | 1.604 | 1.604 | |
| 400 | d [nm] | 15 (1) | 19 (1) |
| 1/d2 × 10−4 [1/nm2] | 44 | 28 | |
| a [Å] | 3.243 (3) | 3.244 (3) | |
| c [Å] | 5.199 (6) | 5.200 (6) | |
| c/a ratio | 1.603 | 1.603 | |
| 500 | d [nm] | 30 (1) | 29 (1) |
| 1/d2 × 10−4 [1/nm2] | 11 | 12 | |
| a [Å] | 3.244 (3) | 3.243 (3) | |
| c [Å] | 5.197 (6) | 5.197 (6) | |
| c/a ratio | 1.602 | 1.603 | |
| 600 | d [nm] | 36 (2) | 31 (1) |
| 1/d2 × 10−4 [1/nm2] | 8 | 10 | |
| a [Å] | 3.243 (3) | 3.243 (3) | |
| c [Å] | 5.193 (6) | 5.194 (6) | |
| c/a ratio | 1.601 | 1.602 |
| Tannealing [°C] | Taverage [%] | Rsheet [Ω/sq] | FOM × 10−4 [Ω−1] |
|---|---|---|---|
| 300 | 88.10 | 380 | 7.42 |
| 500 | 86.74 | 396 | 6.08 |
| 600 | 82.75 | 250 | 6.02 |
| Material | Deposition Method | Taverage [%] | Rsheet [Ω/sq] | FOM [Ω−1] | Reference |
|---|---|---|---|---|---|
| ZnO | Spray pyrolysis | 96.30 | 388 | 1.76 × 10−3 | [58] |
| ZnO:Al | Sol–gel | 84.19 | 0.94 × 10−4 | [43] | |
| ZnO:Al:In | RF sputtering | 88.00 (550 nm) | 9.6 | 2.65 × 10−2 | [59] |
| ZnO:Al (implanted) ZnO | Sol–gel | 82.20 | 156 | 9.03 × 10−4 | [60] |
| ZnO | Sol–gel | 91.75 | 1950 | 2.17 × 10−4 | [61] |
| ZnO:Ga | Atmospheric pressure plasma jet | 83.40 (550 nm) | 12 | 1.48 × 10−2 | [62] |
| ZnO:Li | Sol–gel | 82.75 | 250 | 6.02 × 10−4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B.; Closset, R. Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties. Crystals 2024, 14, 6. https://doi.org/10.3390/cryst14010006
Ivanova T, Harizanova A, Koutzarova T, Vertruyen B, Closset R. Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties. Crystals. 2024; 14(1):6. https://doi.org/10.3390/cryst14010006
Chicago/Turabian StyleIvanova, Tatyana, Antoaneta Harizanova, Tatyana Koutzarova, Benedicte Vertruyen, and Raphael Closset. 2024. "Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties" Crystals 14, no. 1: 6. https://doi.org/10.3390/cryst14010006
APA StyleIvanova, T., Harizanova, A., Koutzarova, T., Vertruyen, B., & Closset, R. (2024). Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties. Crystals, 14(1), 6. https://doi.org/10.3390/cryst14010006









