Thermal Evaporation Synthesis, Optical and Gas-Sensing Properties of ZnO Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of ZnO Nanowires
2.2. Material Characterization
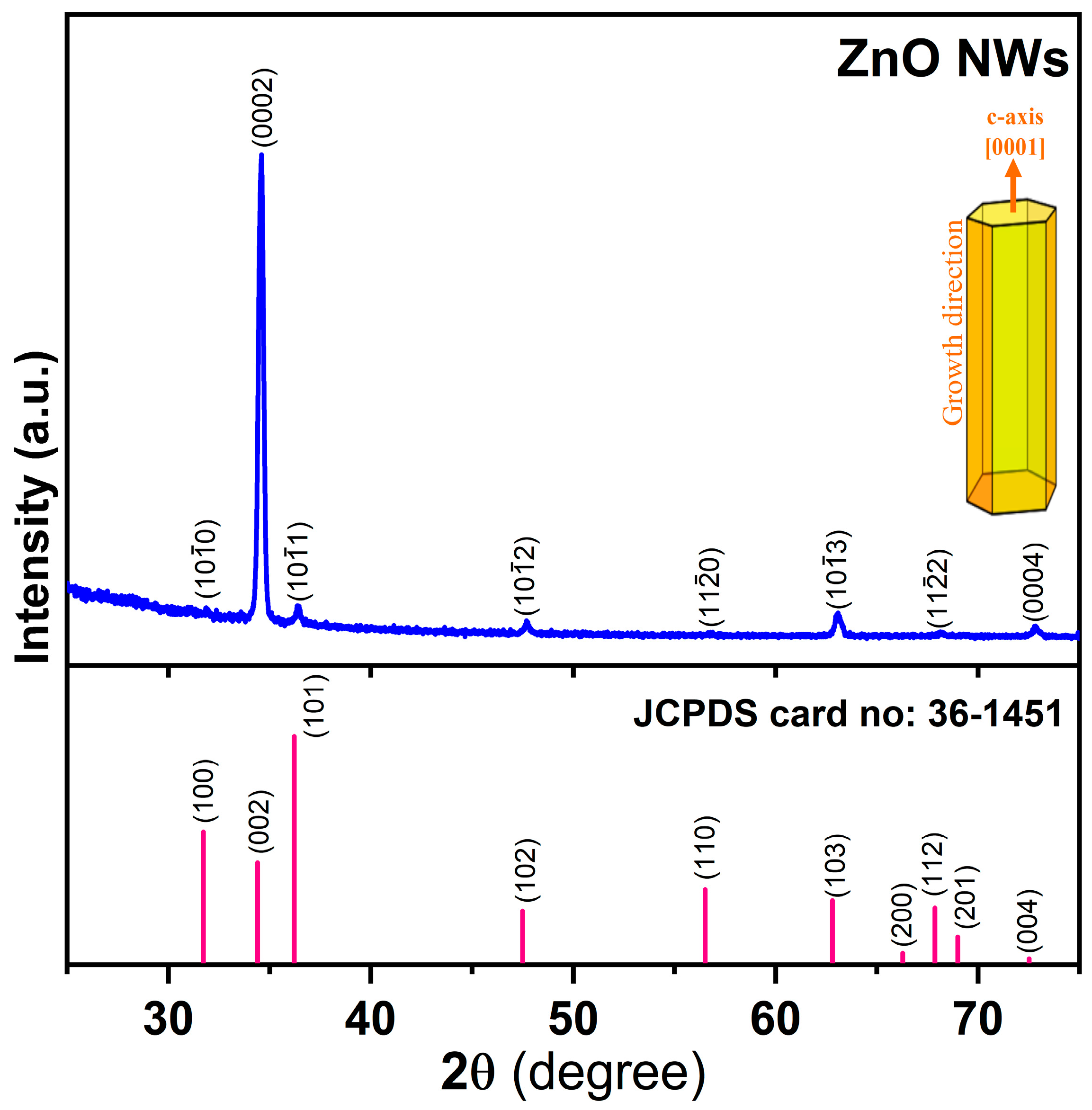
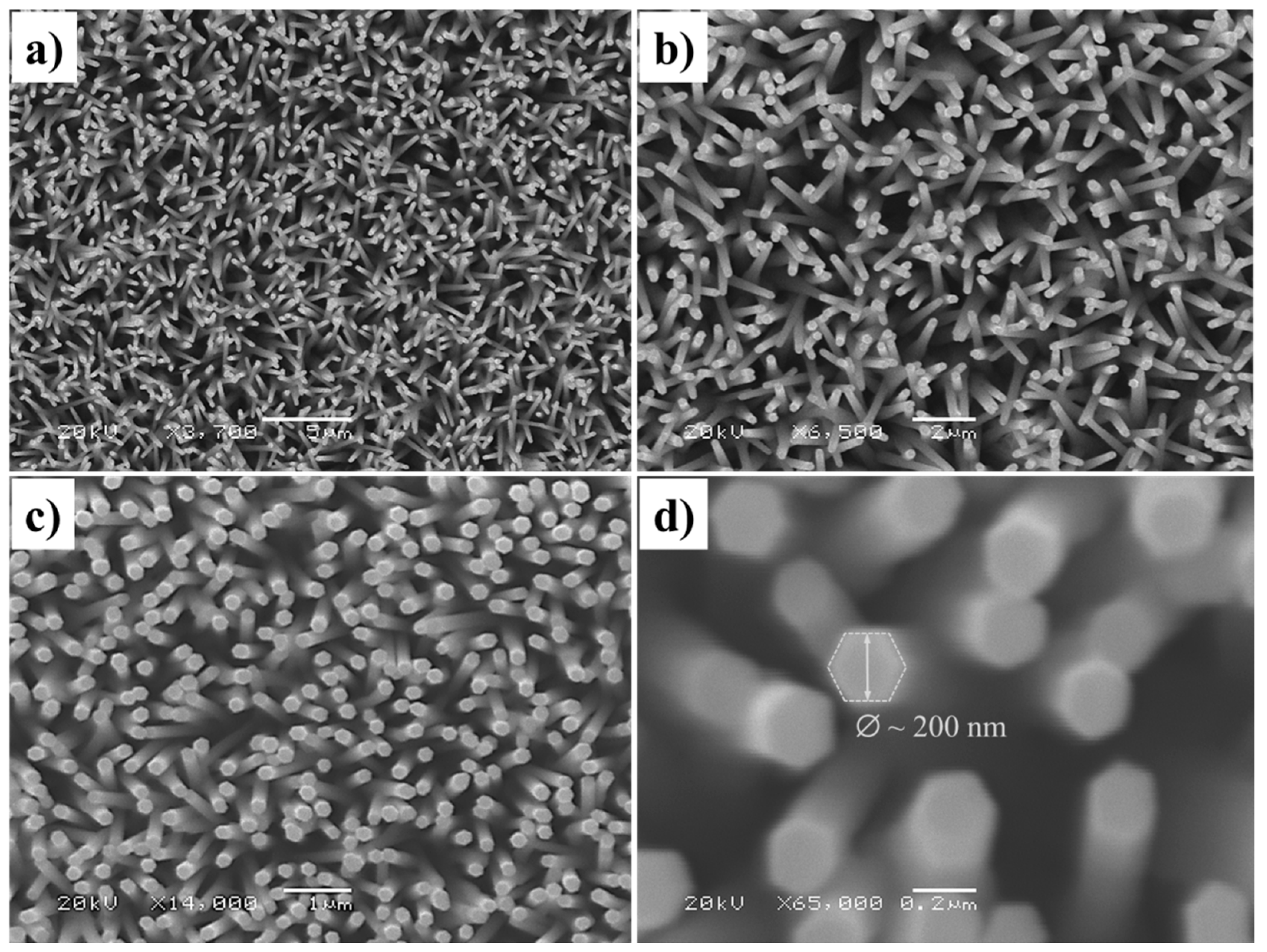
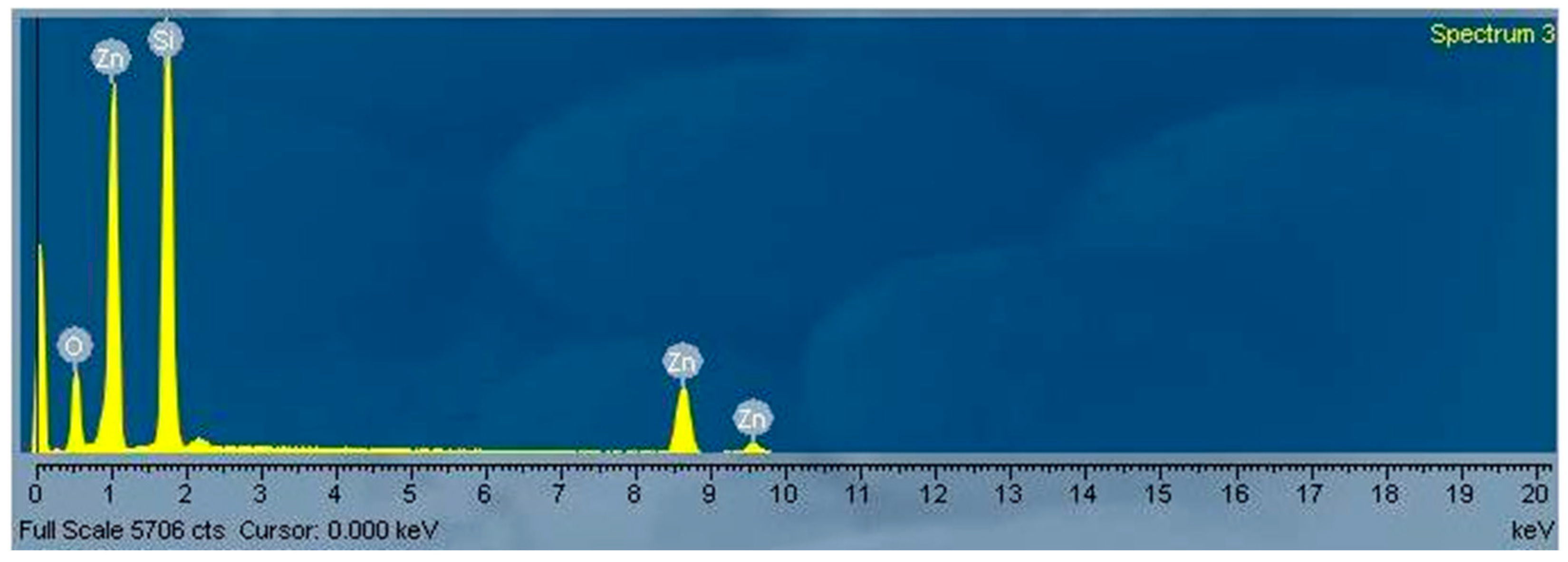
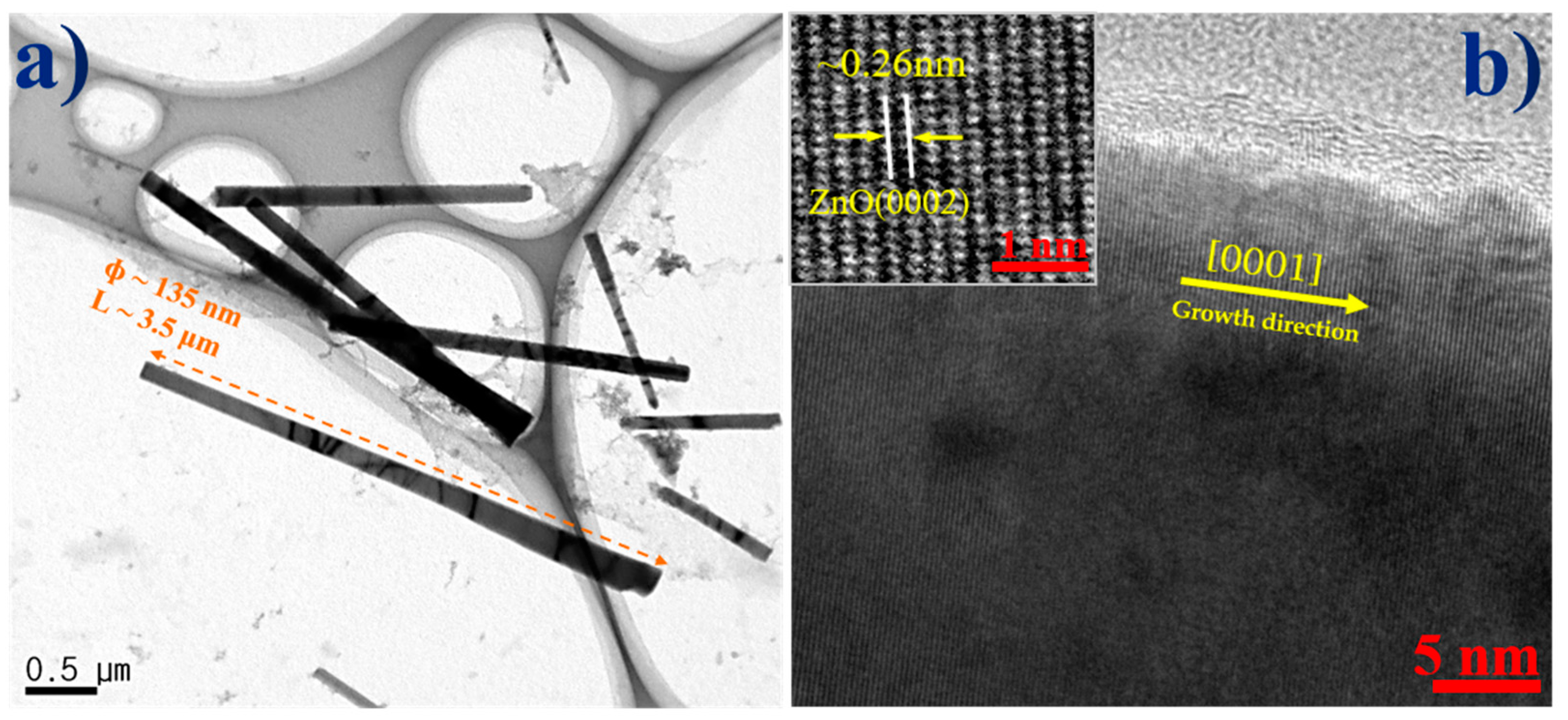
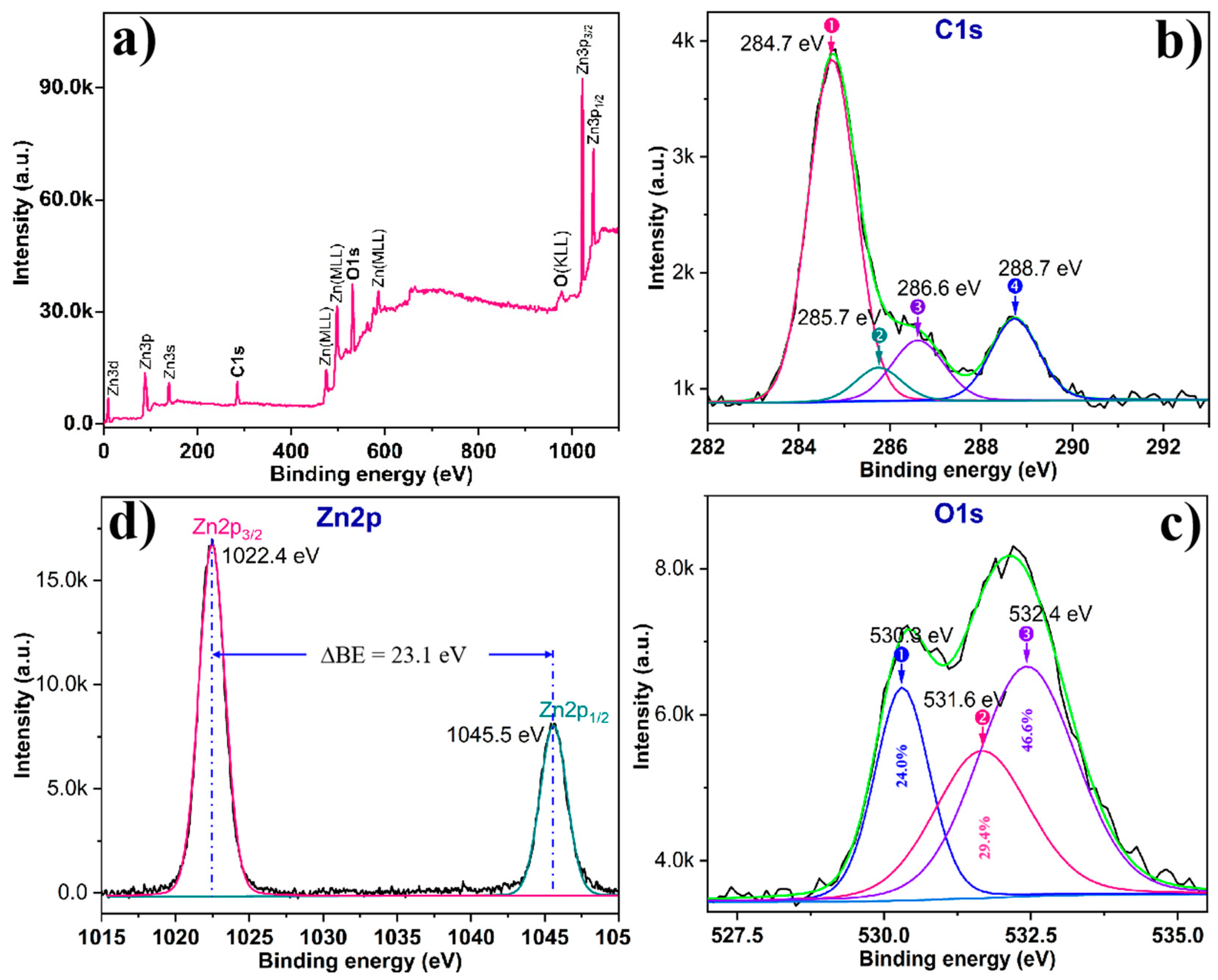
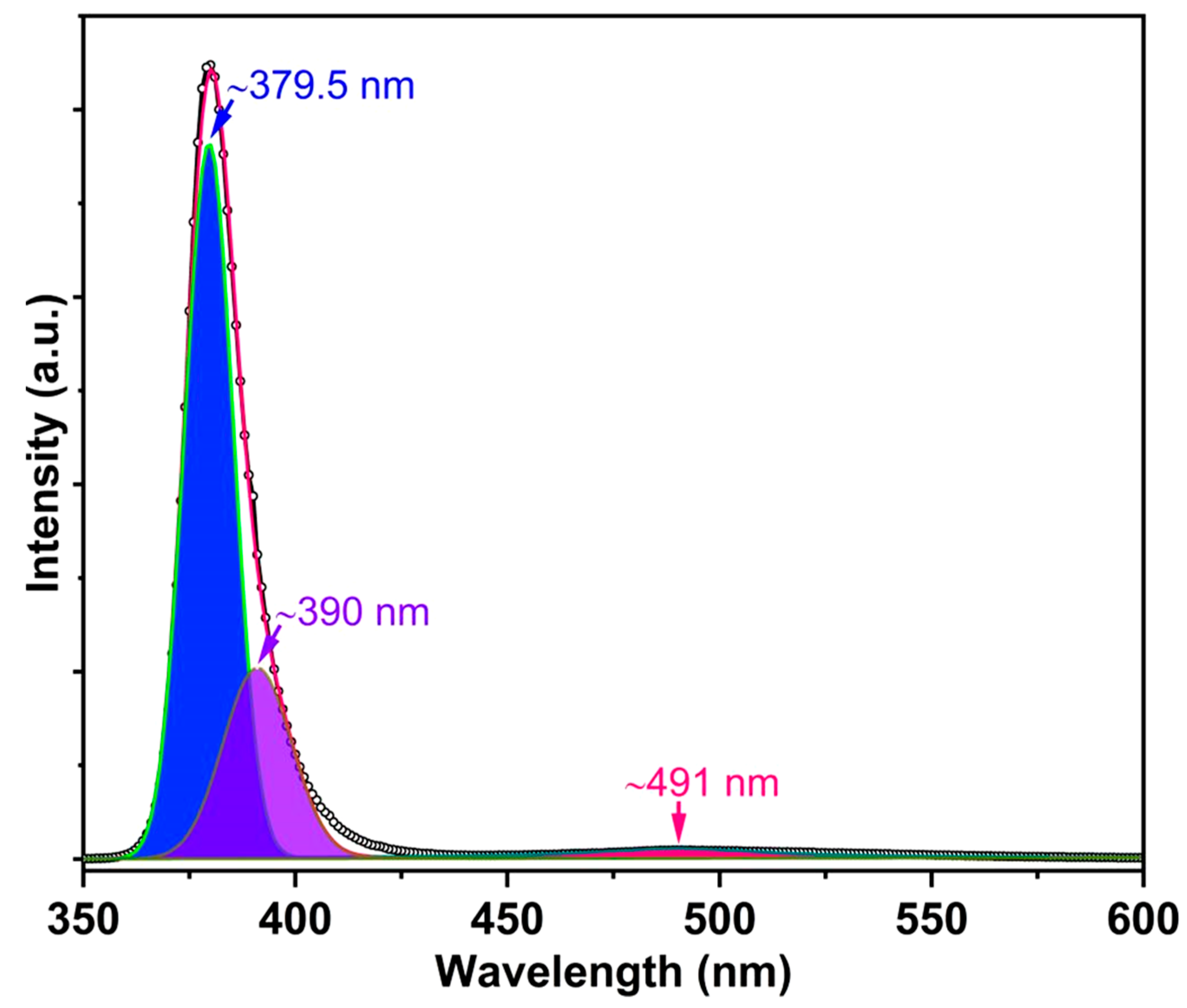
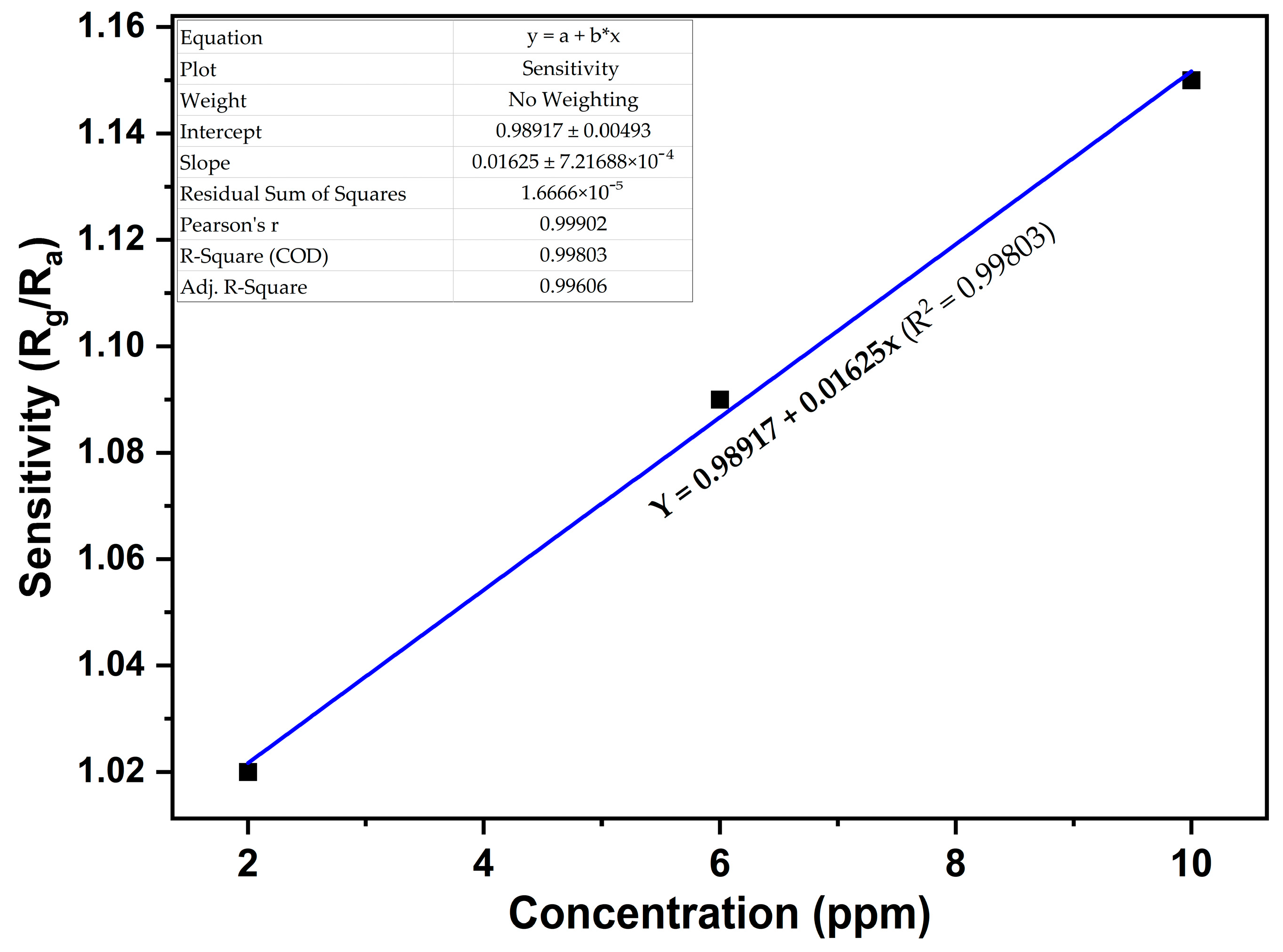
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaniabadi, Y.O.; Goudarzi, G.; Daryanoosh, S.M.; Borgini, A.; Tittarelli, A.; De Marco, A. Exposure to PM10, NO2, and O3 and Impacts on Human Health. Environ. Sci. Pollut. Res. Int. 2017, 24, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Mirzaei, A.; Na, H.G.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Oum, W.; Kim, S.S.; Kim, H.W. Porous Si nanowires for highly selective room temperature NO2 gas sensing. Nanotechnology 2018, 29, 294001. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2007; p. 405. [Google Scholar]
- Brunet, J.; Talazac, L.; Battut, V.; Pauly, A.; Blanc, J.P.; Germain, J.P.; Pellier, S.; Soulier, C. Evaluation of Atmospheric Pollution by Two Semiconductor Gas Sensors. Thin Solid Films 2001, 391, 308–313. [Google Scholar] [CrossRef]
- National Research Council. Emergency and Continuous Exposure Guidance Levels for Selected Submarine Contaminants; National Academies Press: Washington, DC, USA, 2009; Volume 3. [Google Scholar]
- Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide. Sensors 2022, 22, 5316. [Google Scholar] [CrossRef] [PubMed]
- Kamble, D.L.; Harale, N.S.; Patil, V.L.; Patil, P.S.; Kadam, L.D. Characterization and NO2 gas sensing properties of spray pyrolyzed SnO2 thin films. J. Anal. Appl. Pyrolysis 2017, 127, 38–46. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Ji, H.; Li, X.; Cai, S.; Zheng, C. Nanocasting Synthesis of In2O3 with Appropriate Mesostructured Ordering and Enhanced Gas-Sensing Property. ACS Appl. Mater. Interfaces 2014, 6, 401–409. [Google Scholar] [CrossRef]
- Kim, J.W.; Porte, Y.; Ko, K.Y.; Kim, H.; Myoung, J.M. Micropatternable Double-Faced ZnO Nanoflowers for Flexible Gas Sensor. ACS Appl. Mater. Interfaces 2017, 9, 32876–32886. [Google Scholar] [CrossRef]
- Boyadjiev, S.I.; Georgieva, V.; Stefan, N.; Stan, G.E.; Mihailescu, N.; Visan, A.; Mihailescu, I.N.; Besleaga, C.; Szilagyi, I.M. Characterization of PLD grown WO3 thin films for gas sensing. Appl. Surf. Sci. 2017, 417, 218–223. [Google Scholar] [CrossRef]
- Capone, S.; Benkovicova, M.; Forleo, A.; Jergel, M.; Manera, M.G.; Siffalovic, P.; Taurino, A.; Majkova, E.; Siciliano, P.; Vavra, I.; et al. Palladium/gamma-Fe2O3 nanoparticle mixtures for acetone and NO2 gas sensors. Sens. Actuators B 2017, 243, 895–903. [Google Scholar] [CrossRef]
- Luan, V.; Tien, H.N.; Hur, S.H.; Han, J.H.; Lee, W. Three dimensional porous nitrogen-doped NiO nanostructures as highly sensitive NO2 sensors. Nanomaterials 2017, 7, 313. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.; Tan, H.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. Zinc Oxide Nanomaterials for Photocatalytic Degradation of Methyl Orange: A Review. Nanosci. Nanotechnol. Lett. 2014, 6, 631–650. [Google Scholar] [CrossRef]
- Hahn, Y.B. Zinc oxide nanostructures and their applications. Korean J. Chem. Eng. 2011, 28, 1797–1813. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Miro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO Nanostructured Materials for Emerging Solar Cell Applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef]
- Wang, Z.L. ZnO Nanowire and Nanobelt Platform for Nanotechnology. Mater. Sci. Eng. R 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and Characterization of Ni, Co Doped ZnO Nanoparticles for Photocatalytic Applications. Appl. Surf. Sci. 2018, 448, 481–488. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zhuang, J.; Wang, L.; Zhang, J.; Li, H.; Liu, Z.; Han, Y.; Jiang, X.; Zhang, P. Improved selective acetone sensing properties of Co-Doped ZnO nanofibers by electrospinning. Sens. Actuators B 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Tain, S.; Yang, F.; Zeng, D.; Xie, C. Solution-Processed Gas Sensors Based on ZnO Nanorods Array with an Exposed (0001) Facet for Enhanced Gas-Sensing Properties. J. Phys. Chem. C 2012, 116, 10586–10591. [Google Scholar]
- Batzill, M.; Diebold, U. The Surface and Materials Science of Tin Oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B Chem. 2002, 128, 566–573. [Google Scholar] [CrossRef]
- Fioravanti, A.; Marani, P.; Morandi, S.; Lettieri, S.; Mazzocchi, M.; Sacerdoti, M.; Carotta, M.C. Growth Mechanisms of ZnO Micro-Nanomorphologies and Their Role in Enhancing Gas Sensing Properties. Sensors 2021, 21, 1331. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Kwak, W.K.; Yu, Y.T. Solvothermal synthesis of ZnO nanostructures and their morphology-dependent gas-sensing properties. ACS Appl. Mater. Interfaces. 2013, 5, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Obereggera, S.P.; Jonesb, O.A.H.; Spencer, M.J.S. Effect of nanostructuring of ZnO for gas sensing of nitrogen dioxide. Comput. Mater. Sci. 2017, 132, 104–115. [Google Scholar] [CrossRef]
- Carotta, M.C.; Cervi, A.; di Natale, V.; Gherardi, S.; Giberti, A.; Guidi, V.; Puzzovio, D.; Vendemiati, B.; Martinelli, G.; Sacerdoti, M.; et al. ZnO gas sensors: A comparison between nanoparticles and nanotetrapods-based thick films. Sens. Actuators B Chem. 2009, 137, 164–169. [Google Scholar] [CrossRef]
- Öztürk, S.; Kılınç, N.; Taşaltin, N.; Öztürk, Z.Z. A comparative study on the NO2 gas sensing properties of ZnO thin films, nanowires and nanorods. Thin Solid Films 2011, 520, 932–938. [Google Scholar] [CrossRef]
- Bai, S.; Hu, J.; Li, D.; Luo, R.; Chen, A.; Liu, C.C. Quantum-sized ZnO nanoparticles: Synthesis, characterization and sensing properties for NO2. J. Mater. Chem. 2011, 21, 12288–12294. [Google Scholar] [CrossRef]
- Gurav, K.V.; Gang, M.G.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; et al. Gas sensing properties of hydrothermally grown ZnO nanorods with different aspect ratios. Sens. Actuators B Chem. 2014, 190, 439–445. [Google Scholar] [CrossRef]
- Khai, T.V.; Thu, L.V.; Ha, L.T.T.; Thanh, V.M.; Lam, T.D. Structural, optical and gas sensing properties of vertically well-aligned ZnO nanowires grown on graphene/Si substrate by thermal evaporation method. Mater. Charact. 2018, 141, 296–317. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, C.; Zhang, M.; Zhang, Z.; Xie, E.; Zhou, J.; Han, W. Doping effect of In2O3 on structural and ethanol-sensing characteristics of ZnO nanotubes fabricated by electrospinning. Appl. Surf. Sci. 2015, 349, 615–621. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, X.; Wang, X.; Qian, J.; Bai, N.; Li, G.D. Effective detection of trace amount of explosive nitro-compounds by ZnO nanofibers with hollow structure. Sens. Actuators B Chem. 2016, 232, 564–570. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, L.; Yang, T.; Guo, X.; Wu, S.; Wang, S. 3D hierarchically porous ZnO structures and their functionalization by au nanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar] [CrossRef]
- Shouli, B.; Liangyuan, C.; Dianqing, L.; Wensheng, Y.; Pengcheng, Y.; Zhiyong, L.; Aifan, C.; Liu, C.C. Different morphologies of ZnO nanorods and their sensing property. Sens. Actuators B Chem. 2010, 146, 129–137. [Google Scholar] [CrossRef]
- Iversen, K.J.; Spencer, M.J.S. Effect of ZnO Nanostructure Morphology on the Sensing of H2S Gas. J. Phys. Chem. C 2013, 117, 26106–26118. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, S.T.; Nguyen, T.H.; Yang, W.; Noh, J.S. Effect of the Morphology of Solution-Grown ZnO Nanostructures on Gas-Sensing Properties. J. Am. Ceram. Soc. 2017, 100, 5629–5637. [Google Scholar] [CrossRef]
- Gupta, S.K.; Joshi, A.; Kaur, M. Development of gas sensors using ZnO nanostructures. J. Chem. Sci. 2010, 122, 57–62. [Google Scholar] [CrossRef]
- Wei, S.; Wang, S.; Zhang, Y.; Zhou, M. Different morphologies of ZnO and their ethanol sensing property. Sens. Actuators B Chem. 2014, 192, 480–487. [Google Scholar] [CrossRef]
- Cao, Y.; Jia, D.; Wang, R.; Luo, J. Rapid One-Step Room-Temperature Solid-State Synthesis and Formation Mechanism of Zno Nanorods as H2S-Sensing Materials. Solid-State Electron. 2013, 82, 67–71. [Google Scholar] [CrossRef]
- Wang, C.; Chu, X.; Wu, M. Detection of H2S Down to ppb Levels at Room Temperature Using Sensors Based on ZnO Nanorods. Sens. Actuators B Chem. 2006, 113, 320–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Tian, Z.; Hu, P.; Qin, X.; Yun, F. Gas-sensing properties of ITO materials with different morphologies prepared by sputtering. SN Appl. Sci. 2020, 2, 264. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Ko, H.; Jin, C.; Lee, W.I.; Lee, C. Enhanced Gas Sensing Properties of Branched ZnO Nanowires. Thin Solid Films 2013, 547, 241–245. [Google Scholar] [CrossRef]
- Ahn, M.W.; Park, K.S.; Heo, J.H.; Kim, D.W.; Choi, K.J.; Park, J.G. On-chip fabrication of ZnO-nanowire gas sensor with high gas sensitivity. Sens. Actuators B Chem. 2009, 138, 168–173. [Google Scholar] [CrossRef]
- Waclawik, E.R.; Chang, J.; Ponzoni, A.; Concina, I.; Zappa, D.; Comini, E.; Motta, N.; Faglia, G.; Sberveglieri Beilstein, G. Enhanced NO2 gas sensor response by chemical modification of nanowire surfaces. J. Nanotechnol. 2012, 3, 368–377. [Google Scholar]
- Cardoza-Contreras, M.N.; Romo-Herrera, J.M.; Ríos, L.A.; García-Gutiérrez, R.; Zepeda, T.A.; Contreras, O.E. Single ZnO Nanowire-Based Gas Sensors to Detect Low Concentrations of Hydrogen. Sensors 2015, 16, 30539–30544. [Google Scholar] [CrossRef]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Roldan Cuenya, B.; et al. Synthesis and Characterization of ZnO Nanowires for Nanosensor Applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and Ethanol Sensing Characteristics of ZnO Nanowire Gas Sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Wu, W.Y.; Ting, J.M.; Huang, P.J. Electrospun ZnO nanowires as gas sensors for ethanol detection. Nanoscale Res. Lett. 2009, 4, 513–517. [Google Scholar] [CrossRef]
- Hieu, N.V.; Chien, N.D. Low-temperature growth and ethanol-sensing characteristics of quasi-one-dimensional ZnO nanostructures. Phys. B Condens. Matter 2008, 403, 50–56. [Google Scholar] [CrossRef]
- Pivert, M.L.; Martin, N.; Leprince-Wang, Y. Hydrothermally Grown ZnO Nanostructures for Water Purification via Photocatalysis. Crystals 2022, 12, 308. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Yan, B.; Yu, T. Growth mechanism and diameter control of well-aligned small-diameter ZnO nanowire arrays synthesized by a catalyst-free thermal evaporation method. Nanotechnology 2009, 20, 495604. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.J.; Xi, Z.H.; Zhang, X.D.; Song, J.H.; Wang, R.M.; Xu, J.; Xue, Z.Q.; Yu, D.P. Thermal evaporation synthesis of zinc oxide nanowires. Appl. Phys. A 2005, 80, 1527–1530. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhao, S.; Zhou, G.; Zhou, Y.; Qi, J. Synthesis of well-aligned ZnO nanowires by simple physical vapor deposition on c-oriented ZnO thin films without catalysts or additives. Appl. Phys. Lett. 2005, 86, 024108. [Google Scholar] [CrossRef]
- Ashraf, S.; Jones, A.C.; Bacsa, J.; Steiner, A.; Chalker, P.R.; Beahan, P.; Hindley, S.; Odedra, R.; Williams, P.A.; Heys, P.N. MOCVD of vertically aligned ZnO nanowires using bidentate ether adducts of dimethylzinc. Chem. Vap. Depos. 2011, 17, 45–53. [Google Scholar] [CrossRef]
- Woong, L.; Min-Chang, J.; Jae-Min, M. Fabrication and application potential of ZnO nanowires grown on GaAs(002) substrates by metal–organic chemical vapour deposition. Nanotechnology 2004, 15, 254. [Google Scholar]
- Khai, T.V.; Maneeratanasarn, P.; Choi, B.G.; Ham, H.; Shim, K.B. Diameter-and density-controlled synthesis of well-aligned ZnO nanowire arrays and their properties using a thermal evaporation technique. Phys. Status Solidi A 2012, 209, 1498–1510. [Google Scholar] [CrossRef]
- Khai, T.V.; Thu, L.V.; Lam, T.D. Vertically well-aligned ZnO nanowire arrays directly synthesized from Zn vapor deposition without catalyst. J. Electron. Mater. 2016, 45, 2601–2607. [Google Scholar] [CrossRef]
- Yang, Z.; Dou, X.; Zhang, S.; Guo, L.; Zu, B.; Wu, Z.; Zeng, H. A High-Performance Nitro-Explosives Schottky Sensor Boosted by Interface Modulation. Adv. Funct. Mater. 2015, 25, 4039–4048. [Google Scholar] [CrossRef]
- Ham, H.; Shen, G.; Cho, J.; Lee, T.J.; Seo, S.; Lee, C. Vertically Aligned ZnO Nanowires Produced by a Catalyst-Free Thermal Evaporation Method and Their Field Emission Properties. Chem. Phys. Lett. 2005, 404, 69–73. [Google Scholar] [CrossRef]
- Kavakebi, M.; Jamali-sheini, F. Ultrasonic Synthesis of Zn-Doped CdO Nanostructures and Their Optoelectronic Properties. Trans. Nonferrous Met. Soc. China 2018, 28, 2255–2264. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Pearson Education Limited: London, UK, 2014. [Google Scholar]
- Thool, G.S.; Singh, A.K.; Singh, R.S.; Gupta, A.; Susan, A.B.H. Facile synthesis of flat crystal ZnO thin films by solution growth method: A micro-structural investigation. J. Saudi Chem. Soc. 2014, 18, 712–721. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.L.; Pelligra, C.; Chen, C.H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Wagner, C.; Gale, L.; Raymond, R. Two-dimensional chemical state plots: A standardized data set for use in identifying chemical states by X-ray photoelectron spectroscopy. Anal. Chem. 1979, 51, 466–482. [Google Scholar] [CrossRef]
- Saha, T.; Achath Mohanan, A.; Swamy, V.; Guo, N.; Ramakrishnan, N. An Optimal Thermal Evaporation Synthesis of C-Axis Oriented ZnO Nanowires with Excellent UV Sensing and Emission Characteristics. Mater. Res. Bull. 2016, 77, 147–154. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Probing the Dominance of Interstitial Oxygen Defects in ZnO Nanoparticles through Structural and Optical Characterizations. Ceram. Int. 2014, 40, 14569–14578. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and Optical Studies of Different Morphologies of ZnO Nanostructures Prepared by Microwave Methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Das, J.; Pradhan, S.K.; Sahu, D.R.; Mishra, D.K.; Sarangi, S.N.; Nayak, B.B.; Verma, S.; Roul, B.K. Micro-Raman and XPS Studies of Pure ZnO Ceramics. Phys. B Condens. Matter 2010, 405, 2492–2497. [Google Scholar] [CrossRef]
- Zheng, J.H.; Jiang, Q.; Lian, J.S. Synthesis and Optical Properties of Flower-like ZnO Nanorods by Thermal Evaporation Method. Appl. Surf. Sci. 2011, 257, 5083–5087. [Google Scholar] [CrossRef]
- Li, C.C.; Du, Z.F.; Li, L.M.; Yu, H.C.; Wan, Q.; Wang, T.H. Surface-Depletion Controlled Gas Sensing of ZnO Nanorods Grown at Room Temperature. Appl. Phys. Lett. 2007, 91, 032101. [Google Scholar] [CrossRef]
- Ghosh, B.; Ray, S.C.; Pattanaik, S.; Sarma, S.; Mishra, D.K.; Pontsho, M.; Pong, W.F. Tuning of the Electronic Structure and Magnetic Properties of Xenon Ion Implanted Zinc Oxide. J. Phys. D Appl. Phys. 2018, 51, 095304. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišic, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO Nanorods Prepared by a Hydrothermal Method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Karamat, S.; Rawat, R.S.; Lee, P.; Tan, T.; Ramanujan, R. Structural, Elemental, Optical and Magnetic Study of Fe Doped ZnO and Impurity Phase Formation. Prog. Nat. Sci. Mater. 2014, 24, 142–149. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.L.; Zhang, G.H.; Xue, H.; Ma, S.Y. Blue shift of optical band gap in Er-doped ZnO thin films deposited by direct current reactive magnetron sputtering technique. Physica E Low Dimens. Syst. Nanostruct. 2010, 42, 1713–1716. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Qin, Z. ZnO/ZnFe2O4 Nanocomposite as a Broad-Spectrum Photo-Fenton-like Photocatalyst with near-Infrared Activity. Catal. Sci. Technol. 2017, 7, 2236–2244. [Google Scholar] [CrossRef]
- Papari, G.P.; Silvestri, B.; Vitiello, G.; de Stefano, L.; Rea, I.; Luciani, G.; Aronne, A.; Andreone, A. Morphological, Structural, and Charge Transfer Properties of F-Doped ZnO: A Spectroscopic Investigation. J. Phys. Chem. C. 2017, 121, 16012–16020. [Google Scholar] [CrossRef]
- Lisachenko, A.A. Study of self-sensitization of wide-gap oxides to visible light by intrinsic defects: From Terenin to the present days. J. Photochem. Photobiol. A Chem. 2018, 354, 47–60. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
- Hsu, N.E.; Hung, W.K.; Chen, Y.F. Origin of Defect Emission Identified by Polarized Luminescence from Aligned ZnO Nanorods. J. Appl. Phys. 2004, 96, 4671–4673. [Google Scholar] [CrossRef]
- Liu, M.; Kitai, A.H.; Mascher, P. Point Defects and Luminescence Centres in Zinc Oxide and Zinc Oxide Doped with Manganese. J. Lumin. 1992, 54, 35–42. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Jia, Y. Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A Comparative Analysis of Deep Level Emission in ZnO Layers Deposited by Various Methods. J. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, Y.; Zhao, K.; Liu, Z.; Han, P.; Wang, S.; Xiang, W.; Chen, Z.; Lü, H.; Cheng, B.; et al. Violet luminescence emitted from Ag-nanocluster doped ZnO thin films grown on fused quartz substrates by pulsed laser deposition. Phys. B Condens. Matter 2006, 373, 154–156. [Google Scholar] [CrossRef]
- Khai, T.V.; Kwak, D.S.; Kwon, Y.J.; Shim, K.B.; Kim, H.W. Catalyst-free thermally-evaporated growth and optical properties of ZnO nanowires on Si, GaN and sapphire substrates. Cryst. Res. Technol. 2013, 48, 75–86. [Google Scholar] [CrossRef]
- Yousefi, R.; Zak, A.K. Growth and characterization of ZnO nanowires grown on the Si(111) and Si(100) substrates: Optical properties and biaxial stress of nanowires. Mater. Sci. Semicond. 2011, 14, 170–174. [Google Scholar] [CrossRef]
- Hassan, N.K.; Hashim, M.R.; Mahdi, M.A.; Allam, N.K. A catalyst-free growth of ZnO nanowires on Si (100) substrates: Effect of substrate position on morphological, structural and optical properties. ECS J. Solid State Sci. Technol. 2012, 1, P86–P89. [Google Scholar] [CrossRef]
- Umar, A.; Hahn, Y.B. Aligned hexagonal coaxial-shaped ZnO nanocolumns on steel alloy by thermal evaporation. Appl. Phys. Lett. 2006, 88, 173120. [Google Scholar] [CrossRef]
- Ahmad, N.F.; Yasui, K.; Hashim, A.M. Seed/catalyst-free growth of zinc oxide on graphene by thermal evaporation: Effects of substrate inclination angles and graphene thicknesses. Nanoscale Res. Lett. 2015, 10, 10. [Google Scholar] [CrossRef][Green Version]
- ElZein, B.; Yao, Y.; Barham, A.S.; Dogheche, E.; Jabbour, G.E. Toward the Growth of Self-Catalyzed ZnO Nanowires Perpendicular to the Surface of Silicon and Glass Substrates, by Pulsed Laser Deposition. Materials 2020, 13, 4427. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yang, S.; Jing, W.; Jiang, K.C.; Jiang, Z.; Liu, H.; Li, L. Surface Plasmon Enhanced Photoluminescence of ZnO Nanorods by Capping Reduced Graphene Oxide Sheets. Opt. Express 2014, 22, 11436–11445. [Google Scholar] [CrossRef] [PubMed]
- Khai, T.V.; Long, L.N.; Khoi, N.H.T.; Hoc Thang, N. Effects of Hydrothermal Reaction Time on the Structure and Optical Properties of ZnO/Graphene Oxide Nanocomposites. Crystals 2022, 12, 1825. [Google Scholar] [CrossRef]
- Li, L.; Yao, C.; Wu, L.; Jiang, K.; Hu, Z.; Xu, N.; Sun, J.; Wu, J. ZnS Covering of ZnO Nanorods for Enhancing UV Emission from ZnO. J. Phys. Chem. C 2021, 125, 13732–13740. [Google Scholar] [CrossRef]
- Yin, Y.; Sun, Y.; Yu, M.; Liu, X.; Yang, B.; Liu, D.; Liu, S.; Cao, W.; Ashfold, M.N.R. Controlling the hydrothermal growth and the properties of ZnO nanorod arrays by pre-treating the seed layer. RSC Adv. 2014, 4, 44452. [Google Scholar] [CrossRef]
- Shakti, N.; Prakash, A.; Mandal, T.; Katiyar, M. Processing Temperature Dependent Morphological and Optical Properties of ZnO Nanorods. Mater. Sci. Semicond. 2014, 20, 55–60. [Google Scholar] [CrossRef]
- Opoku, C.; Dahiya, A.S.; Cayrel, F.; Poulin-Vittrant, G.; Alquier, D.; Camara, N. Fabrication of Field-Effect Transistors and Functional Nanogenerators Using Hydrothermally Grown ZnO Nanowires. RSC Adv. 2015, 5, 69925–69931. [Google Scholar] [CrossRef]
- Sohn, J.I.; Cha, S.N.; Song, B.G.; Lee, S.; Kim, S.M.; Ku, J.; Kim, H.J.; Park, Y.J.; Choi, B.L.; Wang, Z.L.; et al. Engineering of Efficiency Limiting Free Carriers and an Interfacial Energy Barrier for an Enhancing Piezoelectric Generation. Energy Environ. Sci. 2012, 6, 97–104. [Google Scholar] [CrossRef]
- Kushwaha, A.; Aslam, M. Defect Induced High Photocurrent in Solution Grown Vertically Aligned ZnO Nanowire Array Films. J. Appl. Phys. 2012, 112, 054316. [Google Scholar] [CrossRef]
- Das, S.N.; Kar, J.P.; Choi, J.H.; Lee, T.I.; Moon, K.J.; Myoung, J.M. Fabrication and Characterization of ZnO Single Nanowire-Based Hydrogen Sensor. J. Phys. Chem. C 2010, 114, 1689–1693. [Google Scholar] [CrossRef]
- Selim, F.A.; Weber, M.H.; Solodovnikov, D.; Lynn, K.G. Nature of native defects in ZnO. Phys. Rev. Lett. 2007, 99, 085502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Wang, N.; Quan, X.; Chen, Y. Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep. Purif. Technol. 2008, 62, 727–732. [Google Scholar] [CrossRef]
- Baradaran, M.; Ghodsi, F.; Bittencourt, C.; Llobet, E. The role of Al concentration on improving the photocatalytic performance of nanostructured ZnO/ZnO:Al/ZnO multilayer thin films. J. Alloys Compd. 2019, 788, 289–301. [Google Scholar] [CrossRef]
- Goldberger, J.; Sirbuly, D.J.; Law, M.; Yang, P. ZnO Nanowire Transistors. J. Phys. Chem. B 2005, 109, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, W.; Bai, S.; Qin, Y. Synthesis of High Crystallinity ZnO Nanowire Array on Polymer Substrate and Flexible Fiber-Based Sensor. ACS Appl. Mater. Interfaces 2011, 3, 4197–4200. [Google Scholar] [CrossRef] [PubMed]
- Das, S.N.; Moon, K.J.; Kar, J.P.; Choi, J.H.; Xiong, J.; Lee, T.I.; Myoung, J.M. ZnO Single Nanowire-Based UV Detectors. Appl. Phys. Lett. 2010, 97, 022103. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native Point Defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Ding, M.; Guo, Z.; Zhou, L.; Fang, X.; Zhang, L.; Zeng, L.; Xie, L.; Zhao, H. One-demensional Zinc oxide nanomaterials for application in high-performance advanced opolectronic devices. Crystals 2018, 8, 223. [Google Scholar] [CrossRef]
- Nisha, R.; Madhusoodanan, K.N.; Vimalkumar, T.V.; Vijayakumar, K.P. Gas sensing application of nanocrystalline zinc oxide thin films prepared by spray pyrolysis. Bull. Mater. Sci. 2015, 38, 583–591. [Google Scholar]
- An, W.; Wu, X.; Zeng, X.C. Adsorption of O2, H2, CO, NH3, and NO2 on ZnO Nanotube: A Density Functional Theory Study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors for Detection of Benzene, Toluene and Xylene (BTX) Gases: A Review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. How Shell Thickness Can Affect the Gas Sensing Properties of Nanostructured Materials: Survey of Literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures-Based Gas Sensors: A Review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Aziz, A.A.; Abdullah, M.J.; Ahmed, N.M. Conductometric Gas Sensing Based on ZnO Thin Films: An Impedance Spectroscopy Study. ECS J. Solid State Sci. Technol. 2018, 7, P487–P490. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.; Han, N.; Shi, Y.; Hu, P.; Li, H.; Wang, J. Aerosol assisted chemical vapour deposition of nanostructured ZnO thin films for NO2 and ethanol monitoring. Ceram. Int. 2020, 46, 15152–15158. [Google Scholar] [CrossRef]
- Chen, E.S.; Wentworth, W.E.; Chen, E.C.M. The electron affinities of NO and O2. J. Mol. Struct. 2002, 606, 1–7. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, H.; Liu, R.; Du, B. Study on the structure and property for the NO2 + NO2− electron transfer system. J. Mol. Struct. THEOCHEM 2001, 545, 179–186. [Google Scholar] [CrossRef]
- Šulka, M.; Pitoňák, M.; Neogrády, P.; Urban, M. Electron Affinity of the O2 Molecule: CCSD(T) Calculations Using the Optimized Virtual Orbitals Space Approach. Int. J. Quantum Chem. 2008, 108, 2159–2171. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Inamdar, S.I.; Agawane, G.L.; Kim, J.H.; Rajpure, K.Y. Synthesis of Fast Response, Highly Sensitive and Selective Ni:ZnO Based NO2 Sensor. Chem. Eng. J. 2016, 286, 36–47. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Urchin-like ZnOnanorod arrays for gas sensing applications. Cryst. Eng. Comm. 2010, 12, 3419–3421. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-Temperature Gas Sensing of ZnO-Based Gas Sensor: A Review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, T.; Chuai, M.; Xiao, B.; Zhang, M. Synthesis of ZnO nanosheet arrays with exposed (100) facets for gas sensing applications. Phys. Chem. Chem. Phys. 2016, 18, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Na, H.G.; Mirzaei, A.; Bang, J.H.; Oum, W.; Han, S.; Choi, S.W.; Kim, M.; Jin, C.; Kim, S.S.; et al. Room-temperature NO2 sensor based on electrochemically etched porous silicon. J. Alloys Compd. 2019, 811, 151975. [Google Scholar] [CrossRef]
- Parellada-Monreal, L.; Castro-Hurtado, I.; Martínez Calderón, M.; Presmanes, L.; Mandayo, G.G. Laser-induced periodic surface structures on ZnO thin film for high response NO2 detection. Appl. Surf. Sci. 2019, 476, 569–575. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO Nanowires Synthesized by Vapor Trapping CVD Method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Ahn, M.W.; Park, K.S.; Heo, J.H.; Park, J.G.; Kim, D.W.; Choi, K.J.; Lee, J.H.; Hong, S.H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2008, 93, 263103–263105. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Meng, L.; Sun, Z.; Chen, Z.; Huang, X.; Qin, Y. Adjustment of Oxygen Vacancy States in ZnO and Its Application in Ppb-Level NO2 Gas Sensor. Sci. Bull. 2020, 65, 1650–1658. [Google Scholar] [CrossRef]
- Li, Y.; Zu, B.; Guo, Y.; Li, K.; Zeng, H.; Dou, X. Surface Superoxide Complex Defects-Boosted Ultrasensitive Ppb-Level NO2 Gas Sensors. Small 2016, 12, 1420–1424. [Google Scholar] [CrossRef]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Schaub, R.; Wahlström, E.; Rønnau, A.; Lagsgaard, E.; Stensgaard, I.; Besenbacher, F. Oxygen-Mediated Diffusion of Oxygen Vacancies on the TiO2(110) Surface. Science 2003, 299, 377–379. [Google Scholar] [CrossRef]
- Spencer, M.J.S.; Yarovsky, I. ZnO Nanostructures for Gas Sensing: Interaction of NO2, NO, O, and N with the ZnO Surface. J. Phys. Chem. C 2010, 114, 10881–10893. [Google Scholar] [CrossRef]
- Zou, C.; Liang, F.; Xue, S. Synthesis and oxygen vacancy related NO2 gas sensing properties of ZnO:Co nanorods arrays gown by a hydrothermal method. Appl. Surf. Sci. 2015, 353, 1061–1069. [Google Scholar] [CrossRef]
- Lee, B.W.; Kim, T.S.; Goswami, S.K.; Oh, E. Gas Sensitivity and Point Defects in Sonochemically Grown ZnO Nanowires. J. Korean Phys. Soc. 2012, 60, 415–419. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective Hydrogen Gas Nanosensor Using Individual ZnO Nanowire with Fast Response at Room Temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Choi, S.W.; Park, J.Y.; Kim, S.S. Dependence of Gas Sensing Properties in ZnO Nanofibers on Size and Crystallinity of Nanograins. J. Mater. Res. 2011, 26, 1662–1665. [Google Scholar] [CrossRef]
- Morandi, S.; Fioravanti, A.; Cerrato, G.; Lettieri, S.; Sacerdoti, M.; Carotta, M. Facile synthesis of ZnO nano-structures: Morphology influence on electronic properties. Sens. Actuators B Chem. 2017, 249, 581–589. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Khatibani, A.B. Characterization and Ethanol Sensing Performance of Sol–Gel Derived Pure and Doped Zinc Oxide Thin Films. J. Electron. Mater. 2019, 48, 3784–3793. [Google Scholar] [CrossRef]
- Perekrestov, V.; Latyshev, V.; Kornyushchenko, A.; Kosminska, Y. Formation, Charge Transfer, Structural and Morphological Characteristics of ZnO Fractal-Percolation Nanosystems. J. Electron. Mater. 2019, 48, 2788–2793. [Google Scholar] [CrossRef]
- GuL, M.; Amin, M.; Abbas, M.; Ilyas, S.Z.; Shah, N.A. Synthesis and Characterization of Magnesium Doped ZnO Nanostructures: Methane (CH4) Detection. J. Mater. Sci. Mater. Electron. 2019, 30, 5257–5265. [Google Scholar] [CrossRef]
- Chetna; Kumar, S.; Garg, A.; Chowdhuri, A.; Jain, A.; Kapoor, A. A Novel Method of Electrochemically Growing ZnO Nanorods on Graphene Oxide as Substrate for Gas Sensing Applications. Mater. Res. Express 2019, 6, 075039. [Google Scholar] [CrossRef]
- Aydin, H.; Yakuphanoglu, F.; Aydin, C. Al-Doped ZnO as a Multifunctional Nanomaterial: Structural, Morphological, Optical and Low-Temperature Gas Sensing Properties. J. Alloys Compd. 2019, 773, 802–811. [Google Scholar] [CrossRef]
- Shooshtari, M.; Pahlavan, S.; Rahbarpour, S.; Ghafoorifard, H. Investigating organic vapor sensing properties of composite carbon nanotube-zinc oxide nanowire. Chemosensors 2022, 10, 205. [Google Scholar] [CrossRef]
- Shi, L.; Naik, A.J.T.; Goodall, J.B.M.; Tighe, C.; Gruar, R.; Binions, R.; Parkin, I.; Darr, J. Highly sensitive ZnO nanorod- and nanoprism-based NO2 gas sensors: Size and shape control using a continuous hydrothermal pilot plant. Langmuir 2013, 29, 10603–10609. [Google Scholar] [CrossRef] [PubMed]
- Han, H.V.; Hoa, N.D.; Tong, P.V.; Nguyen, H.; Hieu, N.V. Single-Crystal Zinc Oxide Nanorods with Nanovoids as Highly Sensitive NO2 Nanosensors. Mater. Lett. 2013, 94, 41–43. [Google Scholar] [CrossRef]
- Park, J.; Oh, J.Y. Highly-sensitive NO2 Detection of ZnO Nanorods Grown by a Sonochemical Process. J. Korean Phys. Soc. 2009, 55, 1119–1122. [Google Scholar] [CrossRef]
- Chougule, M.; Sen, S.; Patil, V. Fabrication of Nanostructured ZnO Thin Film Sensor for NO2 Monitoring. Ceram. Int. 2012, 38, 2685–2692. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.Y.; Jung, S.H.; Cho, S.; Kim, J.C.; Lee, K.H.; Kang, S.W.; Kim, J.; Yun, J.Y.; Jeong, S.H. High-Performance NO2 Gas Sensor Based on ZnO Nanorod Grown by Ultrasonic Irradiation. Sens. Actuators B Chem. 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, selectivity, and stability of gas-sensitive metal-oxide nanostructures. In Metal Oxide Nanostructures and Their Applications; Umar, A., Hang, Y.B., Eds.; American Scientific Publishers: Valencia, CA, USA, 2010; pp. 31–35. [Google Scholar]
- Yuan, Z.; Yin, L.; Ding, H.; Huang, W.; Shuai, C.; Deng, J. One-step synthesis of single-crystalline ZnO nanowires for the application of gas sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 11559–11565. [Google Scholar] [CrossRef]
- Priya, S.; Halder, J.; Mandal, D.; Chowdhury, A.; Singh, T.; Chandra, A. Hierarchical SnO2 nanostructures for potential VOC sensor. J. Mater. Sci. 2021, 56, 9883–9893. [Google Scholar] [CrossRef]
- Sahin, Y.; Öztürk, S.; Kılınç, N.; Käsemen, A.; Erkovane, M.; Ozturk, Z.Z. Electrical conduction and NO2 gas sensing properties of ZnO nanorods. Appl. Surf. Sci. 2014, 303, 90–96. [Google Scholar] [CrossRef]
- Öztürk, S.; Kılınç, N.; Öztürk, Z.Z. Fabrication of ZnO nanorods for NO2 sensor applications: Effect of dimensions and electrode position. J. Alloys Compd. 2013, 581, 196–201. [Google Scholar] [CrossRef]

| MFC-1 NO2 (sccm) | MFC-2 Dry Air (sccm) | (ppm) |
|---|---|---|
| 1 | 499 | 2 |
| 3 | 498 | 6 |
| 5 | 495 | 10 |
| Morphology | Synthesis Method | IUV/IDL | References |
|---|---|---|---|
| ZnO NWs | Pulsed laser deposition | ~11.9–45.4 | [94] |
| ZnO nanorods | Hydrothermal | ~0.3 | [95] |
| ZnO nanorods | Hydrothermal | ~0.6 | [96] |
| ZnO nanorods | Hydrothermal | >19 | [97] |
| ZnO nanorods | Hydrothermal | ~0.6–10 | [98] |
| ZnO nanorods | Thermal decomposition | ~1.8 | [99] |
| ZnO NWs | Physical vapor deposition | 2.5–4.7 | [90] |
| ZnO NWs | Thermal evaporation | ~1.4 | [91] |
| ZnO nanocolumns | Thermal evaporation | ~2.4 | [92] |
| ZnO NWs | Thermal evaporation | ~16.2 | [93] |
| ZnO NWs | Thermal evaporation | >77 | Present work |
| NO2 Conc. (ppm) | Sensitivity (S) |
|---|---|
| 2 | 1.02 |
| 6 | 1.09 |
| 10 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thach, P.H.; Khai, T.V. Thermal Evaporation Synthesis, Optical and Gas-Sensing Properties of ZnO Nanowires. Crystals 2023, 13, 1380. https://doi.org/10.3390/cryst13091380
Thach PH, Khai TV. Thermal Evaporation Synthesis, Optical and Gas-Sensing Properties of ZnO Nanowires. Crystals. 2023; 13(9):1380. https://doi.org/10.3390/cryst13091380
Chicago/Turabian StyleThach, Pham Hong, and Tran Van Khai. 2023. "Thermal Evaporation Synthesis, Optical and Gas-Sensing Properties of ZnO Nanowires" Crystals 13, no. 9: 1380. https://doi.org/10.3390/cryst13091380
APA StyleThach, P. H., & Khai, T. V. (2023). Thermal Evaporation Synthesis, Optical and Gas-Sensing Properties of ZnO Nanowires. Crystals, 13(9), 1380. https://doi.org/10.3390/cryst13091380






