Highly Efficient Orange-Red Emission in Sm3+-Doped Yttrium Gallium Garnet Single Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystal Preparation
2.2. Physical Measurements
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Density Measurement
3.3. Absorption Spectra
3.4. Luminescence Properties
3.4.1. PLE Spectrum
3.4.2. PL Spectrum
3.5. Chromaticity Coordinates
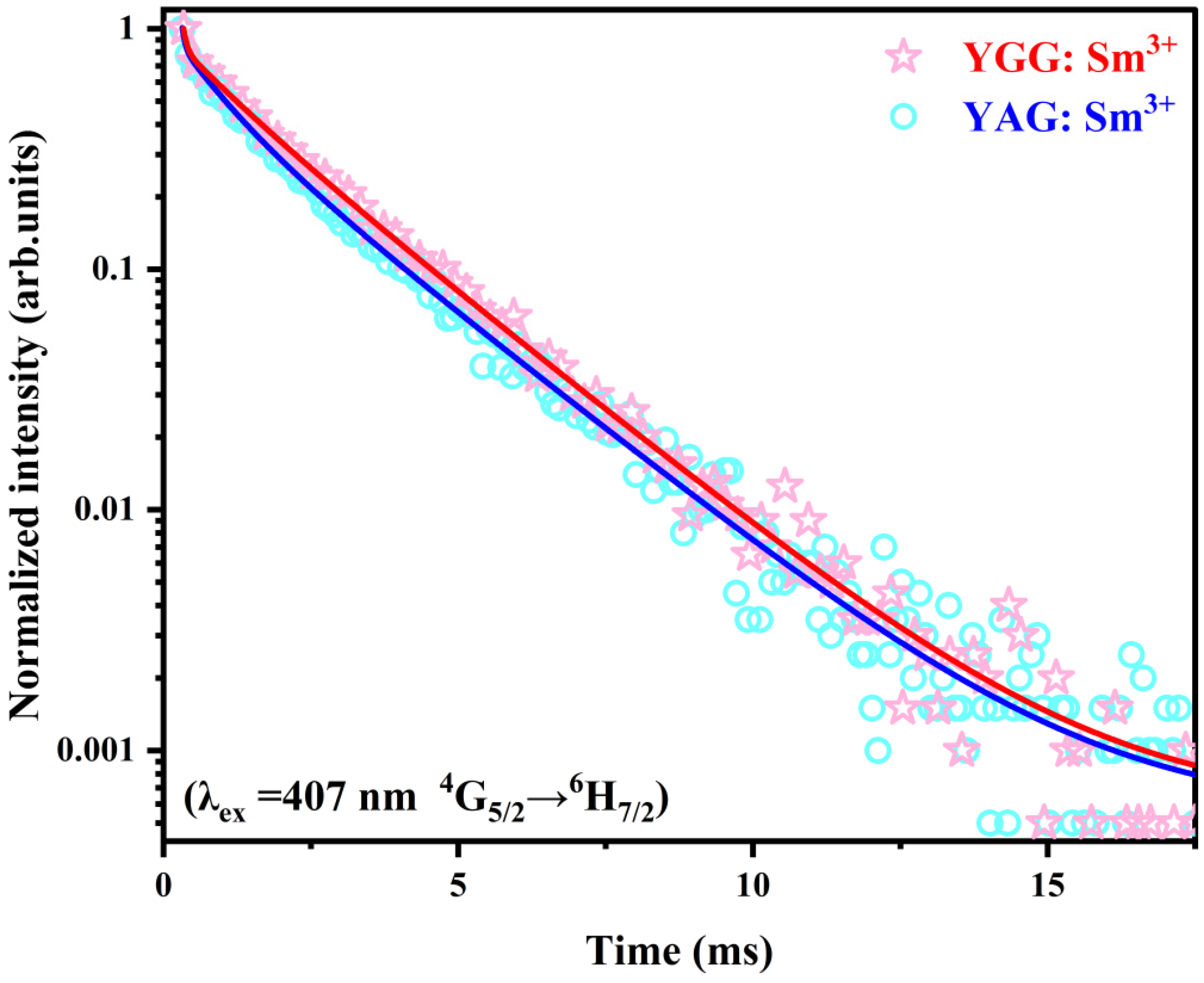
3.6. Fluorescence Lifetime Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhu, Z.; Ta, S.; Cheng, Z.; Zhang, P.; Zeng, N.; Goodman, B.A.; Xu, S.; Deng, W. Optical Properties of Yttria-Stabilized Zirconia Single-Crystals Doped with Terbium Oxide. Crystals 2022, 12, 1081. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, C.; Lou, X.; Zuo, R.; Yang, Y.; Jia, G. Rare earth ions doped Bi2MoO6 luminescent materials: Pechini sol-gel synthesis, down-conversion and up-conversion multicolor emissions, and potential applications. Ceram. Int. 2023, 49, 25987–25997. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Rasool, S.N. Luminescence properties of GdAl3(BO3)4: Dy3+ phosphors for white-LEDs. Mater. Today 2016, 3, 4019–4022. [Google Scholar] [CrossRef]
- Kumar, V.; Som, S.; Dutta, S.; Das, S.; Swart, H.C. Influence of Ho3+ doping on the temperature sensing behavior of Er3+–Yb3+ doped La2CaZnO5 phosphor. RSC Adv. 2016, 6, 84914–84925. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tsai, S.W. Diode-pumped Q-switched Nd: YVO4 yellow laser with intracavity sum-frequency mixing. Opt. Lett. 2002, 27, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, S.; Li, S.; Wu, W.; Pan, Y.; Wang, D.; Hong, X.; Cheng, Z.; Deng, W. Luminescence Properties of Ho2O3-Doped Y2O3 Stabilized ZrO2 Single Crystals. Crystals 2022, 12, 415. [Google Scholar] [CrossRef]
- Yousif, A.; Abbas, B.H.; Vijay, K.; Anurag, P.; Swart, H.C. Luminescence properties of Eu3+ activated Y2O3 red phosphor with incorporation of Ga3+ and Bi3+ trace hertero-cations in the Y2O3 lattice. Vacuum 2018, 155, 73–75. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, V.; Som, S.; Yousif, A.; Kroon, R.E.; Coetsee, E.; Swart, H.C. Photon and electron beam pumped luminescence of Ho3+ activated CaMoO4 phosphor. Appl. Surf. Sci. 2017, 423, 1169–1175. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D.; Chen, M.; Liu, L.; Zhou, Y.; Zeng, F.; Su, Z. Luminescence, energy transfer properties of Dy3+/Eu3+ coactivated neutral and warm white emissions GSBG glasses. J. Lumin. 2021, 237, 118180. [Google Scholar] [CrossRef]
- Çelik Gül, G.; Kurtuluş, F. RE (Y, Er, Gd, La, Nd, Sm, Dy)-doped SrBPO5 colorful phosphors: Definition of structural unit cell parameters and optical properties. Optik 2017, 139, 265–271. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, V.; Kroon, R.E.; Swart, H.C. Temperature induced upconversion behaviour of Ho3+-Yb3+ codoped yttrium oxide films prepared by pulsed laser deposition. J. Alloys Compd. 2016, 672, 190–196. [Google Scholar] [CrossRef]
- Gao, T.; Tian, J.; Liu, Y.; Liu, R.; Zhuang, W. Garnet phosphors for white-light-emitting diodes: Modification and calculation. Dalton Trans. 2021, 50, 3769–3781. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lu, C.; Zhou, G.; Liu, X.; Qin, X.; Liu, G.; Wang, S.; Xu, Z. Transparent YAG:Ce ceramics for WLEDs with high CRI: Ce3+ concentration and sample thickness effects. Ceram. Int. 2016, 42, 6935–6941. [Google Scholar] [CrossRef]
- Du, Q.; Feng, S.; Qin, H.; Hua, H.; Ding, H.; Jia, L.; Zhang, Z.; Jiang, J.; Jiang, H. Massive red-shifting of Ce3+ emission by Mg2+ and Si4+ doping of YAG: Ce transparent ceramic phosphors. J. Mater. Chem. C 2018, 6, 12200–12205. [Google Scholar] [CrossRef]
- Gu, C.; Wang, X.-J.; Xia, C.; Li, S.; Liu, P.; Li, D.; Li, H.; Zhou, G.; Zhang, J.; Xie, R.-J. A new CaF2-YAG: Ce composite phosphor ceramic for high-power and high-color-rendering WLEDs. J. Mater. Chem. C 2019, 7, 8569–8574. [Google Scholar] [CrossRef]
- Yao, Q.; Hu, P.; Sun, P.; Liu, M.; Dong, R.; Chao, K.; Liu, Y.; Jiang, J.; Jiang, H. YAG: Ce3+ transparent ceramic phosphors brighten the next-generation laser-driven lighting. Adv. Mater. 2020, 32, 1907888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Gu, G.R.; Di, X.X.; Xiang, W.D.; Liang, X.J. Optical characteristics of Ce, Eu: YAG single crystal grown by Czochralski method. J. Rare Earths 2019, 37, 145–150. [Google Scholar] [CrossRef]
- Tan, X.; Xu, S.; Wang, X.; Liu, F.; Goodman, B.A.; Xiong, D.; Deng, W. Preparation and optical properties of samaria-doped yttria-stabilized zirconia single crystals. J. Am. Ceram. Soc. 2019, 102, 6863–6871. [Google Scholar] [CrossRef]
- Gu, G.; Xiang, W.; Yang, C.; Liang, X. Synthesis and luminescence properties of a H2 annealed Mn-doped Y3Al5O12: Ce3+ single crystal for WLEDs. CrystEngComm 2015, 17, 4554–4561. [Google Scholar] [CrossRef]
- Demesh, M.; Dernovich, O.; Gusakova, N.; Yasukevich, A.; Kornienko, A.; Dunina, E.; Fomicheva, L.; Pavlyuk, A.; Kuleshov, N. Growth and spectroscopic properties of Sm3+: KY(WO4)2 crystal. Opt. Mater. 2018, 75, 821–826. [Google Scholar] [CrossRef]
- Rezende, M.V.d.S.; Paschoal, C.W.A. Radioluminescence enhancement in Eu3+-doped Y3Al5O12 phosphors by Ga substitution. Opt. Mater. 2015, 46, 530–535. [Google Scholar] [CrossRef]
- Yousif, A.; Som, S.; Kumar, V.; Swart, H. Comparison and analysis of Eu3+ luminescence in Y3Al5O12 and Y3Ga5O12 hosts material for red lighting phosphor. Mater. Chem. Phys. 2015, 166, 167–175. [Google Scholar] [CrossRef]
- You, L.; Zhao, Y.; Lu, D.; Liang, F.; Li, L.; Chen, Y.; Liu, J.; Wang, J.; Yu, H.; Zhang, H. High-efficiency Er-doped yttrium gallium garnet laser resonantly pumped by a laser diode at 1.47 µm. Opt. Lett. 2020, 45, 4361–4364. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Xia, F.; Tian, W.; Cao, F.; Chen, J.; Wu, J.; Song, R.; Mu, S. Single-crystal high-nickel layered cathodes for lithium-ion batteries: Advantages, mechanism, challenges and approaches. Curr. Opin. Electrochem. 2022, 31, 100831. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, S.; Gu, Z.; Wang, N.; Wang, S.; Zhang, Y.; Song, Y. Perovskite single crystals: Dimensional control, optoelectronic properties, and applications. Mater. Today 2023, 62, 225–250. [Google Scholar] [CrossRef]
- Kataoka, K.; Akimoto, J. Lithium-ion conductivity and crystal structure of garnet-type solid electrolyte Li7−xLa3Zr2−xTaxO12 using single-crystal. J. Ceram. Soc. 2019, 127, 521–526. [Google Scholar] [CrossRef]
- Han, Z.; Sun, D.; Zhang, H.; Luo, J.; Quan, C.; Hu, L.; Dong, K.; Cheng, M.; Chen, G.; Hang, Y. Investigation on the growth and properties of six garnet single crystals with large lattice constants. Cryst. Res. Technol. 2021, 56, 2000221. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, L.; Xu, S.; Cheng, Z.; Wang, Y.; Goodman, B.A.; Xiong, D.; Deng, W. White light luminescence in Dy/Tm co-doped yttria stabilized zirconia single crystals. J. Rare Earths 2022. [Google Scholar] [CrossRef]
- Koohpayeh, S.M.; Fort, D.; Abell, J.S. The optical floating zone technique: A review of experimental procedures with special reference to oxides. Prog. Cryst. Growth Charact. Mater. 2008, 54, 121–137. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; Wang, X.; Wang, D.; Goodman, B.A.; Hong, X.; Deng, W. Optical properties of gadolinia-doped cubic yttria stabilized zirconia single crystals. Ceram. Int. 2021, 47, 3346–3353. [Google Scholar] [CrossRef]
- Wolff, N.; Schwaigert, T.; Siche, D.; Schlom, D.G.; Klimm, D. Growth of CuFeO2 single crystals by the optical floating-zone technique. J. Cryst. Growth 2020, 532, 125426. [Google Scholar] [CrossRef]
- Xu, B.; Yang, X.; Cheng, H.; Zhao, J.; Wang, Y.; Zhu, E.; Zhang, J. Preparation, characterization and property of high-quality LaB6 single crystal grown by the optical floating zone melting technique. Vacuum 2019, 168, 108845. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Cheng, L.; Xie, J.; Yu, X.; Mi, X.; Liu, Q. Broadband emission of Lu3Mg2GaSi2O12: Ce3+, Sm3+ phosphors and their potential application for w-LEDs. Ceram. Int. 2021, 47, 26410–26420. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Shi, Y.; Xu, T.; Wang, H.; Fang, J.; Ni, J.; He, H.; Wang, C.; Wan, B.; et al. Fast (Ce,Gd)3Ga2Al3O12 Scintillators Grown by the Optical Floating Zone Method. Cryst. Growth Des. 2022, 22, 180–190. [Google Scholar] [CrossRef]
- Xu, S.; Tan, X.; Liu, F.; Zhang, L.; Huang, Y.; Goodman, B.A.; Deng, W. Growth and optical properties of thulia-doped cubic yttria stabilized zirconia single crystals. Ceram. Int. 2019, 45, 15974–15979. [Google Scholar] [CrossRef]
- Wu, W.; Wang, D.; Pan, Y.; Yang, Y.; Zhang, P.; Xu, S.; Goodman, B.A.; Deng, W. Characterization of interactive up-conversion luminescence mechanisms in Tm3+/Ho3+/Yb3+ doped yttria stabilized zirconia single crystals. Opt. Mater. 2022, 123, 111827. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Brito, D.R.N.; Queiroz, M.N.; Barboza, M.J.; Steimacher, A.; Pedrochi, F. Investigation of optical and spectroscopic properties of Sm3+ ions in CaBAl glasses. Opt. Mater. 2017, 64, 114–120. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Sun, D.; Luo, J.; Gu, C.; Jiang, H.; Yin, S. Crystal growth and spectral properties of Sm: GGG crystal. J. Cryst. Growth 2011, 331, 83–86. [Google Scholar] [CrossRef]
- Jubu, P.R.; Yam, F.K.; Chahrour, K.M. Enhanced red shift in optical absorption edge and photoelectrochemical performance of N-incorporated gallium oxide nanostructures. Vacuum 2020, 182, 109704. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2005. [Google Scholar]
- Pirri, A.; Maksimov, R.N.; Shitov, V.A.; Osipov, V.V.; Sani, E.; Patrizi, B.; Vannini, M.; Toci, G. Continuously tuned (Tm0.05Sc0.252Y0.698)2O3 ceramic laser with emission peak at 2076 nm. J. Alloys Compd. 2021, 889, 161585. [Google Scholar] [CrossRef]
- Han, L.; Hu, Y.; Pan, M.; Xie, Y.; Liu, Y.; Li, D.; Dong, X. A new tactic to achieve Y2O2S: Yb3+/Er3+ up-conversion luminescent hollow nanofibers. CrystEngComm 2015, 17, 2529–2535. [Google Scholar] [CrossRef]
- Wang, D.; Wu, W.; Tan, X.; Goodman, B.A.; Xu, S.; Deng, W. Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals. Crystals 2021, 11, 1328. [Google Scholar] [CrossRef]
- Wu, G.; Xue, J.; Li, X.; Bi, Q.; Sheng, M.; Leng, Z. A novel red-emitting Na5W3O9F5: Eu3+ phosphor with high color purity for blue-based WLEDs. Ceram. Int. 2023, 49, 10615–10624. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, H.; Che, Q.; Liu, B.; Li, J.; Cao, B.; Liu, Z. A novel phosphor of Cu+-doped PbBrOH: Preparation, luminescence mechanism, and outstanding properties. J. Mater. Chem. C 2021, 9, 9178–9187. [Google Scholar] [CrossRef]
- Naresh, V.; Buddhudu, S. Analysis of energy transfer based emission spectra of (Sm3+, Dy3+): Li2O–LiF–B2O3–CdO glasses. J. Lumin. 2014, 147, 63–71. [Google Scholar] [CrossRef]
- Naresh, V.; Gupta, K.; Parthasaradhi Reddy, C.; Ham, B.S. Energy transfer and colour tunability in UV light induced Tm3+/Tb3+/Eu3+: ZnB glasses generating white light emission. Acta A Mol. Biomol. Spectrosc. 2017, 175, 43–50. [Google Scholar] [CrossRef]
- Skaudžius, R.; Enseling, D.; Skapas, M.; Selskis, A.; Pomjakushina, E.; Jüstel, T.; Kareiva, A.; Rüegg, C. Europium–enabled luminescent single crystal and bulk YAG and YGG for optical imaging. Opt. Mater. 2016, 60, 467–473. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A.; Antolín, E.; Tablero, C. Intermediate bands versus levels in non-radiative recombination. Phys. Rev. B Condens. Matter 2006, 382, 320–327. [Google Scholar] [CrossRef]
- Xu, X.; Hu, Z.; Li, R.; Li, D.; Di, J.; Su, L.; Yang, Q.; Sai, Q.; Tang, H.; Wang, Q. Polarized spectral properties of Sm: CaGdAlO4 crystal for reddish-orange laser. Opt. Mater. 2017, 69, 333–338. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Lin, Y.; Gong, X.; Chen, Y.; Luo, Z.; Huang, Y. Spectroscopic properties of Sm3+-doped NaGd(MoO4)2 crystal for visible laser application. J. Lumin. 2017, 187, 235–239. [Google Scholar] [CrossRef]
- Qiao, Z.; Qin, C.; He, W.; Gong, Y.; Li, B.; Zhang, G.; Chen, R.; Gao, Y.; Xiao, L.; Jia, S. Robust micropatterns on graphene oxide films based on the modification of fluorescence lifetime for multimode optical recording. Carbon 2019, 142, 224–229. [Google Scholar] [CrossRef]
- Green, D.C.; Holden, M.A.; Levenstein, M.A.; Zhang, S.; Johnson, B.R.G.; Gala de Pablo, J.; Ward, A.; Botchway, S.W.; Meldrum, F.C. Controlling the fluorescence and room-temperature phosphorescence behaviour of carbon nanodots with inorganic crystalline nanocomposites. Nat. Commun. 2019, 10, 206. [Google Scholar] [CrossRef]

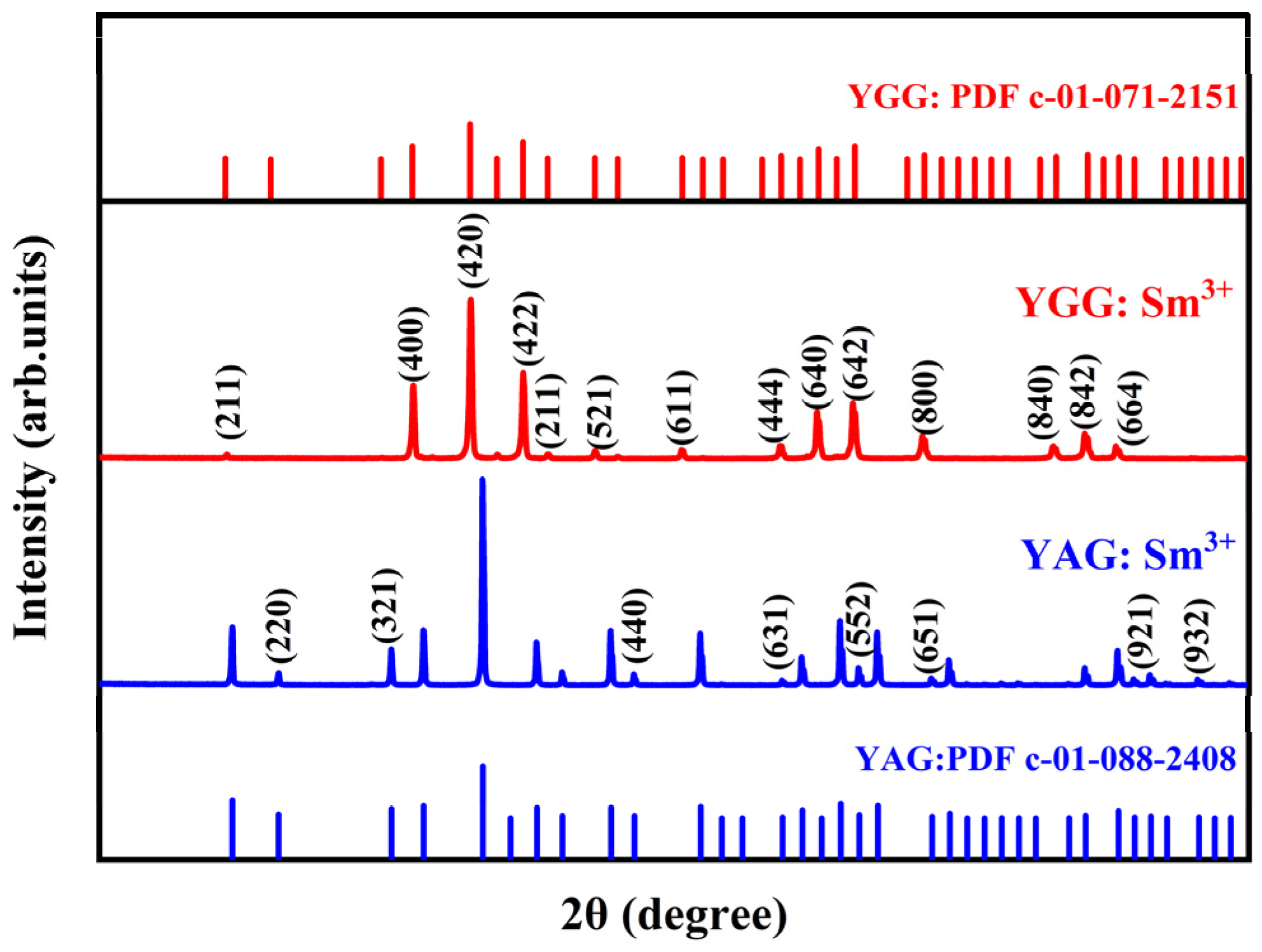
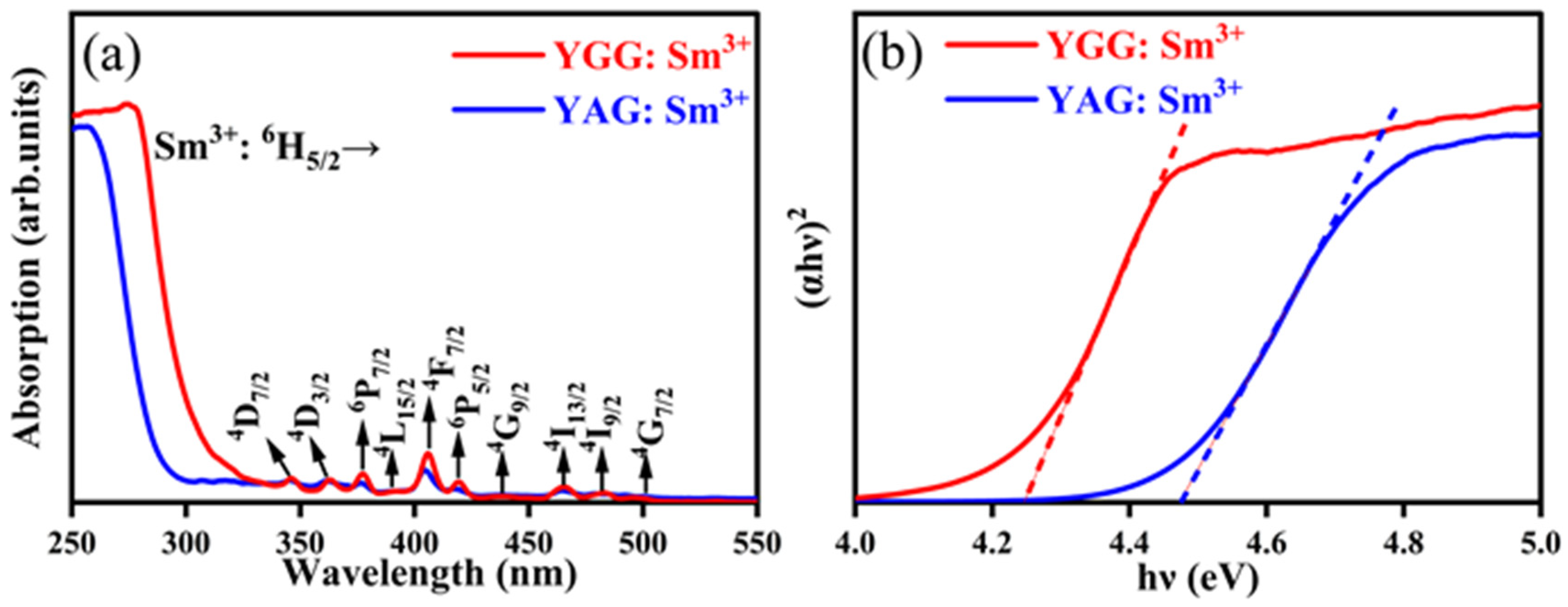
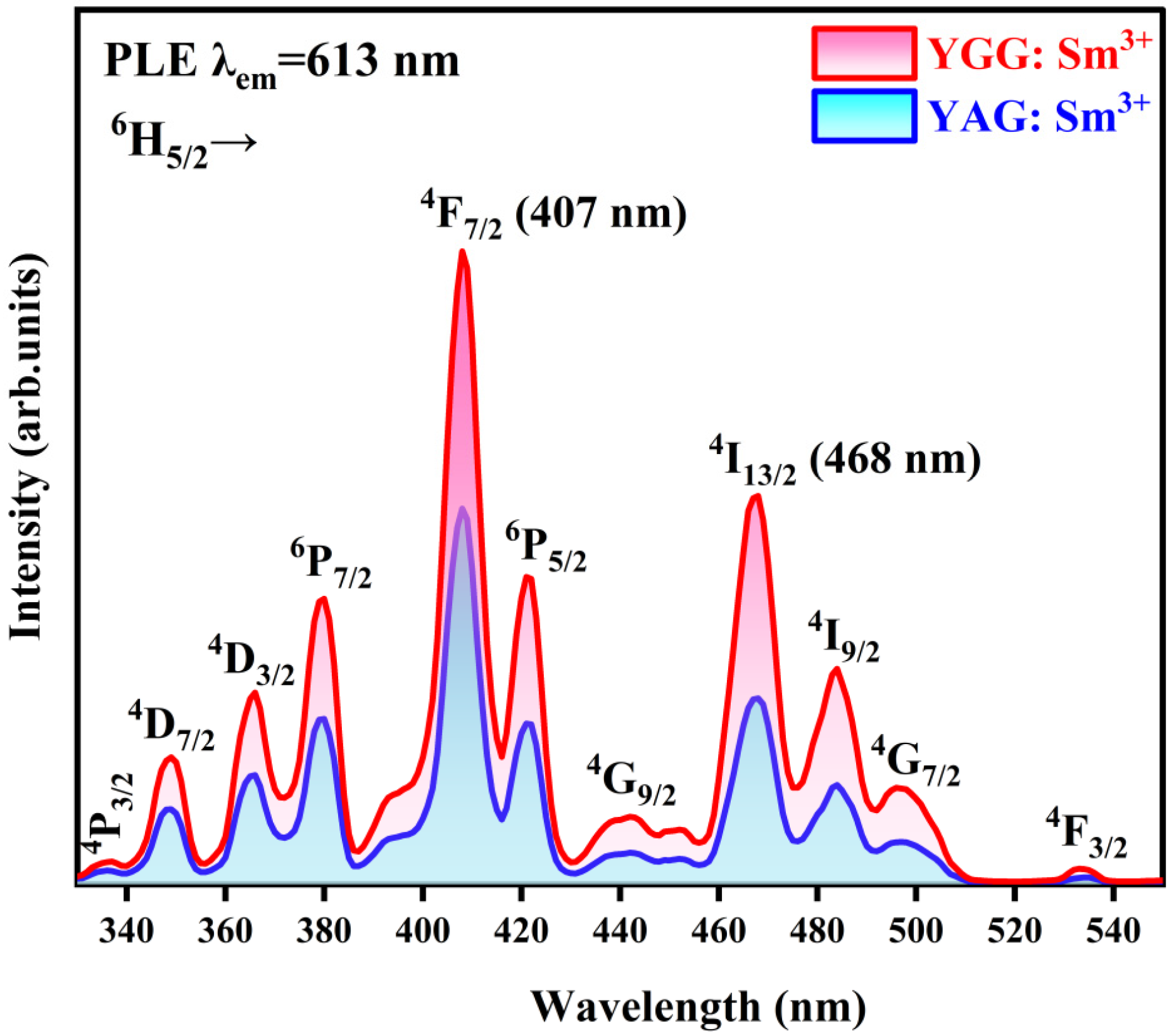

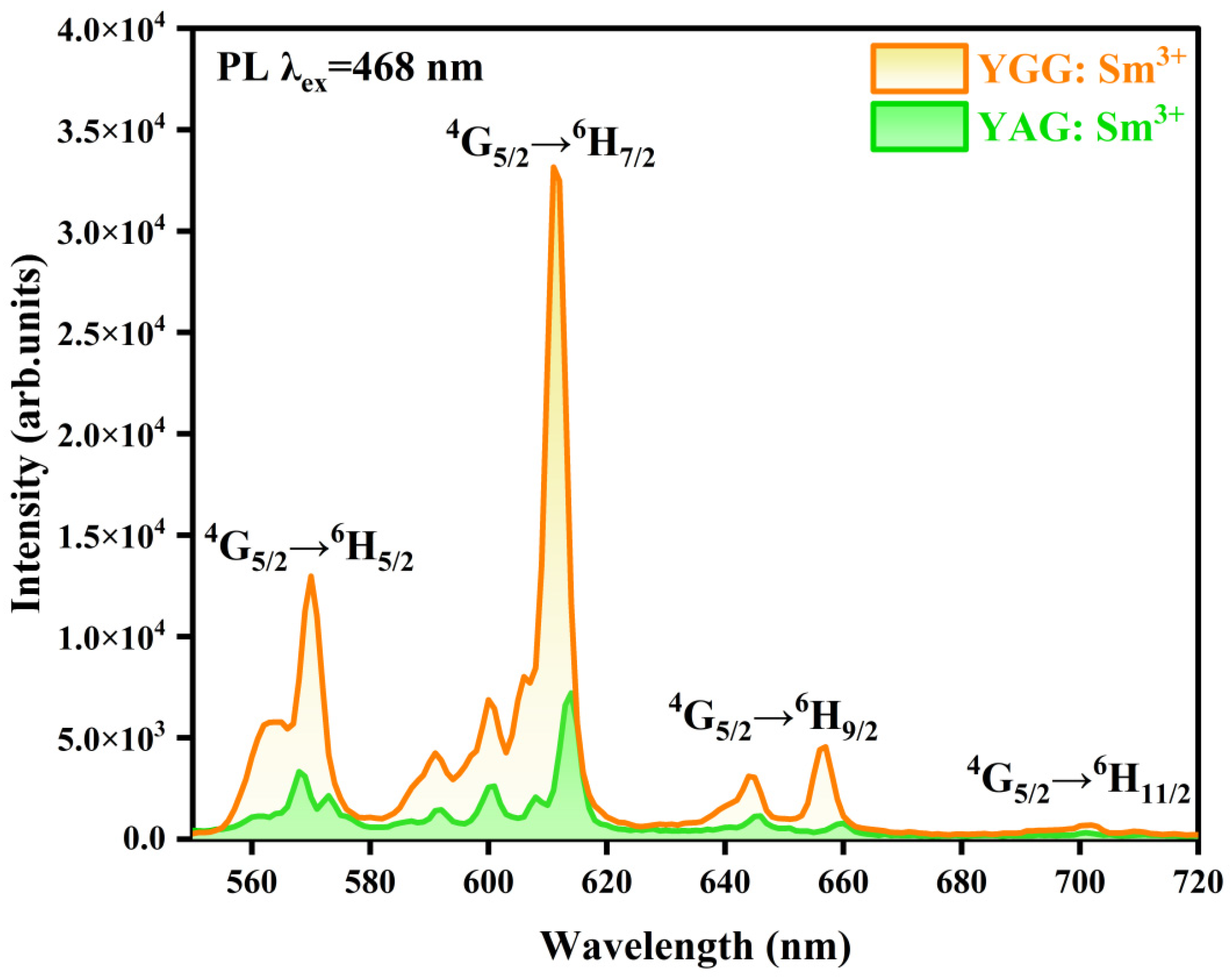

| Sample | Lattice Constant (nm) a = b = c | Cell Volume (nm3) |
|---|---|---|
| YGG: Sm3+ | 1.230 | 1.860 |
| YAG: Sm3+ | 1.201 | 1.732 |
| Crystal | M (g) | V (cm3) | ρ (g/cm3) | ρcal (g/cm3) |
|---|---|---|---|---|
| Y2.96Sm0.04Ga5O12 | 2.498 | 0.434 | 5.756 | 5.765 |
| Y2.96Sm0.04Al5O12 | 2.561 | 0.565 | 4.533 | 4.551 |
| Sample | λex (nm) | (xs, ys) | (xd, yd) | Color Purity |
|---|---|---|---|---|
| YGG: Sm3+ | 407 | (0.590, 0.407) | (0.646, 0.354) | 85% |
| YAG: Sm3+ | 407 | (0.584, 0.405) | (0.647, 0.353) | 83% |
| Crystal | A1 | τ1 | A2 | τ2 | A3 | τ3 | R2 | |
|---|---|---|---|---|---|---|---|---|
| YGG: Sm3+ | 69.89 | 0.06 | 0.24 | 0.95 | 0.76 | 2.21 | 0.705 | 0.998 |
| YAG: Sm3+ | 124.01 | 0.05 | 0.46 | 0.78 | 0.61 | 2.24 | 0.466 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, Z.; Ta, S.; Zeng, N.; Wu, L.; Wu, W.; Zhang, P.; Xu, S.; Goodman, B.A.; Deng, W. Highly Efficient Orange-Red Emission in Sm3+-Doped Yttrium Gallium Garnet Single Crystal. Crystals 2023, 13, 1273. https://doi.org/10.3390/cryst13081273
Zhang H, Zhu Z, Ta S, Zeng N, Wu L, Wu W, Zhang P, Xu S, Goodman BA, Deng W. Highly Efficient Orange-Red Emission in Sm3+-Doped Yttrium Gallium Garnet Single Crystal. Crystals. 2023; 13(8):1273. https://doi.org/10.3390/cryst13081273
Chicago/Turabian StyleZhang, Huiting, Zhonghua Zhu, Shengdi Ta, Ninghan Zeng, Limin Wu, Wenxia Wu, Peng Zhang, Shoulei Xu, Bernard Albert Goodman, and Wen Deng. 2023. "Highly Efficient Orange-Red Emission in Sm3+-Doped Yttrium Gallium Garnet Single Crystal" Crystals 13, no. 8: 1273. https://doi.org/10.3390/cryst13081273
APA StyleZhang, H., Zhu, Z., Ta, S., Zeng, N., Wu, L., Wu, W., Zhang, P., Xu, S., Goodman, B. A., & Deng, W. (2023). Highly Efficient Orange-Red Emission in Sm3+-Doped Yttrium Gallium Garnet Single Crystal. Crystals, 13(8), 1273. https://doi.org/10.3390/cryst13081273






