Influence of Golden Nanoparticles on the Incorporation of Eu2+ into BaI2 and Defect Concentration
Abstract
1. Introduction
2. Experimental
2.1. Preparation of BaI2:Eu Samples with GNPs
2.2. Experimental Techniques
3. Results and Discussion
3.1. Structural Analysis and Morphology of BaI2:Eu/GNP
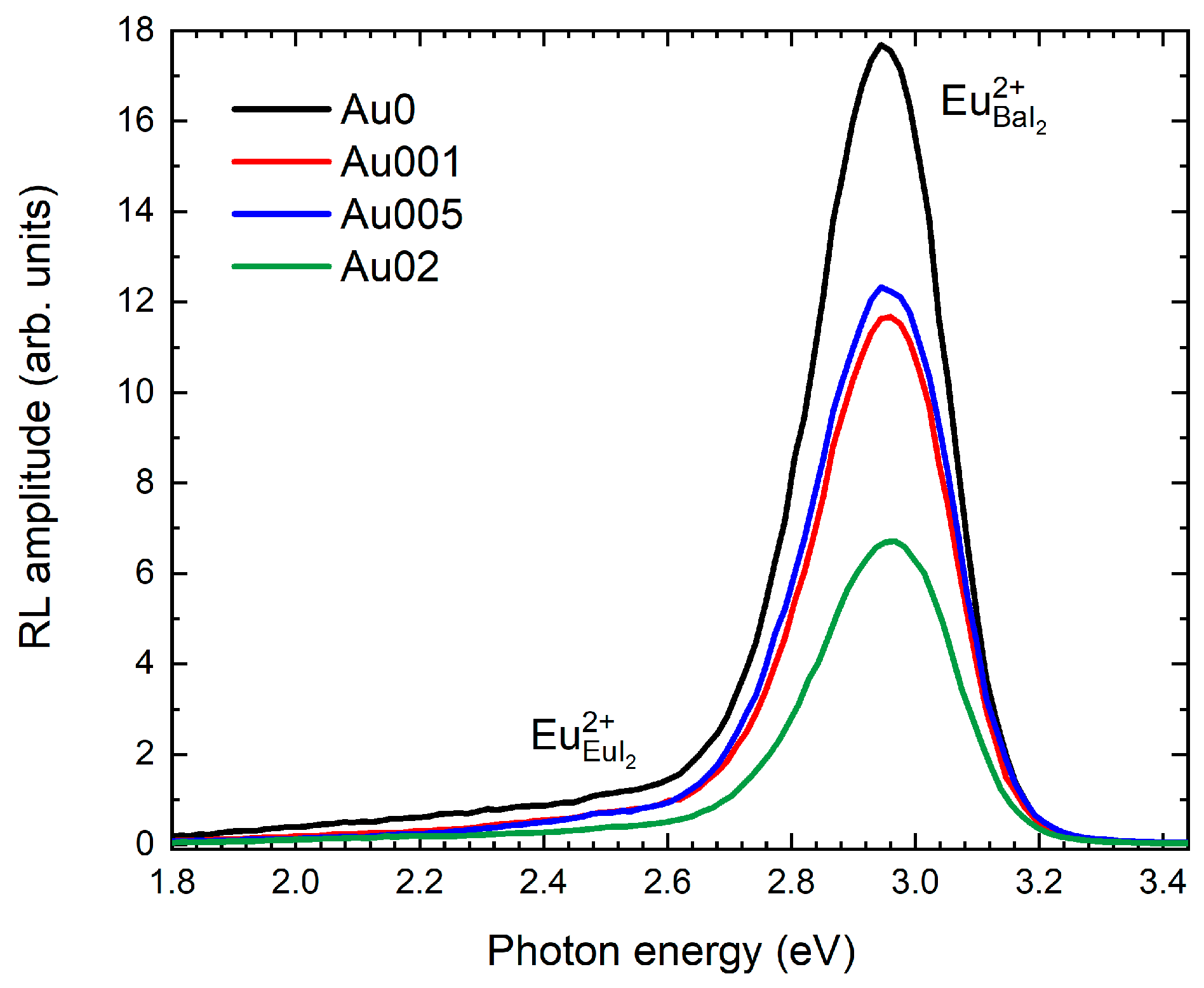
3.2. Luminescence and Timing Characteristics
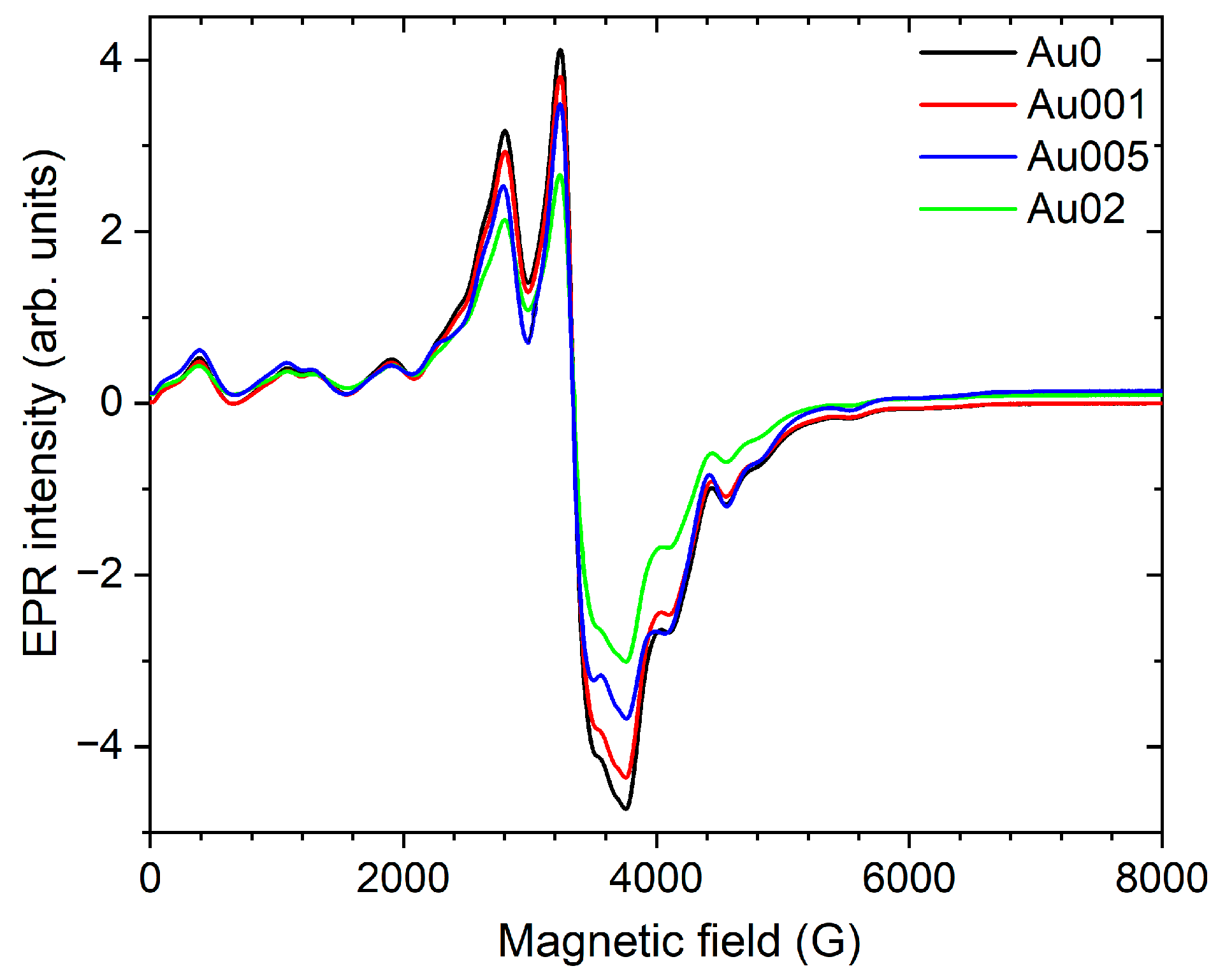
3.3. Eu2+ Incorporation by EPR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldmann, C.; Jüstel, T.; Ronda, C.; Schmidt, P. Inorganic Luminescent Materials: 100 Years of Research and Application. Adv. Funct. Mater. 2003, 13, 511–516. [Google Scholar] [CrossRef]
- Lenus, A.J.; Sornadurai, D.; Rajan, K.G.; Purniah, B. Luminescence behaviour of Eu2+-doped BaClI and BaBrI. Mater. Lett. 2002, 57, 635–638. [Google Scholar] [CrossRef]
- Terraschke, H.; Wickleder, C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+-Doped Nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef] [PubMed]
- Shendrik, R.; Shalaev, A.; Myasnikova, A.; Bogdanov, A.; Kaneva, E.; Rusakov, A.; Vasilkovskyi, A. Optical and structural properties of Eu2+ doped BaBrI and BaClI crystals. J. Lumin 2017, 192, 653–660. [Google Scholar] [CrossRef]
- Pankratov, V.; Popov, A.; Shirmane, L.; Kotlov, A.; Bizarri, G.; Burger, A.; Bhattacharya, P.; Tupitsyn, E.; Rowe, E.; Buliga, V.; et al. Luminescence and ultraviolet excitation spectroscopy of SrI2 and SrI2:Eu2+. Radiat. Meas. 2013, 56, 13–17. [Google Scholar] [CrossRef]
- Ramanantoanina, H.; Merzoud, L.; Muya, J.T.; Chermette, H.; Daul, C. Electronic Structure and Photoluminescence Properties of Eu(η9-C9H9)2. J. Phys. Chem. A 2020, 124, 152–164. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Hull, G.; Niedermayr, T.R.; Drobshoff, A.; Payne, S.A.; Roy, U.N.; Cui, Y.; Bhattacharaya, A.; Harrison, M.; Guo, M.; et al. Barium iodide single-crystal scintillator detectors. Hard X-Ray Gamma-Ray Detect. Phys. IX 2007, 6706, 670616. [Google Scholar] [CrossRef]
- Nikl, M. (Ed.) Nanocomposite Ceramic and Thin Film Scintillators; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017. [Google Scholar]
- Biswas, K.; Sontakke, A.; Sen, R.; Annapurna, K. Luminescence Properties of Dual Valence Eu Doped Nano-crystalline BaF2 Embedded Glass-ceramics and Observation of Eu2+ → Eu3+ Energy Transfer. J. Fluoresc. 2012, 22, 745–752. [Google Scholar] [CrossRef]
- Tratsiak, Y.; Buryi, M.; Babin, V.; Korjik, M.; Trusova, E. The effect of binary glass composition on the Eu-ions luminescence properties. Opt. Mater. 2019, 94, 356–362. [Google Scholar] [CrossRef]
- Gahane, D.; Kokode, N.; Muthal, P.; Dhopte, S.; Moharil, S. Luminescence of Eu2+ in some iodides. Opt. Mater. 2009, 32, 18–21. [Google Scholar] [CrossRef]
- Kalpana, T.; Brik, M.; Sudarsan, V.; Naresh, P.; Kumar, V.R.; Kityk, I.; Veeraiah, N. Influence of Al3+ ions on luminescence efficiency of Eu3+ ions in barium boro-phosphate glasses. J. Non-Crystalline Solids 2015, 419, 75–81. [Google Scholar] [CrossRef]
- Dejneka, M.; Snitzer, E.; Riman, R. Blue, green and red fluorescence and energy transfer of Eu3+ in fluoride glasses. J. Lumin. 1995, 65, 227–245. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.; Lin, J. White light emission from Eu3+ in CaIn2O4 host lattices. Appl. Phys. Lett. 2007, 90, 081904. [Google Scholar] [CrossRef]
- Xie, H.; Lu, J.; Guan, Y.; Huang, Y.; Wei, D.; Seo, H.J. ChemInform Abstract: Abnormal Reduction, Eu3+ → Eu2+, and Defect Centers in Eu3+-Doped Pollucite, CsAlSi2O6, Prepared in an Oxidizing Atmosphere. Cheminform 2014, 45, 827–834. [Google Scholar] [CrossRef]
- Zhai, Y.; You, Z.; Liu, Y.; Sun, Y.; Ji, Q. Properties of red-emitting phosphors Sr2MgSi2O7:Eu3+ prepared by gel-combustion method assisted by microwave. J. Rare Earths 2012, 30, 114–117. [Google Scholar] [CrossRef]
- Selling, J.; Birowosuto, M.D.; Dorenbos, P.; Schweizer, S. Europium-doped barium halide scintillators for x-ray and γ-ray detections. J. Appl. Phys. 2007, 101, 034901. [Google Scholar] [CrossRef]
- Tret’yak, E.V.; Shevchenko, G.P.; Solomakha, T.A.; Korzhik, M.V. Effect of precursor morphology on the structural properties, optical absorption, and luminescence of BaI2:Eu2+,Eu3+. Inorg. Mater. 2017, 53, 307–312. [Google Scholar] [CrossRef]
- Salamakha, T.; Buryi, M.; Tratsiak, Y. Effect of Eu-doping on optical, structural and morphological properties of BaI2·nH2O powders. Opt. Mater. 2018, 78, 352–359. [Google Scholar] [CrossRef]
- Pejchal, J.; Buryi, M.; Babin, V.; Prusa, P.; Beitlerova, A.; Barta, J.; Havlak, L.; Kamada, K.; Yoshikawa, A.; Laguta, V.; et al. Luminescence and scintillation properties of Mg-codoped LuAG:Pr single crystals annealed in air. J. Lumin. 2017, 181, 277–285. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.K.; Park, S.W.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, K.H. Characterization and photoluminescent enhancement of Li+ corporation effect on CaWO4:Eu3+ phosphor. J. Alloy. Compd. 2012, 511, 123–128. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Tong, L.; Ren, X.; Li, Q.; Yang, H. YVO4:Eu3+, Dy3+@Fe3O4 co-doped nanocomposites: Preparation, luminescent, and magnetic properties. J. Nanoparticle Res. 2012, 14, 1216. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, G.; Qin, N.; Bao, D. Dual Enhancement of Photoluminescence and Ferroelectric Polarization in Pr3+/La3+-Codoped Bismuth Titanate Thin Films. J. Am. Ceram. Soc. 2010, 93, 2109–2112. [Google Scholar] [CrossRef]
- Shalapska, T.; Moretti, F.; Bourret, E.; Bizarri, G. Effect of Au codoping on the scintillation properties of BaBrCl:Eu single crystals. J. Lumin. 2018, 202, 497–501. [Google Scholar] [CrossRef]
- Levchenko, V. Luminescence of Europium complex enhanced by surface plasmons of gold nanoparticles for possible application in luminescent solar concentrators. J. Lumin. 2018, 193, 5–9. [Google Scholar] [CrossRef]
- Malashkevich, G.E.; Chukova, O.V.; Nedilko, S.G.; Shevchenko, G.P.; Bokshyts, Y.V.; Kouhar, V.V. Influence of Gold Nanoparticles on Luminescence of Eu3+ Ions Sensitized by Structural Defects in Germanate Films. J. Phys. Chem. C 2016, 120, 15369–15377. [Google Scholar] [CrossRef]
- Wackerow, S.; Seifert, G. Co-doping of glasses with rare earth ions and metallic nanoparticles for frequency up-conversion. Photonics Sol. Energy Syst. III 2010, 7725, 77251H. [Google Scholar] [CrossRef]
- Hussain, T.; Zhong, L.; Danesh, M.; Ye, H.; Liang, Z.; Xiao, D.; Qiu, C.-W.; Lou, C.; Chi, L.; Jiang, L. Enabling low amounts of YAG:Ce3+ to convert blue into white light with plasmonic Au nanoparticles. Nanoscale 2015, 7, 10350–10356. [Google Scholar] [CrossRef]
- Kahane, S.V.; Sudarsan, V.; Mahamuni, S. A study of charge transfer mechanism and optical properties of Au–CdS core–shell nanocrystals. J. Lumin. 2014, 147, 353–357. [Google Scholar] [CrossRef]
- Emtage, P.R.; O’Dwyer, J.J. Richardson-Schottky Effect in Insulators. Phys. Rev. Lett. 1966, 16, 356–358. [Google Scholar] [CrossRef]
- Moores, A.; Goettmann, F. The plasmon band in noble metal nanoparticles: An introduction to theory and applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Salamakha, T.; Trusova, E.E.; Tratsiak, Y.U. Preparation and study of the luminescent glass-ceramics based on barium iodide activated with Eu2+. J. Belarusian State Univ. Chem. 2019, 1, 38–44. [Google Scholar] [CrossRef]
- Mooney, J.; Kambhampati, P. Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Lutz, H.D.; Buchmeier, W.; Engelen, B. Comparative study of the crystal structures of isotypic MX 2.H2O, M = Sr, Ba, and X = Cl, Br, I. Bifurcated H bonds in solid hydrates. Acta Cryst. 1987, B43, 71–75. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Davis, A.; Tran, T.; Young, D. Solution chemistry of iodide leaching of gold. Hydrometallurgy 1993, 32, 143–159. [Google Scholar] [CrossRef]
- Davis, A.; Tran, T. Gold dissolution in iodide electrolytes. Hydrometallurgy 1991, 26, 163–177. [Google Scholar] [CrossRef]
- Elding, L.I.; Skibsted, L.H. Kinetics and mechanism for reduction of ammine and haloammine complexes of gold(III) by iodide. Inorg. Chem. 1986, 25, 4084–4087. [Google Scholar] [CrossRef]
- Yan, Z.; Gundiah, G.; Bizarri, G.A.; Samulon, E.C.; Derenzo, S.E.; Bourret-Courchesne, E.D. Eu2+-activated BaCl2, BaBr2 and BaI2 scintillators revisited. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2014, 735, 83–87. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 2012. [Google Scholar]



| Sample | Au0 | Au001 | Au005 | Au02 |
|---|---|---|---|---|
| Au, wt.% | 0 | 0.01 | 0.05 | 0.2 |
| Sample | a (Å) | b (Å) | c (Å) |
|---|---|---|---|
| Au0 | 12.507 ± 0.003 | 4.768 ± 0.003 | 10.010 ± 0.003 |
| Au001 | 12.508 ± 0.003 | 4.767 ± 0.003 | 10.009 ± 0.003 |
| Au005 | 12.509 ± 0.003 | 4.768 ± 0.003 | 10.008 ± 0.003 |
| Au02 | 12.517 ± 0.003 | 4.772 ± 0.003 | 10.012 ± 0.003 |
| Sample | Decay Time (ns) |
|---|---|
| Au0 | 324.8 ± 0.2 |
| Au001 | 322.5 ± 0.2 |
| Au005 | 322.6 ± 0.2 |
| Au02 | 318.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridzoňová, K.; Buryi, M.; Babin, V.; John, D.; Drahokoupil, J.; Neykova, N.; Salamakha, T.; Tratsiak, Y. Influence of Golden Nanoparticles on the Incorporation of Eu2+ into BaI2 and Defect Concentration. Crystals 2023, 13, 902. https://doi.org/10.3390/cryst13060902
Ridzoňová K, Buryi M, Babin V, John D, Drahokoupil J, Neykova N, Salamakha T, Tratsiak Y. Influence of Golden Nanoparticles on the Incorporation of Eu2+ into BaI2 and Defect Concentration. Crystals. 2023; 13(6):902. https://doi.org/10.3390/cryst13060902
Chicago/Turabian StyleRidzoňová, Katarína, Maksym Buryi, Vladimir Babin, David John, Jan Drahokoupil, Neda Neykova, Tatsiana Salamakha, and Yauhen Tratsiak. 2023. "Influence of Golden Nanoparticles on the Incorporation of Eu2+ into BaI2 and Defect Concentration" Crystals 13, no. 6: 902. https://doi.org/10.3390/cryst13060902
APA StyleRidzoňová, K., Buryi, M., Babin, V., John, D., Drahokoupil, J., Neykova, N., Salamakha, T., & Tratsiak, Y. (2023). Influence of Golden Nanoparticles on the Incorporation of Eu2+ into BaI2 and Defect Concentration. Crystals, 13(6), 902. https://doi.org/10.3390/cryst13060902







