Surface Passivation of Germanium with ALD Al2O3: Impact of Composition and Crystallinity of GeOx Interlayer
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
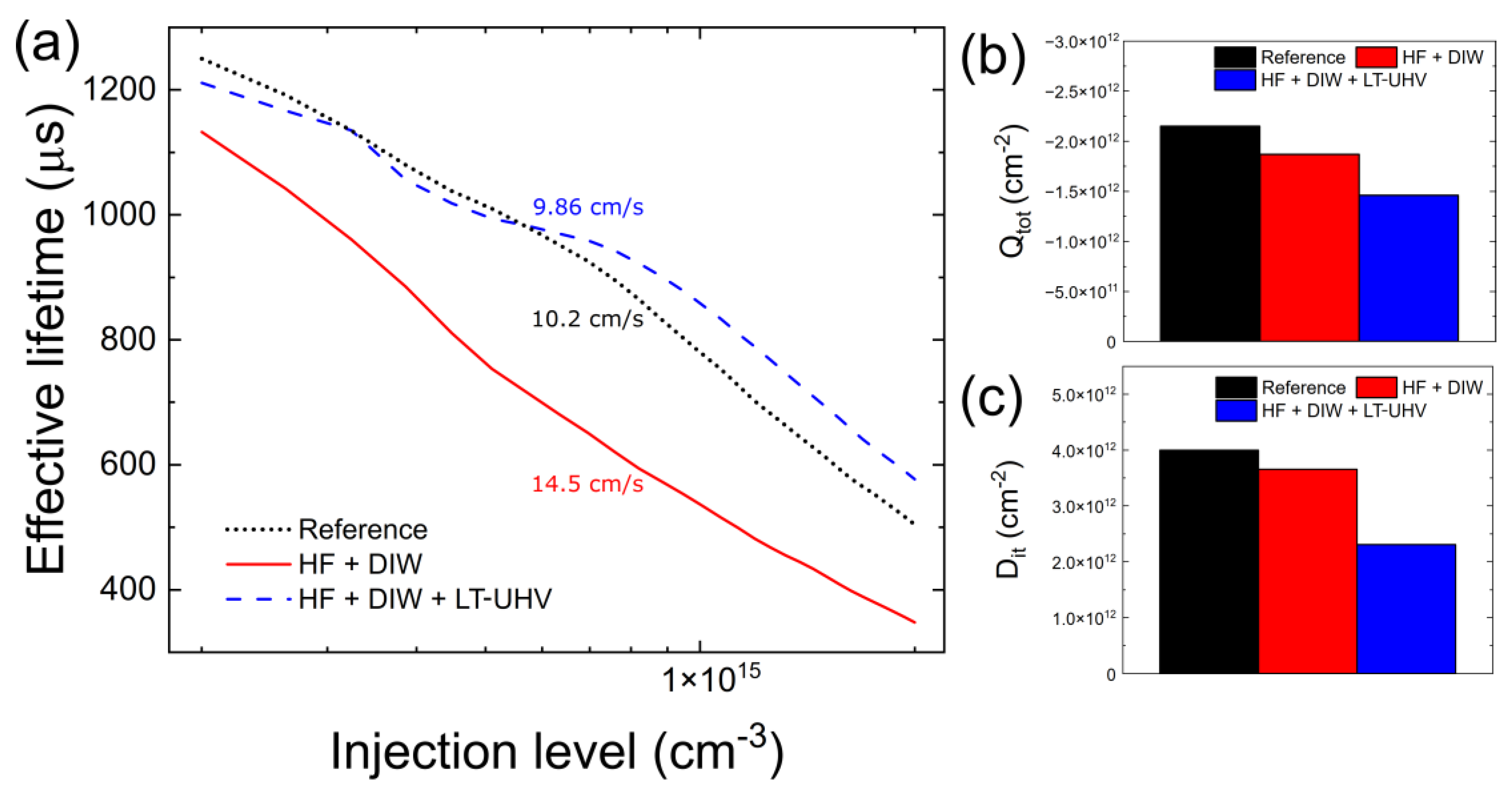
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALD | atomic layer deposition |
| COCOS | corona oxide characterization of semiconductors |
| Dit | interface defect density |
| DIW | deionized water |
| QSS-µPCD | quasi-steady-state microwave-detected photoconductance decay |
| Qtot | fixed charge density |
| LEED | low-energy electron diffraction |
| LT-UHV | low-temperature heating and controlled oxidation in an ultrahigh vacuum |
| SRV | surface recombination velocity |
| XPS | X-ray photoelectron spectroscopy |
| τeff | effective minority carrier lifetime |
References
- Claeys, C.; Simoen, E. Germanium-Based Technologies: From Materials to Devices; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-044953-1. [Google Scholar]
- Knoll, G.F. Radiation Detection and Measurement; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9780470131480. [Google Scholar]
- Lee, M.L.; Leitz, C.W.; Cheng, Z.; Pitera, A.; Langdo, T.; Currie, M.; Taraschi, G.; Fitzgerald, E.A.; Antoniadis, D.A. Strained Ge channel p-type metal-oxide-semiconductor field-effect transistors grown on Si1-xGex/ Si virtual substrates. Appl. Phys. Lett. 2001, 79, 3344–3346. [Google Scholar] [CrossRef]
- Saraswat, K.C.; Chui, C.O.; Krishnamohan, T.; Nayfeh, A.; McIntyre, P. Ge based high performance nanoscale MOSFETs. Microelectron. Eng. 2005, 80, 15–21. [Google Scholar] [CrossRef]
- Xie, D.; Simoen, E.; Chen, H.; Arimura, H.; Horiguchi, N. Impact of Dummy Gate Removal and a Silicon Cap on the Low-Frequency Noise Performance of Germanium nFinFETs. IEEE Trans. Electron. Devices 2020, 67, 4713–4719. [Google Scholar] [CrossRef]
- Chen, K.; Isometsä, J.; Pasanen, T.P.; Vähänissi, V.; Savin, H. Efficient photon capture on germanium surfaces using industrially feasible nanostructure formation. Nanotechnology 2021, 32, 035301. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, T.P.; Isometsä, J.; Garin, M.; Chen, K.; Vähänissi, V.; Savin, H. Nanostructured Germanium with >99% Absorption at 300–1600 nm Wavelengths. Adv. Opt. Mater. 2020, 8, 2000047. [Google Scholar] [CrossRef]
- Du, M.; Yang, T.; Jiao, K. Immobilization-free direct electrochemical detection for DNA specific sequences based on electrochemically converted gold nanoparticles/graphene composite film. J. Mater. Chem. 2010, 20, 9253–9260. [Google Scholar] [CrossRef]
- Hasegawa, B.H.; Stebler, B.; Rutt, B.K.; Martinez, A.; Gingold, E.L.; Barker, C.S.; Faulkner, K.G.; Cann, C.E.; Boyd, D.P. A prototype high-purity germanium detector system with fast photon-counting circuitry for medical imaging. Med. Phys. 1991, 18, 900–909. [Google Scholar] [CrossRef]
- Colace, L.; Assanto, G. Germanium on Silicon for Near-Infrared Light Sensing. IEEE Photonics J. 2009, 1, 69–79. [Google Scholar] [CrossRef]
- Colace, L.; Scacchi, A.; Assanto, G. Noise characterization of Ge/Si photodetectors. In Proceedings of the 8th IEEE International Conference on Group IV Photonics, London, UK, 14–16 September 2011; pp. 290–292. [Google Scholar]
- Soref, R. Mid-infrared photonics in silicon and germanium. Nat. Photonics 2010, 4, 495–497. [Google Scholar] [CrossRef]
- Alcañiz, A.; López, G.; Martín, I.; Jiménez, A.; Datas, A.; Calle, E.; Ros, E.; Gerling, L.G.; Voz, C.; del Cañizo, C.; et al. Germanium photovoltaic cells with MoOx hole-selective contacts. Sol. Energy 2019, 181, 357–360. [Google Scholar] [CrossRef]
- van der Heide, J.; Posthuma, N.E.; Flamand, G.; Geens, W.; Poortmans, J. Cost-efficient thermophotovoltaic cells based on germanium substrates. Sol. Energy Mater. Sol. Cells 2009, 93, 1810–1816. [Google Scholar] [CrossRef]
- An, S.; Liao, Y.; Shin, S.; Kim, M. Black Germanium Photodetector Exceeds External Quantum Efficiency of 160%. Adv. Mater. Technol. 2022, 7, 2100912. [Google Scholar] [CrossRef]
- Fadaly, E.M.T.; Dijkstra, A.; Suckert, J.R.; Ziss, D.; van Tilburg, M.A.J.; Mao, C.; Ren, Y.; van Lange, V.T.; Korzun, K.; Kölling, S.; et al. Direct-bandgap emission from hexagonal Ge and SiGe alloys. Nature 2020, 580, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Lin, Y.; Lee, K.H.; Wang, Y.; Wu, S.; Tan, C.S. High speed and ultra-low dark current Ge vertical p-i-n photodetectors on an oxygen-annealed Ge-on-insulator platform with GeOx surface passivation. Opt. Express 2020, 28, 23978–23990. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Deng, S.; Schaekers, M.; Lin, D.; Caymax, M.; Delabie, A.; Qu, X.P.; Jiang, Y.L.; Deduytsche, D.; Detavernier, C. Germanium surface passivation and atomic layer deposition of high-k dielectrics-A tutorial review on Ge-based MOS capacitors. Semicond. Sci. Technol. 2012, 27, 074012. [Google Scholar] [CrossRef]
- Ponath, P.; Posadas, A.B.; Demkov, A.A. Ge(001) surface cleaning methods for device integration. Appl. Phys. Rev. 2017, 4, 021308. [Google Scholar] [CrossRef]
- Fukuda, Y.; Ueno, T.; Hirono, S.; Hashimoto, S. Electrical characterization of germanium oxide/germanium interface prepared by electron-cyclotron-resonance plasma irradiation. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2005, 44, 6981–6984. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, C. Effects of Sulfur Passivation on Ge MOS Capacitors with High-k Gate Dielectric. In Proceedings of the 2007 International Conference on Solid State Devices and Materials, Ibaraki, Japan, 18–21 September 2007; Volume 28, pp. 976–979. [Google Scholar] [CrossRef]
- Martín, I.; López, G.; Garín, M.; Voz, C.; Ortega, P.; Puigdollers, J. Effect of the thickness of amorphous silicon carbide interlayer on the passivation of c-Ge surface by aluminium oxide films. Surf. Interfaces 2022, 31, 102070. [Google Scholar] [CrossRef]
- Posthuma, N.E.; Flamand, G.; Geens, W.; Poortmans, J. Surface passivation for germanium photovoltaic cells. Sol. Energy Mater. Sol. Cells 2005, 88, 37–45. [Google Scholar] [CrossRef]
- Xie, Q.; Musschoot, J.; Schaekers, M.; Caymax, M.; Delabie, A.; Qu, X.-P.; Jiang, Y.-L.; Van den Berghe, S.; Liu, J.; Detavernier, C. Ultrathin GeOxNy interlayer formed by in situ NH3 plasma pretreatment for passivation of germanium metal-oxide-semiconductor devices. Appl. Phys. Lett. 2010, 97, 222902. [Google Scholar] [CrossRef]
- Chen, J.J.-H.; Bojarezuk, N.A.; Shang, H.; Copel, M.; Hannon, J.B.; Karasinski, J.; Preisler, E.; Banerjee, S.K.; Guha, S. Ultrathin Al2O3 and HfO2 gate dielectrics on surface nitrided Ge. IEEE Trans. Electron. Devices 2004, 51, 1441–1447. [Google Scholar] [CrossRef]
- Isometsä, J. Surface Passivation of Germanium with Atomic Layer Deposited Al2O3; Aalto University: Espoo, Finland, 2019. [Google Scholar]
- Isometsä, J.; Fung, T.H.; Pasanen, T.P.; Liu, H.; Yli-Koski, M.; Vähänissi, V.; Savin, H. Achieving surface recombination velocity below 10 cm/s in n-type germanium using ALD Al2O3. APL Mater. 2021, 9, 3–10. [Google Scholar] [CrossRef]
- Berghuis, W.J.H.; Melskens, J.; Macco, B.; Theeuwes, R.J.; Verheijen, M.A.; Kessels, W.M.M. Surface passivation of germanium by atomic layer deposited Al2O3 nanolayers. J. Mater. Res. 2021, 36, 571–581. [Google Scholar] [CrossRef]
- Berghuis, W.J.H.; Melskens, J.; Macco, B.; Theeuwes, R.J.; Black, L.E.; Verheijen, M.A.; Kessels, W.M.M. Excellent surface passivation of germanium by a-Si:H/Al2O3 stacks. J. Appl. Phys. 2021, 130, 135303. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lei, Z.C.; Abidin, N.I.Z. Surface and interface characteristics of annealed ZrO2/Ge oxide-semiconductor structure in argon ambient. Surf. Interfaces 2021, 23, 101007. [Google Scholar] [CrossRef]
- Ke, M.; Takenaka, M.; Takagi, S. Slow Trap Properties and Generation in Al2O3/GeOx/Ge MOS Interfaces Formed by Plasma Oxidation Process. ACS Appl. Electron. Mater. 2019, 1, 311–317. [Google Scholar] [CrossRef]
- Liu, H.; Pasanen, T.P.; Fung, T.H.; Isometsä, J.; Leiviskä, O.; Vähänissi, V.; Savin, H. Comparison of SiNx-Based Surface Passivation Between Germanium and Silicon. Phys. Status Solidi 2023, 220, 2200690. [Google Scholar] [CrossRef]
- Brunco, D.P.; De Jaeger, B.; Eneman, G.; Mitard, J.; Hellings, G.; Satta, A.; Terzieva, V.; Souriau, L.; Leys, F.E.; Pourtois, G.; et al. Germanium MOSFET Devices: Advances in Materials Understanding, Process Development, and Electrical Performance. J. Electrochem. Soc. 2008, 155, H552. [Google Scholar] [CrossRef]
- Sioncke, S.; Brunco, D.P.; Meuris, M.; Uwamahoro, O.; Van Steenbergen, J.; Vrancken, E.; Heyns, M.M. Etch Rates of Ge, GaAs and InGaAs in Acids, Bases and Peroxide Based Mixtures. ECS Trans. 2008, 16, 451–460. [Google Scholar] [CrossRef]
- Kaur, G.; Dwivedi, N.; Zheng, X.; Liao, B.; Peng, L.Z.; Danner, A.; Stangl, R.; Bhatia, C.S. Understanding Surface Treatment and ALD AlOx Thickness Induced Surface Passivation Quality of c-Si Cz Wafers. IEEE J. Photovolt. 2017, 7, 1224–1235. [Google Scholar] [CrossRef]
- Dingemans, G.; Kessels, W.M.M. Status and prospects of Al2O3-based surface passivation schemes for silicon solar cells. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 040802. [Google Scholar] [CrossRef]
- Rad, Z.J.; Lehtiö, J.P.; Mack, I.; Rosta, K.; Chen, K.; Vähänissi, V.; Punkkinen, M.; Punkkinen, R.; Hedman, H.P.; Pavlov, A.; et al. Decreasing Interface Defect Densities via Silicon Oxide Passivation at Temperatures Below 450 °C. ACS Appl. Mater. Interfaces 2020, 12, 46933–46941. [Google Scholar] [CrossRef] [PubMed]
- Jahanshah Rad, Z.; Lehtiö, J.P.; Chen, K.; Mack, I.; Vähänissi, V.; Miettinen, M.; Punkkinen, M.; Punkkinen, R.; Suomalainen, P.; Hedman, H.P.; et al. Effects of post oxidation of SiO2/Si interfaces in ultrahigh vacuum below 450 °C. Vacuum 2022, 202, 111134. [Google Scholar] [CrossRef]
- Wilson, M.; Findlay, A.; D’Amico, J.; Savtchouk, A.; Lagowski, J. State-of-the-art multiparameter characterization of the chemical and field effect passivation of very high lifetime n-Si with n+ front surface field (FSF). In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015. [Google Scholar]
- Wilson, M.; Savtchouk, A.; Lagowski, J.; Kis-Szabo, K.; Korsos, F.; Toth, A.; Kopecek, R.; Mihailetchi, V. QSS-μPCD measurement of lifetime in silicon wafers: Advantages and new applications. Energy Procedia 2011, 8, 128–134. [Google Scholar] [CrossRef]
- Wilson, M.; Lagowski, J.; Jastrzebski, L.; Savtchouk, A.; Faifer, V. COCOS (corona oxide characterization of semiconductor) non-contact metrology for gate dielectrics. In Proceedings of the AIP Conference Proceedings, American Institute of Physics, College Park, MD, USA, 28 March 2001; Volume 550, pp. 220–225. [Google Scholar]
- Klesse, W.M.; Scappucci, G.; Capellini, G.; Simmons, M.Y. Preparation of the Ge(001) surface towards fabrication of atomic-scale germanium devices. Nanotechnology 2011, 22, 145604. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Zhang, R.; Taoka, N.; Huang, P.C.; Takenaka, M.; Takagi, S. 1-nm-thick EOT high mobility Ge n- and p-MOSFETs with ultrathin GeOx/Ge MOS interfaces fabricated by plasma post oxidation. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011. [Google Scholar] [CrossRef]
- Wang, X.; Nishimura, T.; Yajima, T.; Toriumi, A. Thermal oxidation kinetics of germanium. Appl. Phys. Lett. 2017, 111, 052101. [Google Scholar] [CrossRef]
- Kobayashi, M.; Thareja, G.; Ishibashi, M.; Sun, Y.; Griffin, P.; McVittie, J.; Pianetta, P.; Saraswat, K.; Nishi, Y. Radical oxidation of germanium for interface gate dielectric GeO2 formation in metal-insulator-semiconductor gate stack. J. Appl. Phys. 2009, 106, 104117. [Google Scholar] [CrossRef]
- Wada, A.; Zhang, R.; Takagi, S.; Samukawa, S. Formation of thin germanium dioxide film with a high-quality interface using a direct neutral beam oxidation process. Jpn. J. Appl. Phys. 2012, 51, 125603. [Google Scholar] [CrossRef]
- Toriumi, A.; Nishimura, T. Germanium CMOS potential from material and process perspectives: Be more positive about germanium. Jpn. J. Appl. Phys. 2018, 57, 010101. [Google Scholar] [CrossRef]
- Lu, C.; Lee, C.H.; Zhang, W.; Nishimura, T.; Nagashio, K.; Toriumi, A. Structural and thermodynamic consideration of metal oxide doped GeO2 for gate stack formation on germanium. J. Appl. Phys. 2014, 116, 174103. [Google Scholar] [CrossRef]
- Molle, A.; Bhuiyan, M.N.K.; Tallarida, G.; Fanciulli, M. In situ chemical and structural investigations of the oxidation of Ge(001) substrates by atomic oxygen. Appl. Phys. Lett. 2006, 89, 083504. [Google Scholar] [CrossRef]
- Owens, A. Semiconductor Radiation Detectors; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351629171. [Google Scholar]
- Savin, H.; Repo, P.; von Gastrow, G.; Ortega, P.; Calle, E.; Garín, M.; Alcubilla, R. Black silicon solar cells with interdigitated back-contacts achieve 22.1% efficiency. Nat. Nanotechnol. 2015, 10, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Steglich, M.; Käsebier, T.; Kley, E.B.; Tünnermann, A. Black Germanium fabricated by reactive ion etching. Appl. Phys. A Mater. Sci. Process. 2016, 122, 836. [Google Scholar] [CrossRef]
- Schicho, S.; Jaouad, A.; Sellmer, C.; Morris, D.; Aimez, V.; Arès, R. Black germanium produced by inductively coupled plasma etching. Mater. Lett. 2013, 94, 86–88. [Google Scholar] [CrossRef]
- Fung, T.H.; Isometsä, J.; Lehtiö, J.-P.; Pasanen, T.P.; Liu, H.; Leiviskä, O.; Laukkanen, P.; Savin, H.; Vähänissi, V. Efficient Passivation of Nanostructured Germanium Surfaces. Nanotechnology, 2023; under review. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isometsä, J.; Jahanshah Rad, Z.; Fung, T.H.; Liu, H.; Lehtiö, J.-P.; Pasanen, T.P.; Leiviskä, O.; Miettinen, M.; Laukkanen, P.; Kokko, K.; et al. Surface Passivation of Germanium with ALD Al2O3: Impact of Composition and Crystallinity of GeOx Interlayer. Crystals 2023, 13, 667. https://doi.org/10.3390/cryst13040667
Isometsä J, Jahanshah Rad Z, Fung TH, Liu H, Lehtiö J-P, Pasanen TP, Leiviskä O, Miettinen M, Laukkanen P, Kokko K, et al. Surface Passivation of Germanium with ALD Al2O3: Impact of Composition and Crystallinity of GeOx Interlayer. Crystals. 2023; 13(4):667. https://doi.org/10.3390/cryst13040667
Chicago/Turabian StyleIsometsä, Joonas, Zahra Jahanshah Rad, Tsun H. Fung, Hanchen Liu, Juha-Pekka Lehtiö, Toni P. Pasanen, Oskari Leiviskä, Mikko Miettinen, Pekka Laukkanen, Kalevi Kokko, and et al. 2023. "Surface Passivation of Germanium with ALD Al2O3: Impact of Composition and Crystallinity of GeOx Interlayer" Crystals 13, no. 4: 667. https://doi.org/10.3390/cryst13040667
APA StyleIsometsä, J., Jahanshah Rad, Z., Fung, T. H., Liu, H., Lehtiö, J.-P., Pasanen, T. P., Leiviskä, O., Miettinen, M., Laukkanen, P., Kokko, K., Savin, H., & Vähänissi, V. (2023). Surface Passivation of Germanium with ALD Al2O3: Impact of Composition and Crystallinity of GeOx Interlayer. Crystals, 13(4), 667. https://doi.org/10.3390/cryst13040667





