Abstract
Cyclotriphosphazenes, a variety of inorganic rings together with a curing ingredient, 4,4′-methylene dianiline (MDA), are mainly used to enhance the thermal conductivity and mechanical characteristics of epoxy resin (DGEBA). Three DGEBA@MDA, HGCP@MDA, and thermosets were produced, and their curing behaviors were investigated. Using a molecular dynamics (MD) approach, the impact of cyclotriphosphazene on the characteristics of DGEBA composites is thoroughly explored in this paper. Results indicated that the glass transition temperatures (Tg) of DGEBA containing HGCP had slightly decreased compared to DGEBA. With the addition of HGCP to DGEBA, epoxy resin (DGEBA@HGCP@MDA) has a high thermal conductivity of 0.215284 W/m·K, with an increase of 116.04% more than pure DGEBA (0.185524 W/m·K). Moreover, the DGEBA@HGCP@MDA composite has high mechanical strength with a specific Young’s modulus of 5.4902 GPa. In order to forecast and analyze certain performances directly associated with the microstructure characteristics of the various cross-linked resin systems and their composite materials, an MD simulation approach will be quite valuable.
1. Introduction
Due to their low weight and superior tensile strength, polymer matrix composite materials are widely employed as structural materials in the aerospace, automotive, and chemical sectors [1,2,3,4]. Epoxy resins (EP), on the other hand, are polymeric substances that are well-known for having high mechanical and outstanding adhesive qualities. As a result, they are frequently utilized in industry for things like adhesives, coatings, laminates, and construction materials, as well as applications in composites and electronic encapsulation [5,6,7,8,9].
Phosphazene-based epoxy resin has recently attracted considerable interest in chemical engineering and technology. As a result, a new family of cyclotriphosphazene that exhibited extraordinary flame-retardant efficiency and high thermal and chemical stability was generated. The presence of a phosphazene-based motif also provided a noticeable enhancement in the mechanical and electrical properties [10,11]. Currently, many cyclotriphosphazene derivatives have been synthesized on the basis of hexachlorocyclotriphosphazene. They can provide improved thermal and flame retardant properties to epoxy resin. Hexachlorocyclotriphosphazene (HCCP), due to its high potential for substitution of the P-Cl group, is a good starting molecule for the synthesis of novel cyclotriphosphazene-based compounds [12].
A significant amount of experimental and computational work has been done to explore the characteristics of epoxy resins and epoxy-based composites. In the study of epoxy systems, molecular dynamics (MD) modeling has been frequently employed to compute parameters as opposed to labor-intensive experiments with several constraints, including the glass transition temperature (Tg), the mean square displacement (MSD), the coefficient of thermal expansion, and others [13,14]. Using molecular dynamics simulations, Jeyranpour et al. [15], Wu et al. [16], and Yang et al. [17] proposed that the cross-linking density had a significant impact on the properties of the epoxy system. The force fields used in the molecular dynamics of epoxy resins are Dreiding [14], PCFF [16], and COMPASS [15], respectively. The impact of simulation size and the comparison of force fields, however, were not investigated.
Recently, researchers have paid a lot of attention to epoxy-based nanocomposites. Li et al. [18] and Hadden et al. [19] postulated that at the interface region of carbon nanofiber/epoxy composites, the molecule’s energy would drop as the epoxy resin’s crosslinking density increases. Zhang et al. [20] evaluated the elastic modulus of single-walled nanotubes (SWCNT)/epoxy composite across a wide temperature range using MD modeling and experimental verification. The rheological and thermal characteristics of BNNS/epoxy composites have been examined by Liu et al. [21] using the molecular dynamics simulation, and the mechanism by which Boron nitride nanosheet (BNNS) increases the heat conductivity of composite materials has been explored. The findings demonstrated that adding BNNS greatly raised the viscosity of the composites while also significantly enhancing their heat conductivity. The thermal conductivity of composites rises, and the viscosity reduces as BNNS size increases. Yang et al. [22] used molecular dynamics modeling to examine the impact of adding carbon nanobud (CNB) to cross-linked epoxy resins based on DGEBA and methyl tetrahydrophthalic anhydride (MTHPA) on their mechanical characteristics and thermal conductivities. However, the use of short SWCNTs/CNBs limits the improvement in thermal conductivity.
This current work uses MD simulations to examine the temperature, thermal conductivity, and mechanical characteristics of the thermosets DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA. Using the program Materials Studio (MS), simulations were carried out. The computational and analytical findings demonstrate that the interaction between cyclotriphosphazene and epoxy resin is strongly influenced by the presence of cyclotriphosphazene next to DGEBA.
2. Simulation Techniques and Experimentation
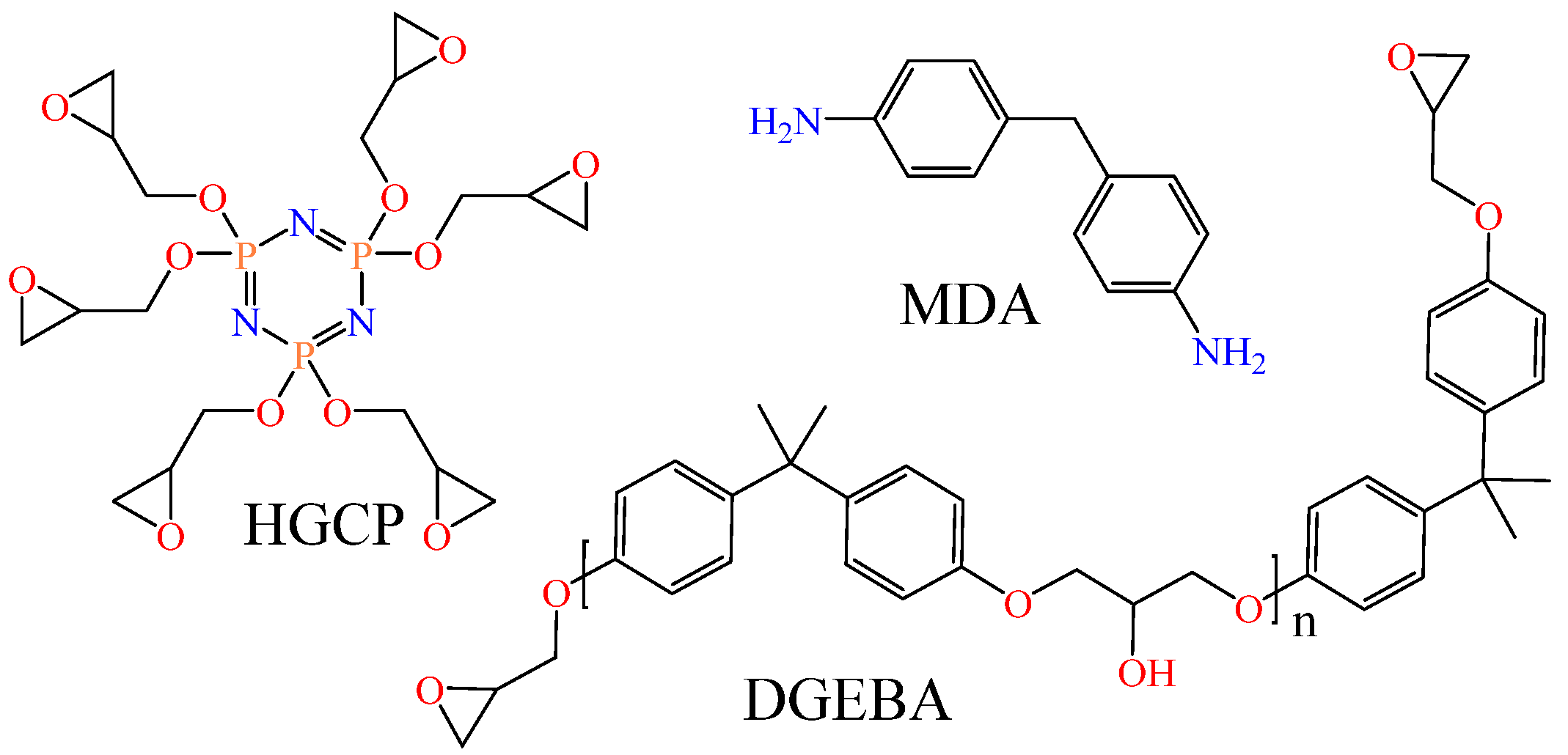
In this study, properties such as bulk density, Tg, Young’s modulus, and thermal conductivity of DGEBA@HGCP@MDA, HGCP@MDA, as well as DGEBA@MDA materials were analyzed by molecular dynamics. We investigated bisphenol-A epoxy resin diglycidyl ether (DGEBA) type Epon 828 and cyclotriphosphazene hexaglycidyl (HGCP) as epoxy resins and 4,4′-methylene dianiline (MDA) as a curing agent (Figure 1). According to the literature report, the HGCP resin synthesis process has been developed [23,24,25].
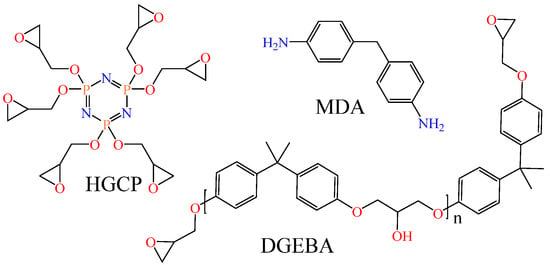
Figure 1.
The study’s main elements of the epoxy composite materials.
The epoxy resins (HGCP, DGEBA and their blend) were warmed to melt, and the curing agent (MDA) was added and mixed until homogeneous. The resin hardener mixture was then poured into preheated molds and cured in a forced convection oven to make samples (Figure S1 see Supplementary Materials) [26]. The mixture of the epoxy resin with the 4,4′-methylene-dianiline (MDA) curing agent is created before the crosslinking is carried out according to the protocol adopted by Levan [27]. The formulations and cure schedules were as follows:
- -
- The samples were prepared by mixing stoichiometric amounts of MDA and epoxy resins HGCP, DGEBA and their blend.
- -
- The samples thus prepared underwent the cycle of heating: one night at 70 °C, three hours at l00 °C, two hours at 120 °C, one hour at 140 °C and 30 min at 150 °C.
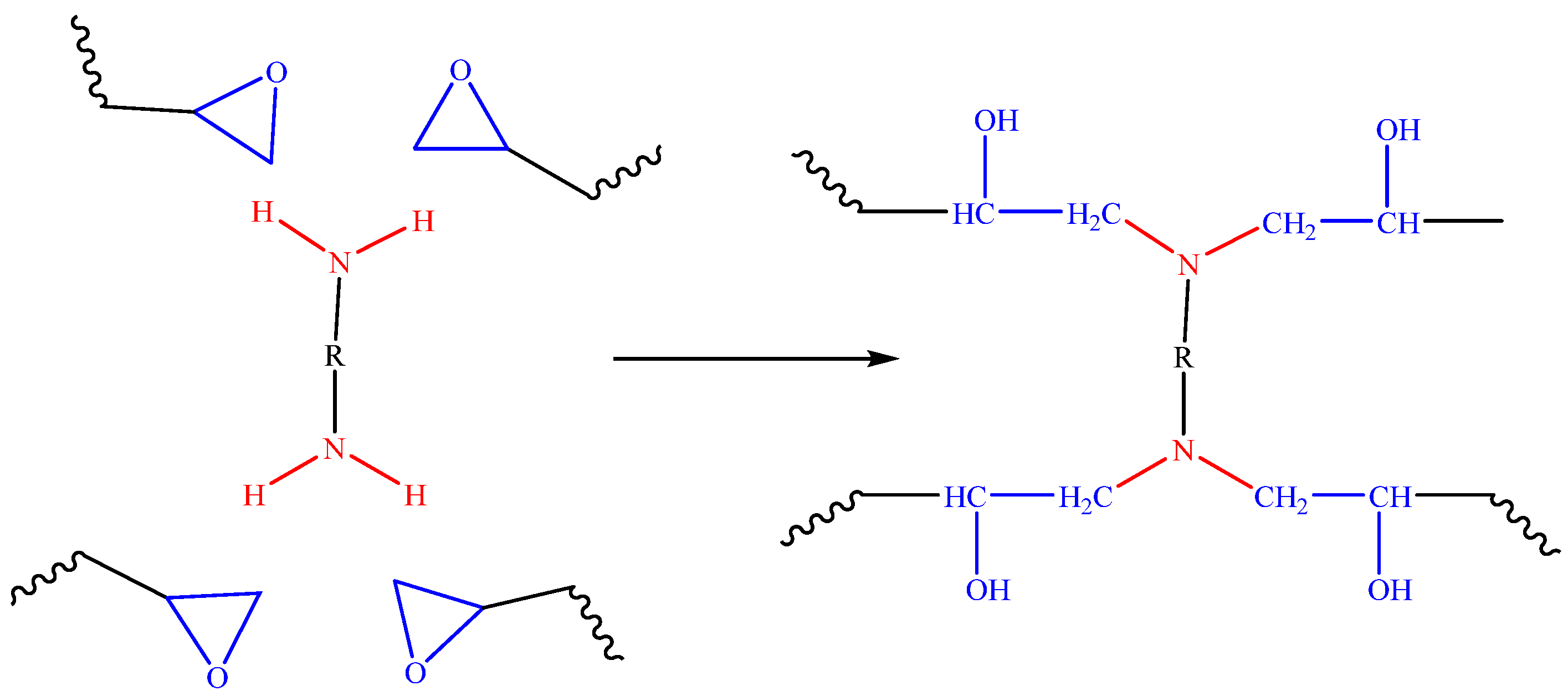
The polymerization of the epoxy resins and the curing agent is a nucleophilic ring-opening reaction [28,29]. The hardening process usually initiated by heat turns into a spontaneous process generating heat [30]. The generally accepted scheme of MDA-epoxy cure involving three main reactions is shown in Figure 2.
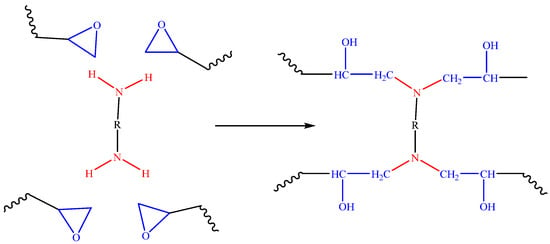
Figure 2.
A proposed mechanism of the reaction of an epoxy resin with a diamine.
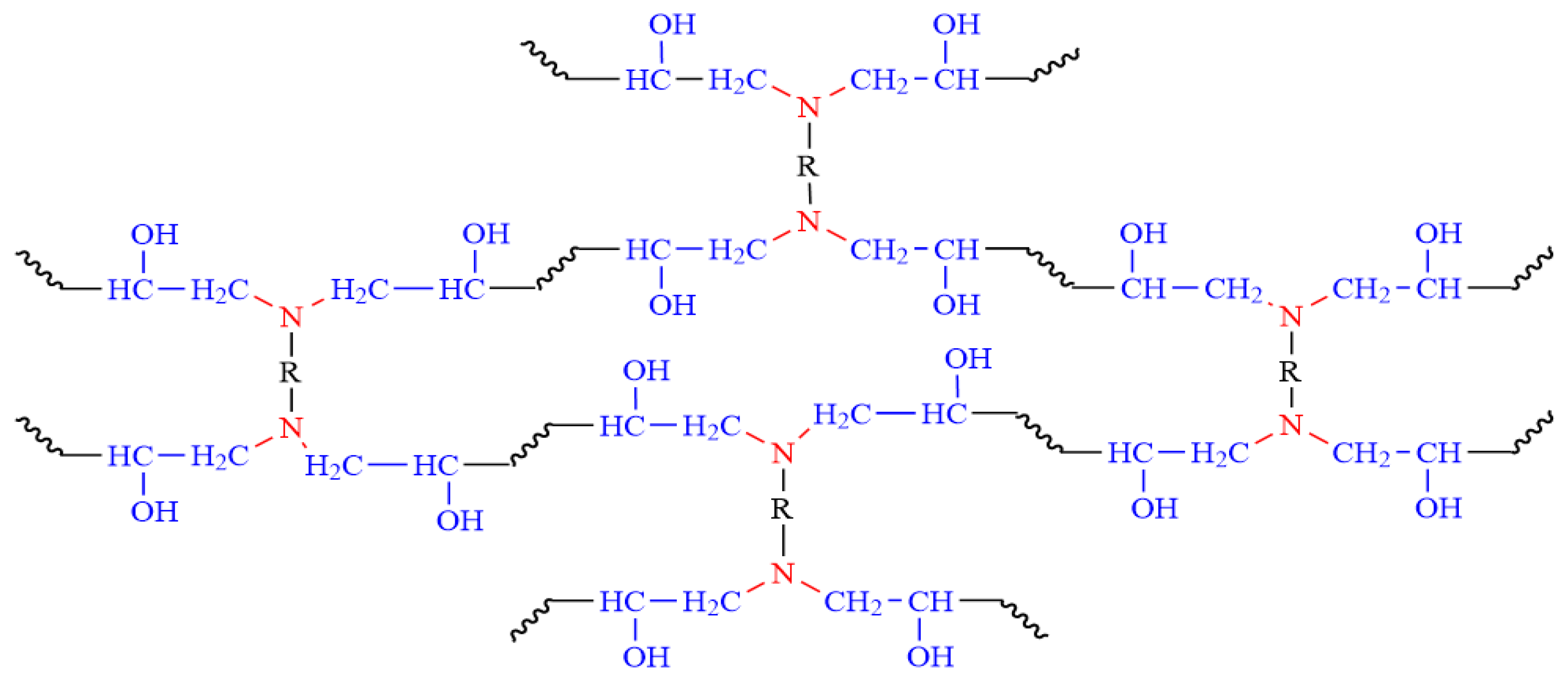
The primary amine initially reacts and links with the epoxide group resulting in a secondary amine. Then, the latter reacts further with the epoxide group. The hydroxyl formed proceeds to react with the epoxide group until a three-dimensional network is formed (Figure 3) [30].
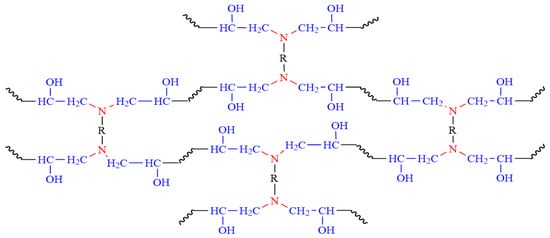
Figure 3.
The curing reaction of epoxy resins with hardener.
Calculation of the epoxy/hardener mixture ratio
In order to obtain optimum properties when curing a multifunctional epoxy resin with a hardener (especially amines), it is important to react the resin and the hardener with stoichiometric quantities. In order to determine the ratio of the mixture, the calculation adopted in all the technical manuals of epoxy resin formulations is made as follows:
Epoxy Equivalent Weight: EEW
The equivalent epoxy (Epoxy Equivalent Weight: EEW) of DGEBA resins of n = 0 and HGCP was calculated as follows:
Case of DGEBA resin:
- M: Molar mass of the DGEBA (M = 340 g/mol).
- f: Functionality of the DGEBA (f = 2).
Case of HGCP resin:
- M: Molar mass of the HGCP (M = 573 g/mol).
- f: Functionality of the HGCP (f = 6).
Amine hydrogen equivalent weights (AHEW) of the hardener were calculated using Equation (1).
- M: Molar mass of the hardener used.
- f: Functionality of the hardener.
Calculation of the Ratio to Weight
The weight ratio of hardener to resin is calculated, in most cases, for 100 parts of resin (or part by weight) or PHR (Parts per Hundred of Resin).
The parts by weight of hardener per hundred parts resin (phr) were calculated using Equation (2).
The resin (DGEBA) with an equivalent weight of 170 g/eq epoxy is mixed with an amine hardener (MDA) with an equivalent weight of 49.56 g/eq amine. For a complete reaction with no unreacted components left over, it is important that one equivalent of epoxy must react with one equivalent of N-H amine. One equivalent epoxy weighs 170 g of the DGEBA and 95.50 g of the HGCP. One equivalent amine weighs 49.56 g. The mix ratio by weight is 170 g epoxy per 49.56 g amine of the DGEBA and 95.50 g epoxy per 49.56 amines of the HGCP. Consequently, the amount of the required MDA for the hardening of 100 g DGEBA is 29.15 g and 51.89 g of the HGCP.
Case of a Mixture (DGEBA@HGCP)
When dealing with formulations consisting of a mixture of epoxy resins on one side and a mixture of amines on the other, which is often the case, it is first necessary to calculate the average EEW and the Average AHEW (Table 1). For two epoxy resins, DGEBA and HGCP mixed were calculated using Equation (3):

Table 1.
Formulations prepared of (DGEBA/HGCP) thermosets with MDA curing agent.
Total Weight (Wt) is the total mass of all products in the epoxy formulation, whether reactive or not. Wt (DGEBA) and Wt (HGCP) are the formulation masses of epoxy resins DGEBA and HGCP, respectively. The same for a mixture of hardener was calculated using Equation (4):
Moreover, the ratio of the mixture will then be:
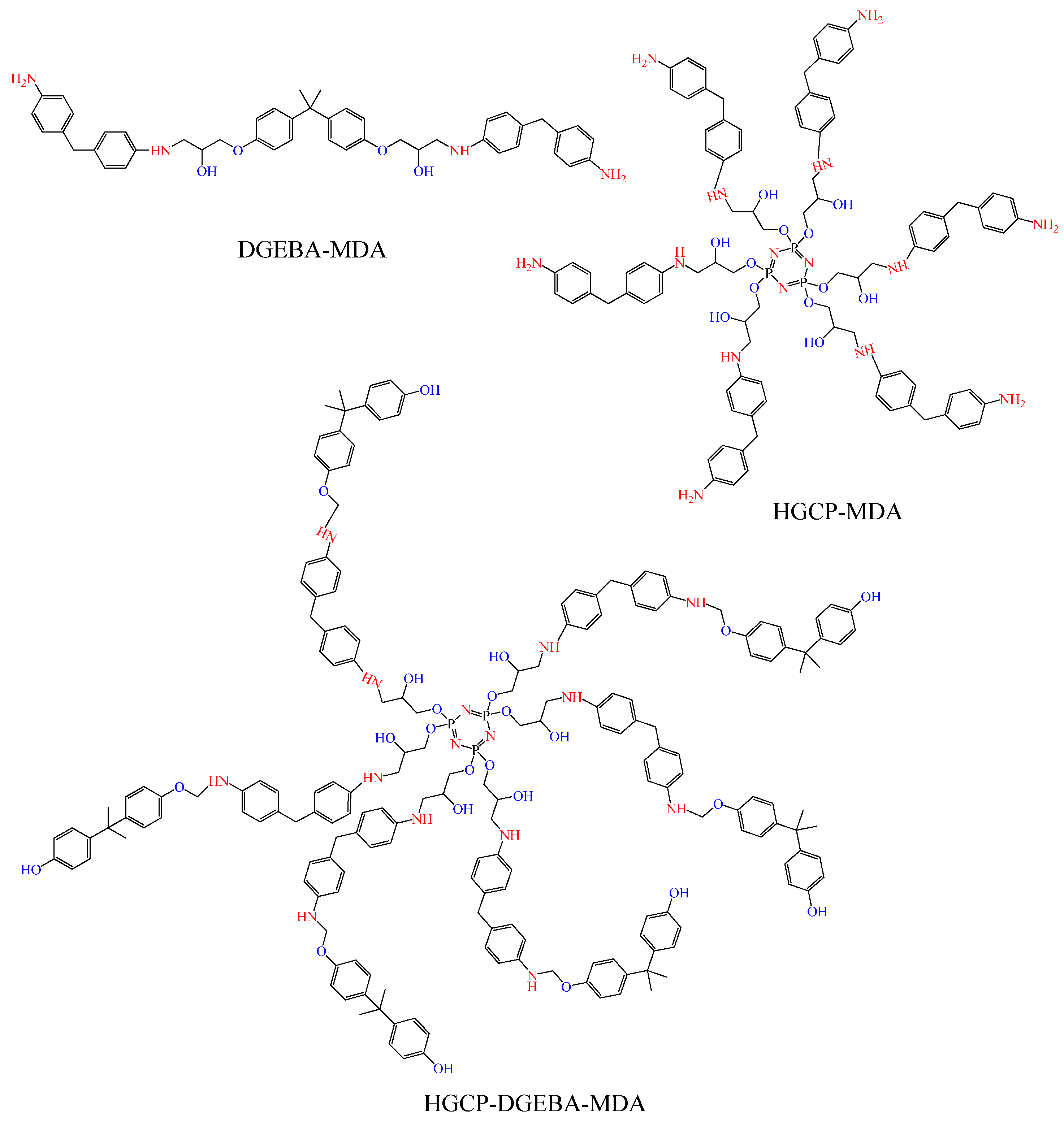
The curing agent (MDA) was introduced and well mixed after the epoxy resins (DGEBA, HGCP, and their combination) had melted (Figure 4). In order to create samples, the epoxy resin hardener mixture was then poured into heated molds and cured in a forced convection oven. Prior to crosslinking, the epoxy resin and MDA hardener were combined in accordance with Levan’s procedure [28,29,30]. The following were the formulas and curing schedules: the samples were made by combining MDA, DGEBA, HGCP, and their combination in a stoichiometric quantity. The samples were then sealed in molds and proceeded through the following cycle of curing: 12 h at 70 °C.
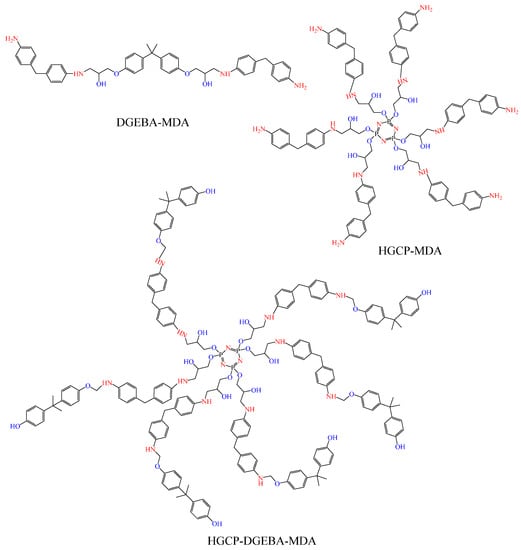
Figure 4.
Epoxy resins (DGEBA, HGCP, and their mixture) crosslinked by MDA.
Several studies have already demonstrated that the degree of dispersion of nanoparticles within polymer matrices greatly affects the physical characteristics. Most notably, it is widely known that the degree of dispersion affects rheological and mechanical properties. While a strong interfacial binding may efficiently transfer the load from the matrix to the reinforcement, the lack of chemical bonding and compatibility between the polymer and the particle, which has a dispersion relationship, might affect the mechanical characteristics of the composites. The chemical makeup of the polymer, as well as the surface area of both the nanoparticles, influence the interactions between the chemical groups of the polymer and even the particles. As the DGEBA is indeed an epoxy resin and the HGCP is coated with organic groups like epoxy groups, excellent dispersion was anticipated. The compatibility of the two species is the reason for this.
Aggregations are seen in the composite (HGCP-DGEBA-MDA) as opposed to the former ([NPCl2]3). He asserts that the improved compatibility between the DGEBA resin as well as the reticulated structure of cyclophosphazene may lead to the chlorocyclophosphazen modification being well disseminated in the DGEBA. This study will concentrate on the impact of HGCP on the physical properties of substances based on DGEBA epoxy resin since it has excellent dispersion in the DGEBA epoxy resin.
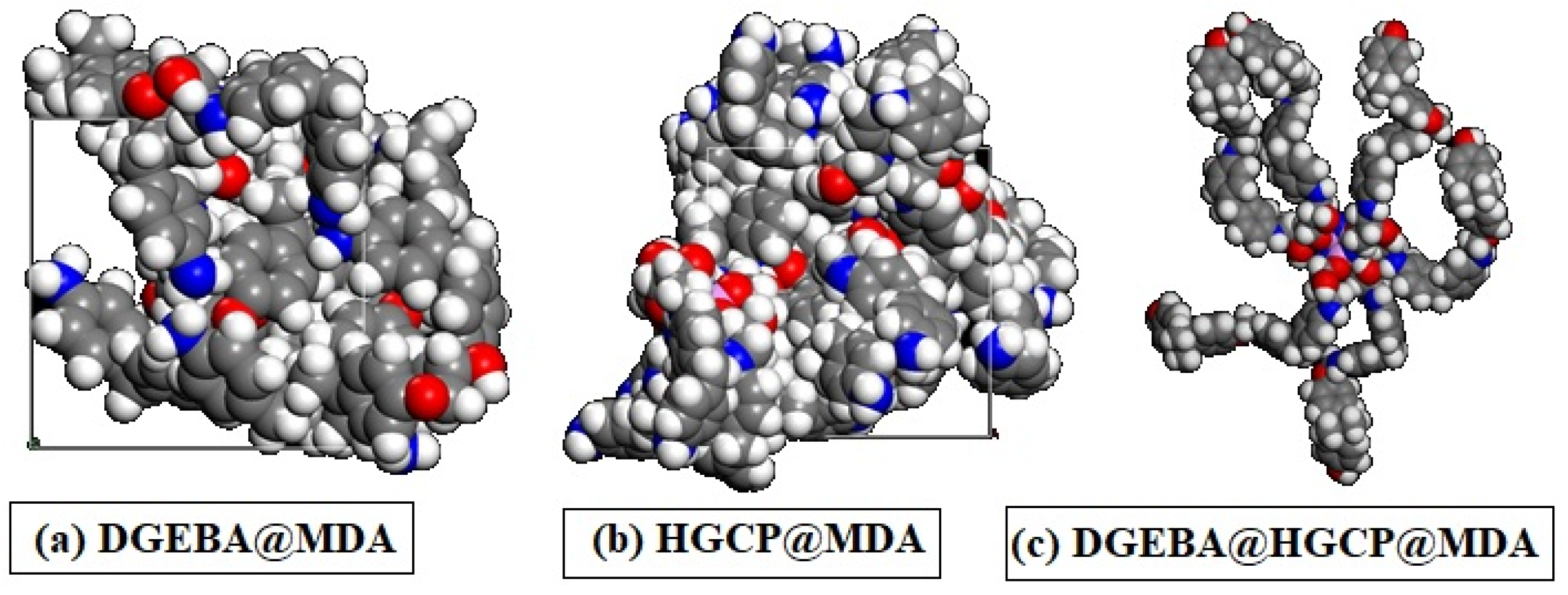
Before the molecular dynamics stage, the Forcite module included in the Biovia software package was used to improve the simulation cell’s form (tolerance for energy convergence of 0.0001 kcal/mol; with a cut-off distance of 15.5 Å, spline width of 1 Å, and buffer of 0.5 Å, the atom-based summing approach was used for electrostatic and Van der Waals interaction). The molecular model for the DGEBA@HGCP@MDA, HGCP@MDA and DGEBA@MDA composite was established in Figure 5.
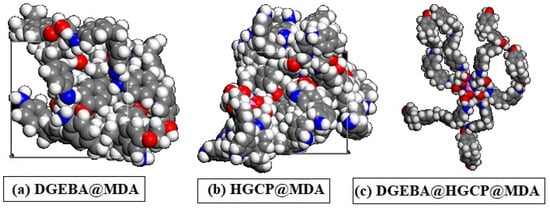
Figure 5.
Schematic description of modeling system of (a) DGEBA@MDA, (b) HGCP@MDA, and (c) DGEBA@HGCP@MDA.
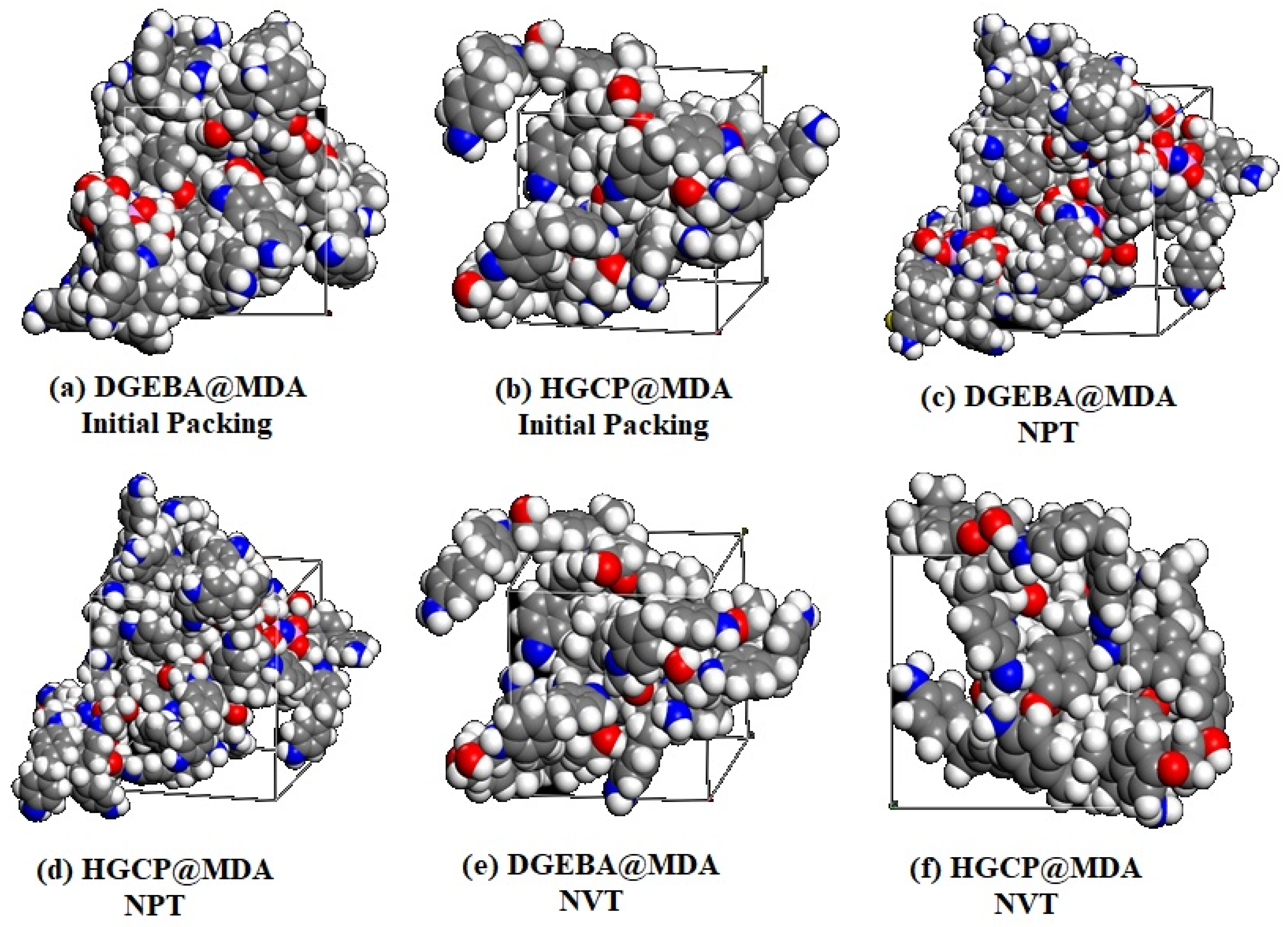
All dynamic simulations in this work use the force field COMPASS II (Version 1.0) [31]. A box containing the values 29.06 Å × 29.06 Å × 29.06 Å HGCP and DGEBA@HGCP20%@MDA was created using the Forcite module and the Build Tool. Berendsen thermostat at 298 K using the COMPASS II (Version 1.0) force field [2,32,33,34], as summarized in Table 2, was established in Figure 6.

Table 2.
Dynamics parameters for (a) DGEBA@MDA, (b) HGCP@MDA, and (c) DGEBA@HGCP@MDA with ensemble NPT and NVT.
Figure 6.
Schematic description of modeling systems of (a) DGEBA@MDA Initial Packing, (b) HGCP@MDA Initial Packing, (c) DGEBA@MDA with NPT, (d) HGCP@MDA with NPT, (e) DGEBA@MDA with NVP and (f) HGCP@MDA with NVP.
3. Results and Discussion
3.1. Analysis of Glass Transition Temperature
Using stoichiometric proportions using the MDA of DGEBA@20wt%, a DSC analysis was carried out to determine the effects of HGCP sap and DGEBA tar on the combination. In our earlier work, we reported on HGCP@MDA, HGCP@MDA, and DGEBA@MDA [24]. Thermograms of HGCP@MDA, DGE-BA@20wt%HGCP@MDA, and DGE-BA@MDA (neat EP) are presented in Table 3. Following are the calculated Tg values: 368 K, 350 K, and 366 K, respectively. Peaks of exothermic hardening were observed in three materials in Figure S2. The temperatures of exothermic hardening began at a higher temperature, i.e., about 90 °C, for materials DGEBA@MDA and DGE-BA@20wt%HGCP@MDA; however, the peak of exothermic hardening for material HGCP@MDA is seen commencing at around 80 °C (353.15 K) (363.15 K). The temperature is reduced by around 3 °C (276.15), far below tidy DGEBA@MDA temperature when HGCP is present in the DGEBA lattice. The oxirane gatherings of the HGCP gum are in direct contact with both the hardener amine (NH2), and this clarifies that the amine/oxirane responses for HGCP@MDA cause faster curing than for the DGEBA@20wt%HGCP@MDA load. It can therefore be concluded that the HGCP pitch is much more responsive to MDA when it is blended alone. The electronic impact may be responsible for this responsiveness. It is acknowledged that the atomic attack of the amino nitrogen molecule on the methylene carbon iota of both the oxirane gathering causes the polymerization of epoxy tar well with an amino relieving operator. The HGCP resin’s inorganic cyclotriphosphazene ring has an electron-attracting action that lowers the electronic density of the oxirane group’s methylene group, which facilitates the amine’s (NH2) nucleophilic assault on the oxirane group. The peak of exothermic crosslinking for the material DGEBA@20wt%HGCP@MDA is prolonged by up to 220 °C (493.15) because the hydroxyl groups (-OH) and residual oxirane groups of something like the DGEBA and HGCP resins are unable to react together readily in the crosslinked structure.
Figure S3 shows a stack plot of the products’ DSC thermograms. The thermograms showed distinct single endothermic changes that lined up with the Tg transitions. The samples (DGEBA@5wt%HGCP@MDA), (DGEBA@10wt%HGCP@MDA), and (DGEBA@15wt%HGCP@MDA) had respective Tg values of 62 °C (335.15 K), 60 °C (333.15 K), and 70 °C (343.15 K).
The occurrence of glycidyl-pendant groups, therefore, increases the free volume and causes Tg to decrease, which is responsible for the lower of the two Tg values. Nevertheless, the occurrence of six reactive groups (epoxy) in the HGCP’s backbone caused the matrix’s nodes to slightly raise the Tg when the HGCP concentration in DGEBA grew.
In light of this, it can be argued that variations in Tg are caused by a complex balance here between free volume shown by the HGCP itself as well as the nodes produced by (HGCP@MDA@DGEBA) crosslinking, which is influenced by the quantity of HGCP [35].
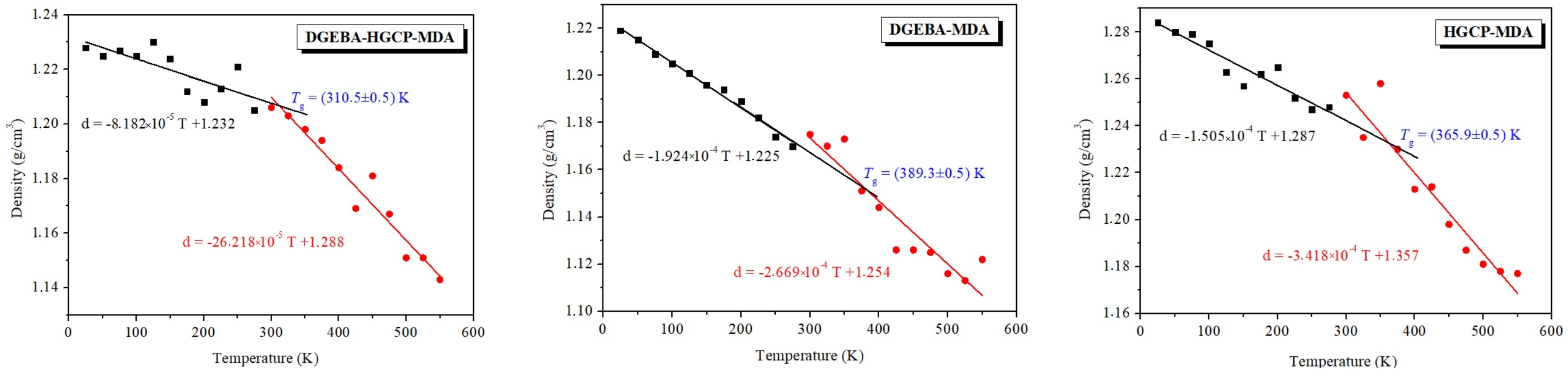
By calculating the mass density as a function of temperature, one may get the glass transition temperature from MD. With differing slopes in the high-temperature (rubbery regime) and low-temperature ranges, a bilinear response is anticipated (glassy regime). These two slopes’ intersection temperature is Tg [36]. An MD simulation is run under NPT circumstances with air pressure and a temperature gradually varying from 25 K to 550 K in order to produce the density vs. temperature curves. The outcomes are displayed in Figure 7. The curves show a bilinear response with a large slope change. By locating the intersection of linear regression that fits the data points above and below the slope change, Tg is calculated.
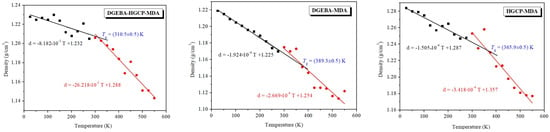
Figure 7.
Temperature-dependent mass density of DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA materials. The outcomes of MD simulations.
From the density vs. temperature slopes, Tgs were defined as the temperature corresponding to the points of gradient changes. The Tg values of HGCP@MDA and DGEBA@MDA obtained were 365.9 K and 389.3 K, respectively, and these values are far greater than that of DGEBA@HGCP@MDA (313 K), indicating that thermosets made of DGEBA and HGCP exhibited good heat resistance [12,23,24,37].
There are three potential causes for the observed phenomena. Firstly, HGCP, a non-reactive phosphorus-containing addition, can raise the epoxy resin’s free volume, which lowers Tg values [38]. Secondly, HGCP with a low melting temperature has a greater plasticization impact on DGEBA, which lowers the reported Tg. Lastly, the observed outcome is also a result of the rod-like geometry, bridging, and stiff molecular structures of HGCP, which restrict the thermal movement of the polymer backbone and the mobility of macromolecular chains [39]. Due to this, adjusted DGEBA’s Tg values somewhat dropped after being added to HGCP loadings.

Table 3.
Theoretical and experimental Tg values for the DGEBA@HGCP@MDA, HGCP@MDA, as well as DGEBA@MDA.
Table 3.
Theoretical and experimental Tg values for the DGEBA@HGCP@MDA, HGCP@MDA, as well as DGEBA@MDA.
| Samples | Tg Values (K) | ||
|---|---|---|---|
| Theoretical Values | Experimental Values | Ref | |
| DGEBA@MDA | 389.30 | 368.00 | [24] |
| 431.55 | [12] | ||
| 425.00 | [40] | ||
| 429.15 | [41] | ||
| 428.15 | [42] | ||
| 405.85 | [43] | ||
| HGCP@MDA | 365.90 | 350.00 | [24] |
| DGEBA@HGCP@MDA | 310.50 | 366.00 | [24] |
3.2. Thermal Conductivity
Theory for Calculation Details of Thermal Conductivity
The thermal conductivity (Wm−1K−1) can be calculated as follows [22,44]:
where (Wm−2) is the heat flux in one direction and ∇T is the gradient temperature (Km−1). The heat flux can be calculated based on Fourier’s law, as follows [22,44]:
where is the energy transferred, S (m2) is the cross-section area, and Δt is the time between the two positions one and two. The temperature (K) is created by the kinetic energy of atoms in every region and can be calculated as follows [22]:
where kB, mi (kg) and vi (m·s−1) are Boltzmann’s constant (cal K−1), masses, and velocities of atoms i, respectively. Equation (4) can be used to calculate the temperature T in each region [45]:
The energy flow and temperature gradient patterns of the DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA materials were calculated using Equations (2) and (3). Equation (1) was subsequently used to obtain the thermal conductivity.
The thermal conductivities of the DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA materials are presented in Table 4. According to the results, the thermal conductivity of the materials DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA is 0.215284, 0.301005, and 0.185524 (W/m·K), respectively. For both DGEBA@HGCP@MDA and HGCP@MDA, their thermal conductivities of thermosets are much greater [46,47,48].

Table 4.
Thermal conductivity, energy flux, and temperature gradient of DGEBA@MDA, HGCP@MDA, and DGEBA@HGCP@MDA.
The pure DGEBA@MDA has a thermal conductivity of just 0.185524 W/m·K [49,50]. The HGCP@MDA’s thermal conductivity improves by 162% (0.301005 W/m·K), whereas the DGEBA@HGCP@MDA’s thermal conductivity increases by 116.04% (0.215284 W/m·K). The findings demonstrate that epoxy thermosets’ thermal conductivity is significantly influenced by the HGCP form.
3.3. Technical Features
Calculating tiny deformations at various static pressures in specified directions can yield mechanical characteristics. In order to generate 12 units of strains well with the extent of strain maintained under 0.01 in line with Hooke’s law, the xy, xz, and yz planes are each subjected to minor shear deformation along the x-, y-, as well as z-axis directions, as illustrated in Equation (5) [15]:
The following are several ways to express the stress component [22,48]:
The Lamé coefficients λ and μ could be determined using the formula below [22,49]:
The following equations can be used to calculate the elastic modulus (E), volume modulus (K), and shear modulus (G) [22]:
The five models’ mechanical performance specifications at room temperature (T = 298 K) were also calculated. Table 5 shows Young’s modulus, Bulk modulus, Shear modulus, Poisson’s ratio, and compressibility of DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA. As shown, the mechanical properties of the DGEBA material modified by the addition of the HGCP (DGEBA@HGCP@MDA) composite were significantly higher than that of both materials, DGEBA and pure HGCP epoxy [47].

Table 5.
Mechanical properties of DGEBA@MDA, HGCP@MDA, and DGEBA@HGCP@MDA.
4. Conclusions
In this study, the thermal stability, thermal conductivity, and mechanical properties of different polymeric materials DGEBA@MDA and HGCP@MDA and their composite (DGEBA@HGCP@MDA) based on DGEBA epoxy resin thermoset with MDA and HGCP epoxy resin as additives were evaluated. Three DGEBA@MDA, HGCP@MDA, and thermosets were produced, and their curing behaviors were investigated.
Molecular dynamics (MD) approach were carried out to look at the thermal conductivity and mechanical properties of the DGEBA@HGCP@MDA, HGCP@MDA, and DGEBA@MDA materials. The computational and analytical findings demonstrate that the interaction between cyclotriphosphazene and epoxy resin is strongly influenced by the presence of cyclotriphosphazene next to DGEBA.
The following findings were made through an analysis of the studied materials:
- -
- The Tg values of DGEBA@MDA and HGCP@MDA are 389.3 K and 365.9 K, respectively, which far exceeded those of DGEBA@HGCP@MDA (310.5 K), demonstrating a strong heat resistance of HGCP thermosets. The early breakdown of DGEBA was produced by the introduction of HGCP, and the Tg of DGEBA was somewhat lowered.
- -
- HGCP@MDA’s thermal conductivity improves by 162% (0.301005 W/m·K), whereas DGEBA@HGCP@MDA’s thermal conductivity increases by 116.04% (0.215284 W/m·K).
- -
- Young’s modulus for DGEBA@HGCP@MDA is 5.4902 GPa, which rose by 103% compared to HGCP@MDA (5.3074 Gpa) and by 114.28% versus pure DGEBA@MDA (4.804 Gpa). The DGEBA-based epoxy materials’ mechanical characteristics were improved when HGCP was included. By improving the compatibility of the cyclophosphazene with the organic matrix, the complexation of cyclophosphazene enabled enhanced dispersion. Shear modulus, Bulk modulus, Young’s modulus, and Poisson’s ratio all significantly improved when HGCP was included in the DGEBA resin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13030478/s1, Figure S1: A samples preparation procedure [26]; Figure S2: DSC thermograms of materials DGEBA@MDA, HGCP@MDA and DGEBA@HGCP20%@MDA [24]; Figure S3: DSC thermograms of materials DGEBA@HGCP5%@MDA, DGEBA@HGCP10%@MDA and DGEBA@HGCP15%@MDA [37].
Author Contributions
Conceptualization, formal analysis, and investigation, O.D. and L.E.G.; methodology, E.B.; software, A.B.; validation, O.H. and S.J.; resources, E.E.E.; writing—original draft preparation, O.D. and R.H; writing—review and editing, E.D.A.; visualization, and supervision, S.-C.K., R.H. and E.E.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korean National Research Foundation (NRF), which is funded by the Ministry of Education, grant number 2020R1I1A3052258.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DGEBA | Bisphenol A diglycidyl ether |
| MDA | 4,4′-Methylene dianiline |
| HGCP | Hexaglycidyl cyclotriphosphazene |
| COMPASS | Condensed-phase optimized molecular potentials for atomistic simulation |
| Studies | |
| MSD | Mean square displacement |
| PCFF | Polymer consistent force-field |
| BNNS | Boron nitride nanosheets |
| MD | Molecular dynamics |
| MTHPA | Methyl tetrahydrophthalic anhydride |
| SWCNT | Single-walled carbon nanotubes |
| CNB | Carbon nanobud |
| NPT | Constant-temperature, constant-pressure ensemble |
| NVT | Constant-temperature, constant-volume ensemble |
| EEW | Epoxy Equivalent Weight |
| AHEW | Amine hydrogen equivalent weights |
| M | Molar mass |
| f | Functionality |
| PHR | Parts per Hundered of Resin |
| Wt | Total Weight |
| EP | Epoxy resins |
References
- Zhao, W.; Zhao, W.; Huang, Z.; Liu, G.; Wu, B. Tribological performances of epoxy resin composite coatings using hexagonal boron nitride and cubic boron nitride nanoparticles as additives. Chem. Phys. Lett. 2019, 732, 136646. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Safi, Z.; Hamed, O.; Jodeh, S.; Verma, C.; Ebenso, E.E.; El Harfi, A. DGEBA-polyaminoamide as effective anti-corrosive material for 15CDV6 steel in NaCl medium: Computational and experimental studies. J. Appl. Polym. Sci. 2020, 137, 48402. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Safi, Z.; Dagdag, S.; Berrani, M.; Jodeh, S.; Verma, C.; Ebenso, E.E.; Wazzan, N.; El Harfi, A. Highly durable macromolecular epoxy resin as anticorrosive coating material for carbon steel in 3% NaCl: Computational supported experimental studies. J. Appl. Polym. Sci. 2020, 137, 49003. [Google Scholar] [CrossRef]
- Dagdag, O.; Hanbali, G.; Khalaf, B.; Jodeh, S.; El Harfi, A.; Deghles, A. Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel. Coatings 2019, 9, 463. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Zhang, J. Curing behavior and thermal properties of trifunctional epoxy resin cured by 4, 4′-diaminodiphenyl sulfone. Express Polym. Lett. 2009, 3, 501–509. [Google Scholar] [CrossRef]
- Dagdag, O.; Essamri, A.; El Gana, L.; El Bouchti, M.; Hamed, O.; Cherkaoui, O.; Jodeh, S.; El Harfi, A. Synthesis, characterization and rheological properties of epoxy monomers derived from bifunctional aromatic amines. Polym. Bull. 2019, 76, 4399–4413. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cai, Z. Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins. Polymers 2020, 12, 317. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; El Gana, L.; Hlimi, Z.; Erramli, H.; Hamed, O.; Jodeh, S. The role of zinc phosphate pigment in the anticorrosion properties of bisphenol A diglycidyl ether-polyaminoamide coating for aluminum alloy AA2024-T3. J. Bio-Tribo-Corros. 2019, 5, 7. [Google Scholar] [CrossRef]
- Dagdag, O.; El Gouri, M.; El Mansouri, A.; Outzourhit, A.; El Harfi, A.; Cherkaoui, O.; El Bachiri, A.; Hamed, O.; Jodeh, S.; Hanbali, G.; et al. Rheological and Electrical Study of a Composite Material Based on an Epoxy Polymer Containing Cyclotriphosphazene. Polymers 2020, 12, 921. [Google Scholar] [CrossRef]
- El Gouri, M.; El-Harfi, A. Modifications chimiques de l’hexachlorocyclotriphosphazène-Préparation de retardateurs de flame et de matériaux polymères ignifuges écologiques (Chemical modification of hexachlorocyclotriphosphazene-Preparation of flame retardants and ecological flame retardant polymers). J. Mater. Environ. Sci. 2012, 3, 17–33. [Google Scholar]
- Liang, W.J.; Zhao, B.; Zhang, C.Y.; Jian, R.K.; Liu, D.Y.; Liu, Y.Q. Enhanced flame retardancy of DGEBA epoxy resin with a novel bisphenol-A bridged cyclotriphosphazene. Polym. Degrad. Stab. 2017, 144, 292–303. [Google Scholar] [CrossRef]
- Yarovsky, I.; Evans, E. Computer simulation of structure and properties of crosslinked polymers: Application to epoxy resins. Polymer 2002, 43, 963–969. [Google Scholar] [CrossRef]
- Wu, C.; Xu, W. Atomistic molecular modelling of crosslinked epoxy resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Arab, B. Comparative investigation of thermal and mechanical properties of cross-linked epoxy polymers with different curing agents by molecular dynamics simulation. J. Mol. Graph. Model. 2015, 62, 157–164. [Google Scholar] [CrossRef]
- Fan, H.B.; Yuen, M.M. Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation. Polymer 2007, 48, 2174–2178. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Li, C.; Browning, A.R.; Christensen, S.; Strachan, A. Atomistic simulations on multilayer graphene reinforced epoxy composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1293–1300. [Google Scholar] [CrossRef]
- Hadden, C.; Jensen, B.; Bandyopadhyay, A.; Odegard, G.; Koo, A.; Liang, R. Molecular modeling of EPON-862/graphite composites: Interfacial characteristics for multiple crosslink densities. Compos. Sci. Technol. 2013, 76, 92–99. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Gao, L.; Zhang, Q.; Zhong, W.; Sui, G.; Yang, X. Molecular simulation and experimental analysis on thermal and mechanical properties of carbon nanotube/epoxy resin composites with different curing agents at high-low temperature. Polym. Compos. 2018, 39, E945–E954. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Zhou, C.; Zhu, W. A molecular dynamics study on thermal and rheological properties of BNNS-epoxy nanocomposites. Int. J. Heat Mass Transf. 2018, 126, 353–362. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Wang, X.; Fu, Y.; Huang, Z.; Xie, Q. Molecular dynamics studies of the mechanical behaviors and thermal conductivity of the DGEBA/MTHPA/CNB composites. Compos. Part B Eng. 2019, 164, 659–666. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Dagdag, O.; El Gouri, M.; Safi, Z.S.; Wazzan, N.; Safi, S.K.; Jodeh, S.; Hamed, O.; Haldhar, R.; Verma, C.; Ebenso, E.E. Flame retardancy of an intumescent epoxy resin containing cyclotriphosphazene: Experimental, computational and statistical studies. Iran. Polym. J. 2021, 30, 1169–1179. [Google Scholar] [CrossRef]
- Dagdag, O.; Bachiri, A.E.; Hamed, O.; Haldhar, R.; Verma, C.; Ebenso, E.; Gouri, M.E. Dendrimeric epoxy resins based on hexachlorocyclotriphosphazene as a reactive flame retardant polymeric materials: A review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3240–3261. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Ziraoui, R.; Rafik, M.; El Harfi, A. A phosphazene compound multipurpose application-Composite material precursor and reactive flame retardant for epoxy resin materials. J. Mater. Environ. Sci. 2011, 2, 319–334. [Google Scholar]
- Levan, Q. Thèse de Docteur-Ingénieur; INP: Toulouse, France, 1981. [Google Scholar]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-based epoxy cured with a polyaminoamide as an anticorrosive coating for aluminum 2024-T3 surface: Experimental studies supported by computational modeling. J. Bio-Tribo-Corros. 2019, 5, 58. [Google Scholar] [CrossRef]
- Dagdag, O.; Hamed, O.; Erramli, H.; El Harfi, A. Anticorrosive performance approach combining an epoxy polyaminoamide–zinc phosphate coatings applied on sulfo-tartaric anodized aluminum alloy 5086. J. Bio-Tribo-Corros. 2018, 4, 52. [Google Scholar] [CrossRef]
- Dagdag, O.; El Bouchti, M.; Cherkaoui, O.; Hamed, O.; El Gouri, M.; Dagdag, S.; El Harfi, A. A study on thermal and rheological cure characterization of a sulfur-containing epoxy resin. J. Chem. Technol. Metall. 2019, 54, 881–888. [Google Scholar]
- Tang, Y.; Tang, C.; Hu, D.; Gui, Y. Effect of aminosilane coupling agents with different chain lengths on thermo-mechanical properties of cross-linked epoxy resin. Nanomaterials 2018, 8, 951. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182–192. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Berisha, A.; Safi, Z.; Verma, C.; Ebenso, E.; Touhami, M.E.; El Gouri, M. Fabrication of polymer based epoxy resin as effective anti-corrosive coating for steel: Computational modeling reinforced experimental studies. Surf. Interfaces 2020, 18, 100454. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Chandra, R.; Singh, S.P.; Mandal, A.; Dondapati, R.S. Overview of BIOVIA Materials Studio, LAMMPS, and GROMACS; Molecular Dynamics Simulation of Nanocomposites Using BIOVIA Materials Studio, LAMMPS and GROMACS; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–100. [Google Scholar]
- El Gouri, M.; El Mansouri, A.; El Gouri, R.; Hadik, N.; Cherkaoui, O.; Outzourhit, A.; El Harfi, A. Physical behaviour of epoxy resin material flame retarded with a reactive flame retardant based on cyclophosphazene. J. Mater. Environ. Sci. 2014, 5, 400–407. [Google Scholar]
- Fan, J.; Anastassiou, A.; Macosko, C.W.; Tadmor, E.B. Molecular dynamics predictions of thermomechanical properties of an epoxy thermosetting polymer. Polymer 2020, 196, 122477. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, C.; Zhai, X.; Qu, L.; Mu, J. Effect of hexaphenoxycyclotriphosphazene combined with octapropylglycidylether polyhedral oligomeric silsesquioxane on thermal stability and flame retardancy of epoxy resin. High Perform. Polym. 2014, 26, 744–752. [Google Scholar] [CrossRef]
- Zang, L.; Wagner, S.; Ciesielski, M.; Mueller, P.; Döring, M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym. Adv. Technol. 2011, 22, 1182–1191. [Google Scholar] [CrossRef]
- Ciesielski, M.; Schäfer, A.; Döring, M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym. Adv. Technol. 2008, 19, 507–515. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. Improvement of the flame retardant and thermomechanical properties of epoxy resins by a vanillin-derived cyclotriphosphazene-cored triazole compound. Polym. Degrad. Stab. 2022, 204, 110088. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Yang, S.; Yang, L.; Cao, J.; Yang, K. The synthesis, curing kinetics, thermal properties and flame rertardancy of cyclotriphosphazene-containing multifunctional epoxy resin. Thermochim. Acta 2019, 680, 178348. [Google Scholar] [CrossRef]
- Xu, G.-R.; Xu, M.-J.; Li, B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym. Degrad. Stab. 2014, 109, 240–248. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Su, C.-H.; Chen, H.-L.; Ju, S.-P.; Liao, B.-Y.; Pan, C.-T. Thermal conductivity and stability for ultrathin SiO2 nanowires: Insight from molecular dynamics simulation. Mater. Res. Express 2019, 6, 105073. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, C.; Wu, D.; Rao, Z. Influence of chemical bonding on thermal contact resistance at silica interface: A molecular dynamics simulation. Comput. Mater. Sci. 2018, 149, 316–323. [Google Scholar] [CrossRef]
- Jin, W.; Yuan, L.; Liang, G.; Gu, A. Multifunctional cyclotriphosphazene/hexagonal boron nitride hybrids and their flame retarding bismaleimide resins with high thermal conductivity and thermal stability. ACS Appl. Mater. Interfaces 2014, 6, 14931–14944. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Taylor, J.P. Phosphorylation of phosphazenes and its effects on thermal properties and fire retardant behavior. Polym. Eng. Sci. 2000, 40, 1177–1189. [Google Scholar] [CrossRef]
- Dieck, E.J.; Quinn, R.L. Low Smoke Polyphosphazene Compositions. U.S. Patent No 4,083,820, 11 April 1978. [Google Scholar]
- Zhu, M.; Li, J.; Chen, J.; Song, H.; Zhang, H. Improving thermal conductivity of epoxy resin by filling boron nitride nanomaterials: A molecular dynamics investigation. Comput. Mater. Sci. 2019, 164, 108–115. [Google Scholar] [CrossRef]
- Aghadavoudi, F.; Golestanian, H.; Tadi Beni, Y. Investigating the effects of resin crosslinking ratio on mechanical properties of epoxy-based nanocomposites using molecular dynamics. Polym. Compos. 2017, 38, E433–E442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).