Synthesis and Luminescence Properties of Green-to-Red Color-Tunable Upconverting K2Gd(PO4)(WO4):Yb3+,Tb3+,Eu3+ Phosphors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
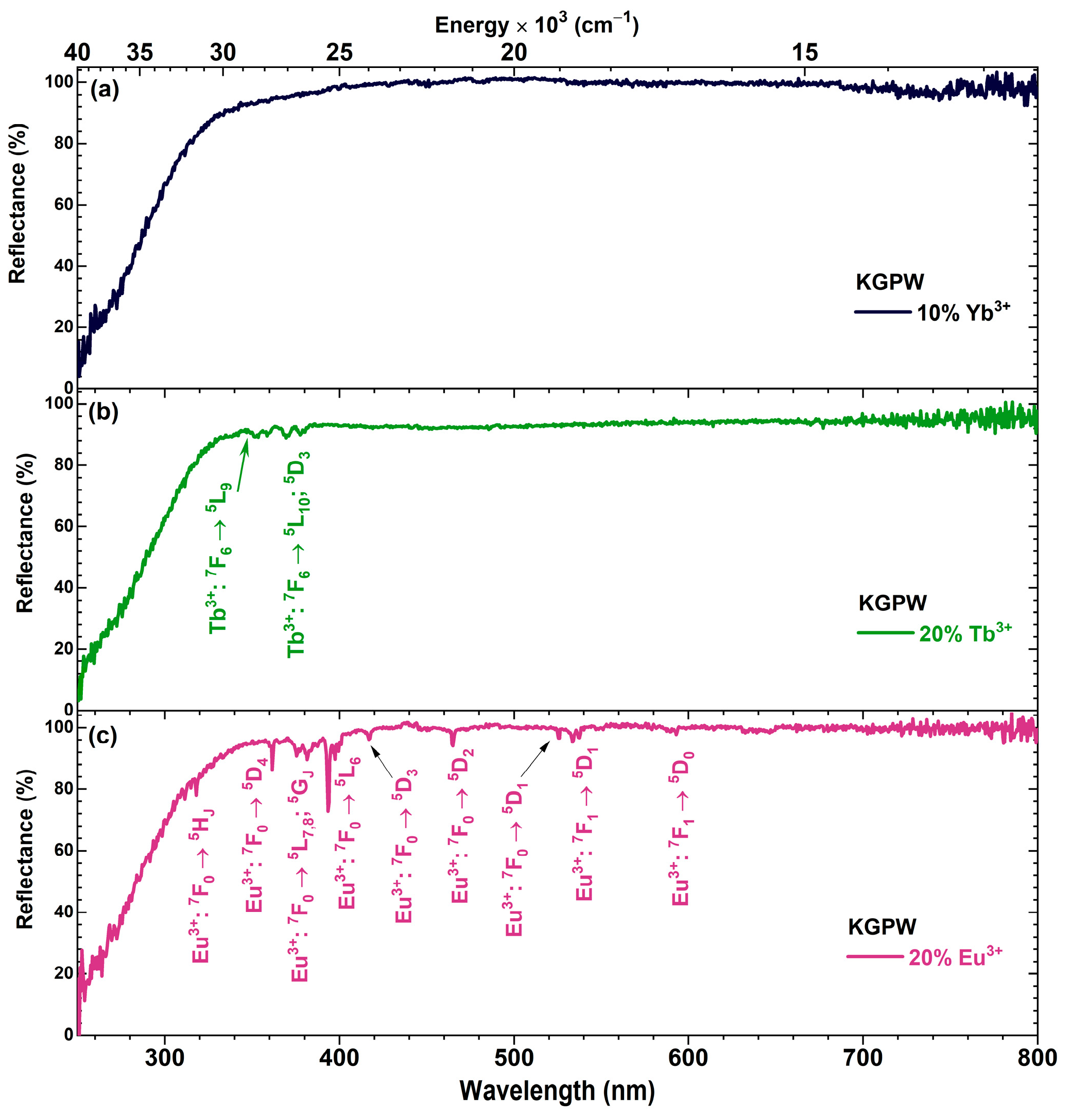
3.1. Structural Analysis
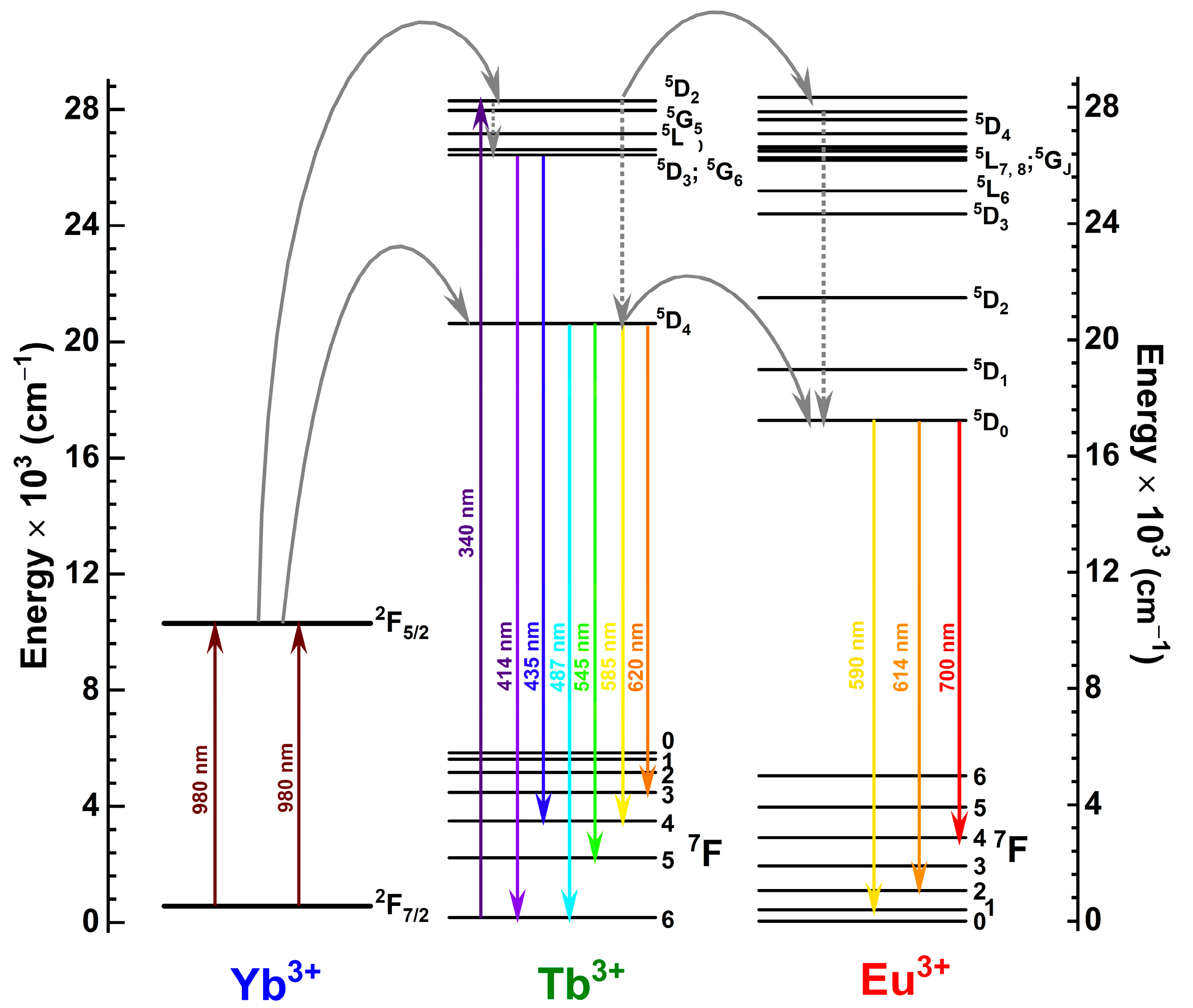
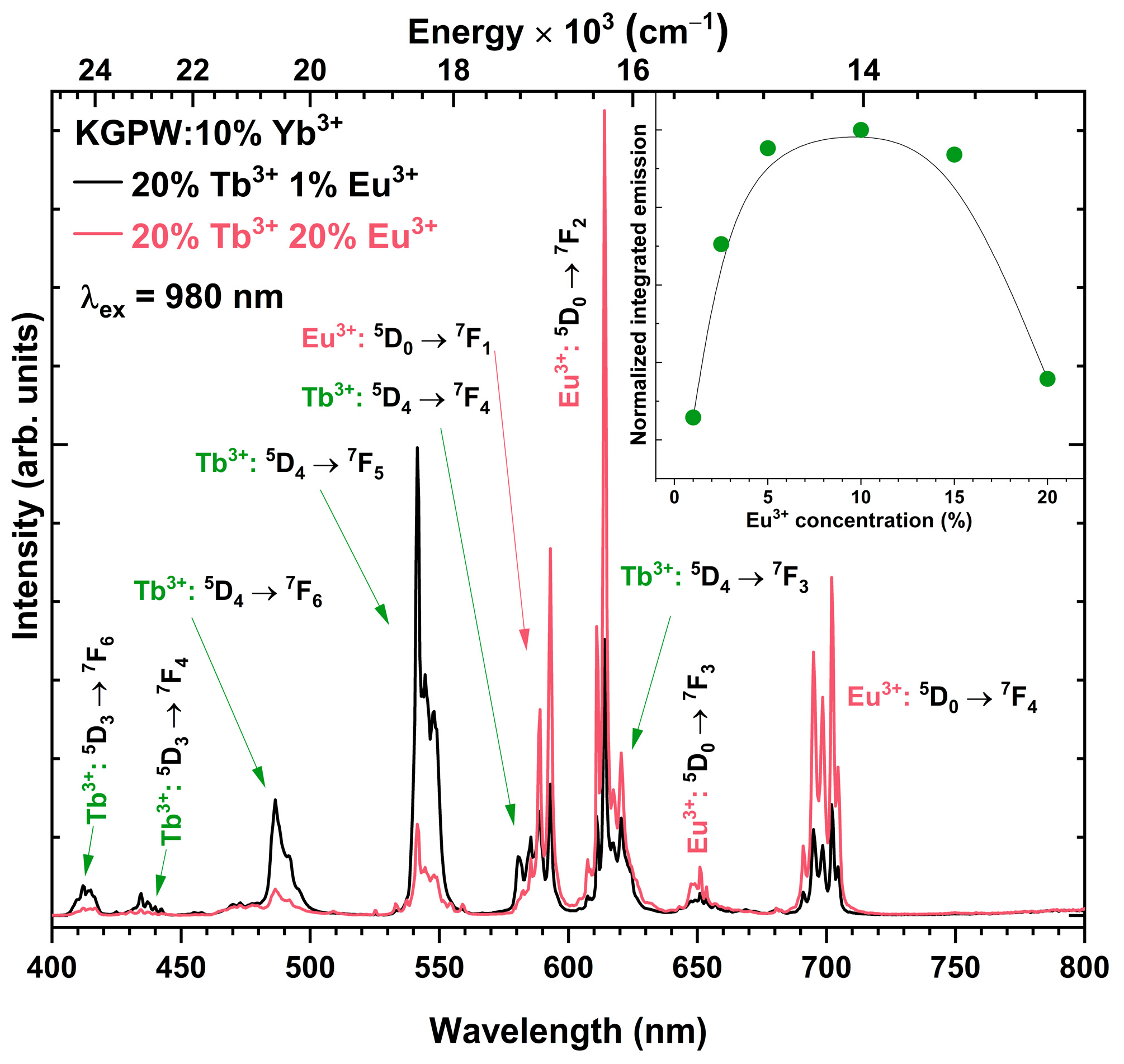
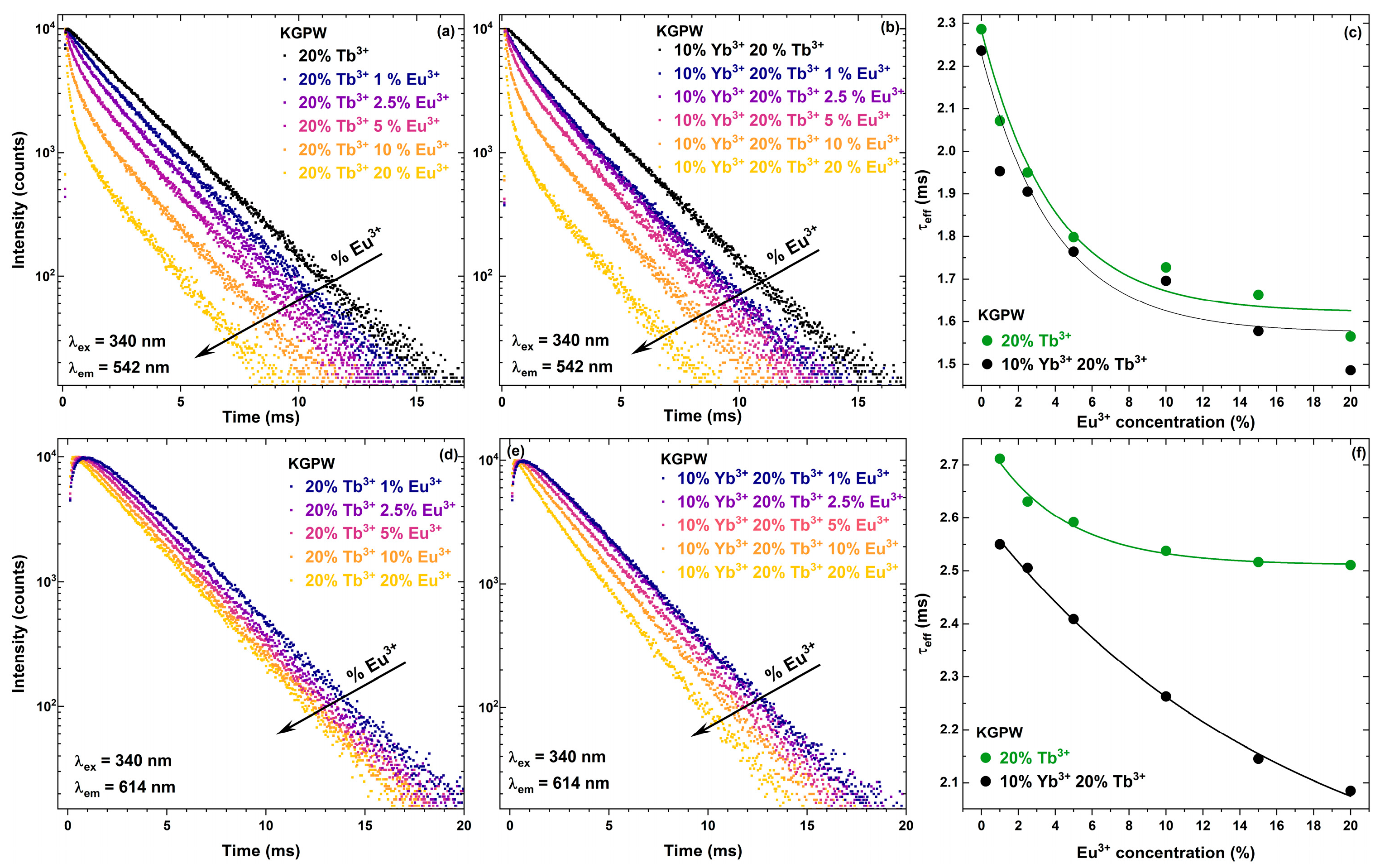
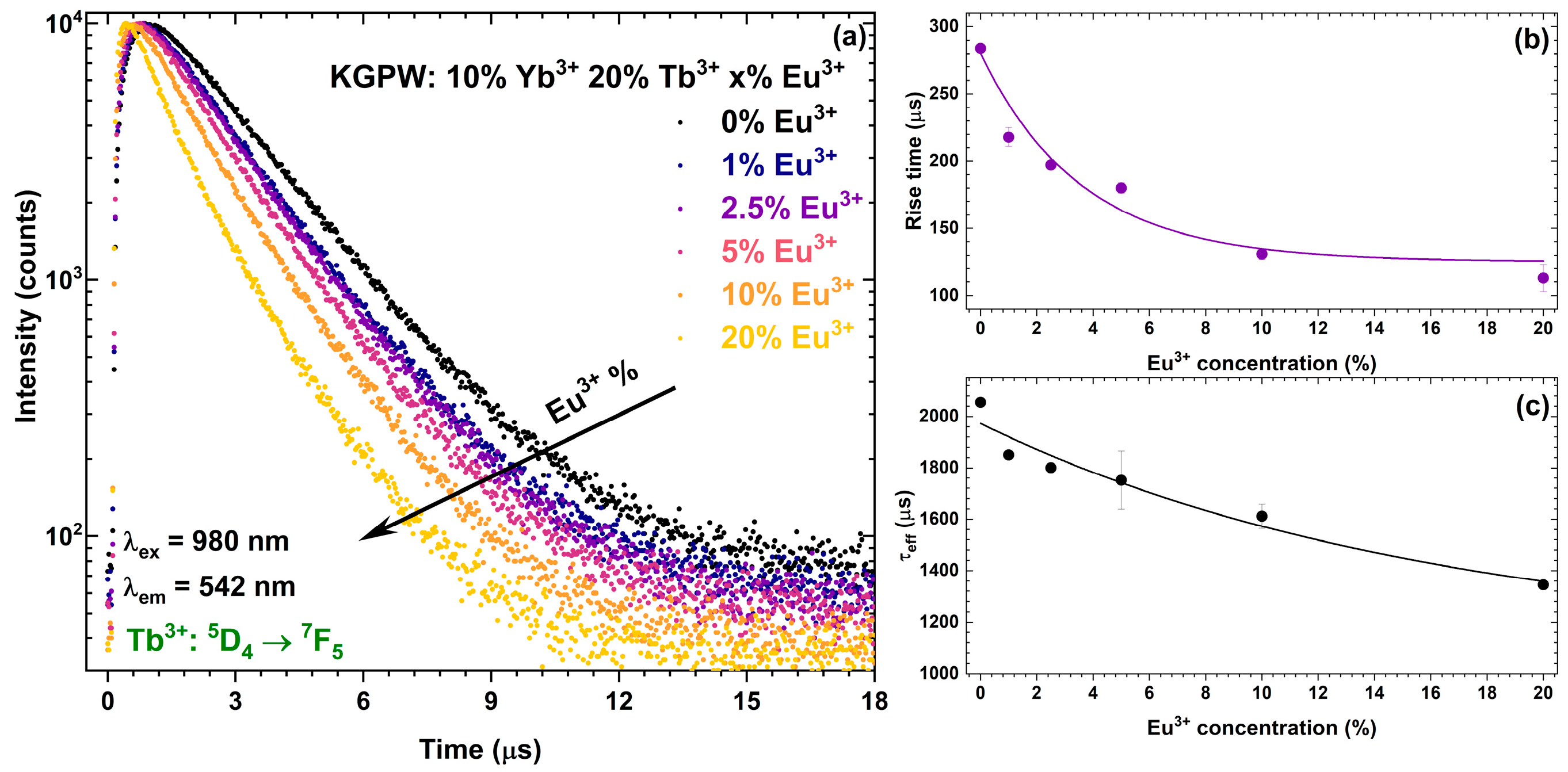
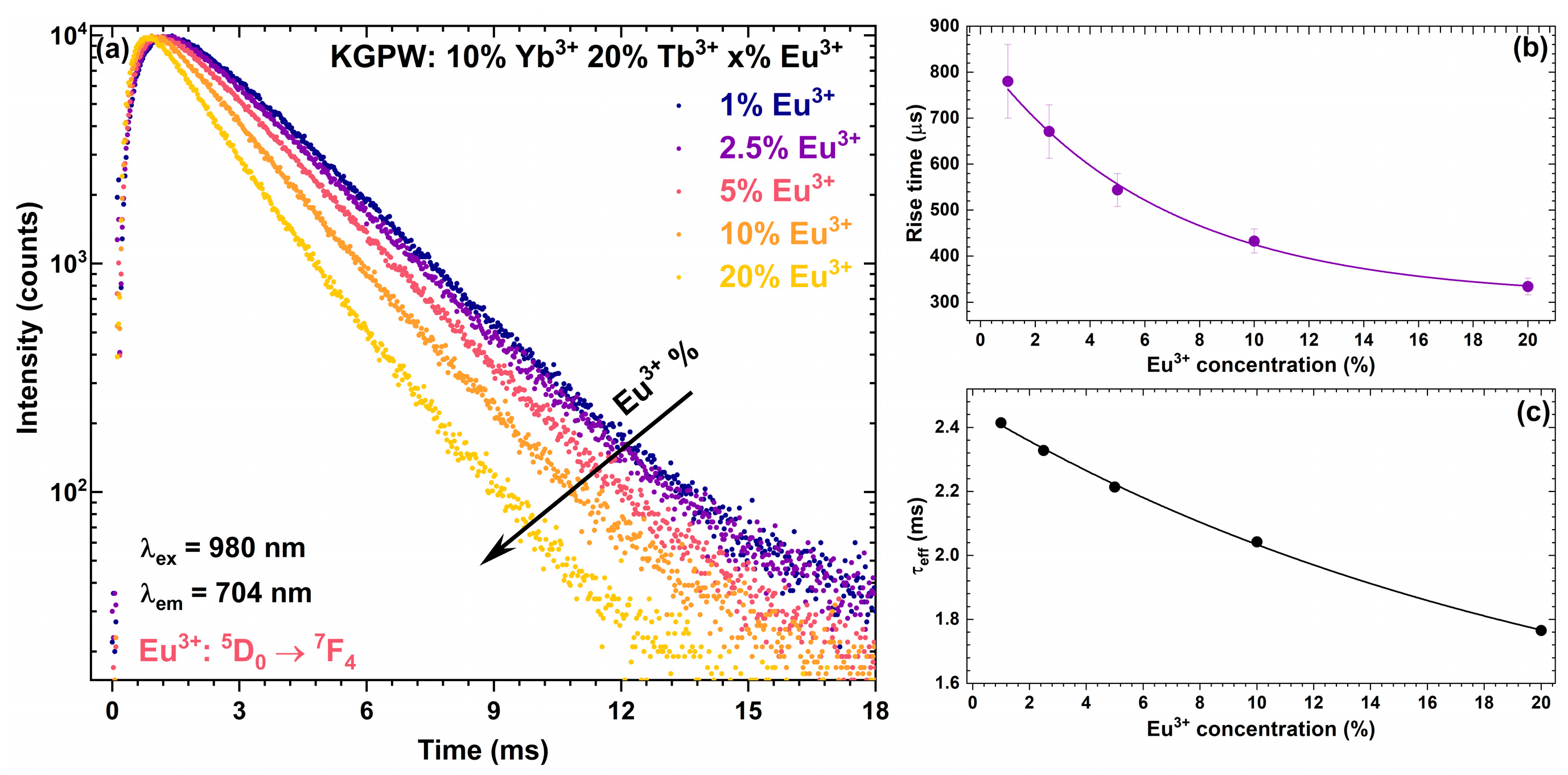
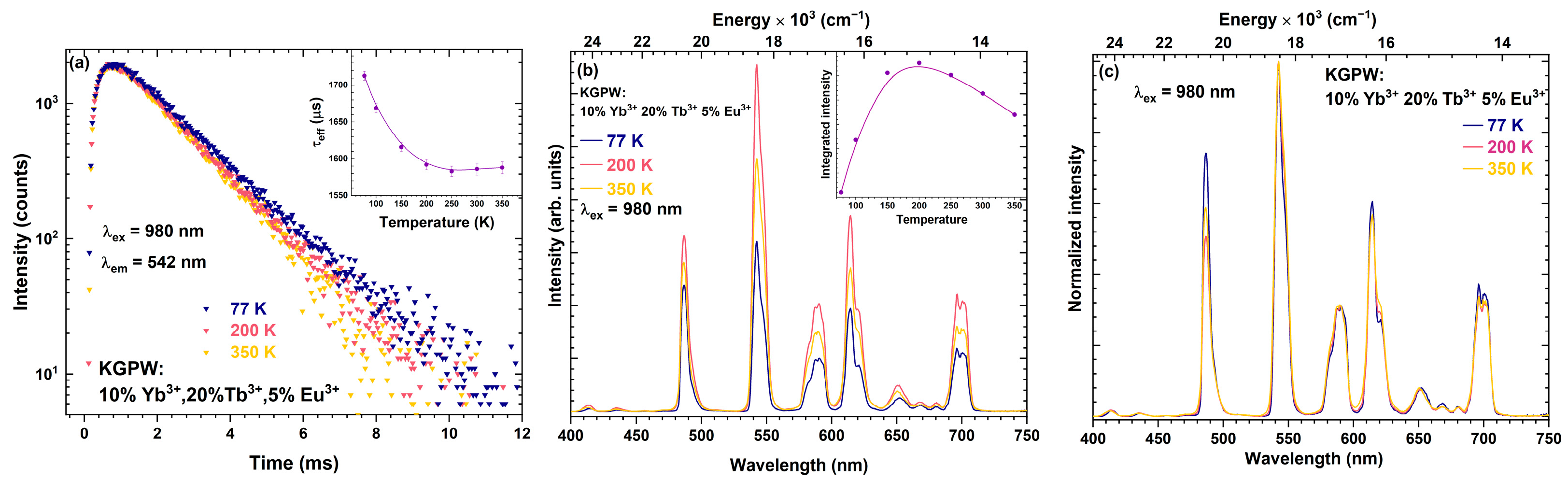
3.2. Photoluminescence Studies
3.3. Photometric
3.4. Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kataria, V.; Mehta, D.S. Multispectral harvesting rare-earth oxysulphide based highly efficient transparent luminescent solar concentrator. J. Rare Earth 2022, 40, 41–48. [Google Scholar] [CrossRef]
- Alekhin, M.S.; Renger, J.; Kasperczyk, M.; Douissard, P.-A.; Martin, T.; Zorenko, Y.; Vasil’ev, D.A.; Stiefel, M.; Novotny, L.; Stampanoni, M. STED properties of Ce3+, Tb3+, and Eu3+ doped inorganic scintillators. Opt. Express 2017, 25, 1251–1261. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Ezerskyte, E.; Paterek, J.; Saitzek, S.; Zabiliute-Karaliune, A.; Vitta, P.; Enseling, D.; Justel, T.; Katelnikovas, A. Luminescence and luminescence quenching of K2Bi(PO4)(MoO4):Sm3+ phosphors for horticultural and general lighting applications. Mater. Adv. 2020, 1, 1427–1438. [Google Scholar] [CrossRef]
- Sujecki, S. Modelling and Design of Lanthanide Ion-Doped Chalcogenide Fiber Lasers: Progress towards the Practical Realization of the First MIR Chalcogenide Fiber Laser. Fibers 2018, 6, 25. [Google Scholar] [CrossRef]
- Cheng, F.R.; Xia, Z.G.; Jing, X.P.; Wang, Z.Y. Li/Ag ratio dependent structure and upconversion photoluminescence of LixAg1-xYb0.99(MoO4)2:0.01Er3+ phosphors. Phys. Chem. Chem. Phys. 2015, 17, 3689–3696. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Katelnikovas, A. Synthesis and optical properties investigation of blue-excitable red-emitting K2Bi(PO4)(MoO4):Pr3+ powders. J. Mater. Res. Technol. 2020, 9, 15779–15787. [Google Scholar] [CrossRef]
- Lamon, S.; Wu, Y.; Zhang, Q.; Liu, X.; Gu, M. Nanoscale optical writing through upconversion resonance energy transfer. Sci. Adv. 2021, 7, eabe2209. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lu, Y.Q.; Yang, X.S.; Zheng, X.L.; Wen, S.H.; Wang, F.; Vidal, X.; Zhao, J.B.; Liu, D.M.; Zhou, Z.G.; et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mahata, M.K.; Hofsass, H.C.; Vetter, U. Photon-Upconverting Materials: Advances and Prospects for Various Emerging Applications. In Luminescence-An Outlook on the Phenomena and Their Applications; InTech: Rijeka, Croatia, 2016; pp. 109–131. [Google Scholar] [CrossRef]
- Suyver, J.F.; Aebischer, A.; Biner, D.; Gerner, P.; Grimm, J.; Heer, S.; Kramer, K.W.; Reinhard, C.; Gudel, H.U. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt. Mater. 2005, 27, 1111–1130. [Google Scholar] [CrossRef]
- Giang, L.T.K.; Trejgis, K.; Marciniak, L.; Vu, N.; Minh, L.Q. Fabrication and characterization of up-converting beta-NaYF4:Er3+,Yb3+@NaYF4 core-shell nanoparticles for temperature sensing applications. Sci. Rep. 2020, 10, 14672. [Google Scholar] [CrossRef]
- Mikalauskaite, I.; Pleckaityte, G.; Skapas, M.; Zarkov, A.; Katelnikovas, A.; Beganskiene, A. Emission spectra tuning of upconverting NaGdF4:20% Yb, 2% Er nanoparticles by Cr3+ co-doping for optical temperature sensing. J. Lumin. 2019, 213, 210–217. [Google Scholar] [CrossRef]
- Gunaseelan, M.; Yamini, S.; Kumar, G.A.; Senthilselvan, J. Highly efficient upconversion luminescence in hexagonal NaYF4:Yb3+, Er3+ nanocrystals synthesized by a novel reverse microemulsion method. Opt. Mater. 2018, 75, 174–186. [Google Scholar] [CrossRef]
- Ma, Y.R.; Qiu, P.S.; Xu, D.F.; Lin, J.R.; Tang, Y.X.; Wang, F.F.; He, X.Y.; Zhou, Z.Y.; Sun, N.X.; Zhang, X.Y.; et al. Controllable synthesis and upconversion luminescence of NaYF4:Yb3+, Er3+ nanocrystals. Ceram Int. 2015, 41, S713–S718. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Chen, G.Y. Controlled Synthesis of Monodisperse Hexagonal NaYF4:Yb/Er Nanocrystals with Ultrasmall Size and Enhanced Upconversion Luminescence. Molecules 2017, 22, 2113. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, P.; Przybylska, D.; Klima, G.; Grzyb, T. Improvement in Luminescence Intensity of beta-NaYF4: 18%Yb3+, 2%Er3+@β-NaYF4 Nanoparticles as a Result of Synthesis in the Presence of Stearic Acid. Nanomaterials 2022, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, T. Bright and tunable up-conversion luminescence through cooperative energy transfer in Yb3+, Tb3+ and Eu3+ co-doped LaPO4 nanocrystals. Rsc. Adv. 2014, 4, 2590–2595. [Google Scholar] [CrossRef]
- Neto, A.N.C.; Jr, R.T.M.; Shyichuk, A.; Paterlini, V.; Piccinelli, F.; Bettinelli, M.; Malta, O.L. Theoretical and Experimental Investigation of the Tb3+ → Eu3+ Energy Transfer Mechanisms in Cubic A3Tb0.90Eu0.10(PO4)3 (A = Sr, Ba) Materials. J. Phys. Chem. C 2020, 124, 10105–10116. [Google Scholar] [CrossRef]
- Mi, R.Y.; Chen, J.; Liu, Y.G.; Fang, M.H.; Mei, L.F.; Huang, Z.H.; Wang, B.C.; Zhaob, C.L. Luminescence and energy transfer of a color tunable phosphor: Tb3+ and Eu3+ co-doped ScPO4. Rsc. Adv. 2016, 6, 28887–28894. [Google Scholar] [CrossRef]
- Wang, B.X.; Ren, Q.; Hai, O.; Wu, X.L. Luminescence properties and energy transfer in Tb3+ and Eu3+ co-doped Ba2P2O7 phosphors. Rsc. Adv. 2017, 7, 15222–15227. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Z.Y.; Chen, Z.; Zhao, D.; Qin, G.S.; Qin, W.P. Upconversion emissions from high-energy states of Eu3+ sensitized by Yb3+ and Ho3+ in β-NaYF4 microcrystals under 980 nm excitation. Opt Express 2011, 19, 25471–25478. [Google Scholar] [CrossRef]
- Suo, H.; Zhu, Q.; Zhang, X.; Chen, B.; Chen, J.K.; Wang, F. High-security anti-counterfeiting through upconversion luminescence. Mater. Today Phys. 2021, 21, 100520. [Google Scholar] [CrossRef]
- Maciel, G.S.; Biswas, A.; Prasad, P.N. Infrared-to-visible Eu3+ energy upconversion due to cooperative energy transfer from an Yb3+ ion pair in a sol-gel processed multi-component silica glass. Opt. Commun. 2000, 178, 65–69. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Katelnikovas, A. Optical spectroscopy studies of K2Bi(PO4)(MoO4):20%Yb3+,Er3+ phosphors for upconversion applications. J. Lumin. 2022, 252, 119384. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Katelnikovas, A. Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing. Materials 2023, 16, 917. [Google Scholar] [CrossRef] [PubMed]
- Grigorjevaite, J.; Katelnikovas, A. Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors. Materials 2023, 16, 1305. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Terebilenko, K.V.; Zatovsky, I.V.; Baumer, V.N.; Slobodyanik, N.S.; Shishkin, O.V. K2Ho(PO4)(WO4). Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, i75. [Google Scholar] [CrossRef] [PubMed]
- Demesh, M.; Gorbachenya, K.; Kisel, V.; Volkova, E.; Maltsev, V.; Koporulina, E.; Dunina, E.; Kornienko, A.; Fomicheva, L.; Kuleshov, N. Transitions intensities and cross-sections of Tb3+ ions in YAI3(BO3)4 crystal. Osa Contin. 2021, 4, 822–830. [Google Scholar] [CrossRef]
- Li, Y.C.; Chang, Y.H.; Chang, Y.S.; Lin, Y.J.; Laing, C.H. Luminescence and energy transfer properties of Gd3+ and Tb3+ in LaAlGe2O7. J. Phys. Chem. C 2007, 111, 10682–10688. [Google Scholar] [CrossRef]
- Huang, X.Y.; Li, B.; Guo, H. Highly efficient Eu3+-activated K2Gd(WO4)(PO4) red-emitting phosphors with superior thermal stability for solid-state lighting. Ceram Int. 2017, 43, 10566–10571. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Wagh, A.; Kamath, S.D.; Dahshan, A.; Hegazy, H.H.; Marzec, M.; Kityk, I.V.; Lee, D.E.; Yoon, J.; Park, T. Eu3+-doped fluoro-telluroborate glasses as red-emitting components for W-LEDs application. Opt. Mater. 2020, 99, 109555. [Google Scholar] [CrossRef]
- Lahoz, F.; Martin, I.R.; Mendez-Ramos, J.; Nunez, P. Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: An infrared-laser induced upconversion study. J. Chem. Phys. 2004, 120, 6180–6190. [Google Scholar] [CrossRef] [PubMed]
- Paulose, P.I.; Jose, G.; Thomas, V.; Unnikrishnan, N.V.; Warrier, M.K.R. Sensitized fluorescence of Ce3+/Mn2+ system in phosphate glass. J. Phys. Chem. Solids 2003, 64, 841–846. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Katelnikovas, A. Luminescence and Luminescence Quenching of K2Bi(PO4)(MoO4):Eu3+ Phosphors with Efficiencies Close to Unity. Acs. Appl. Mater. Inter. 2016, 8, 31772–31782. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorjevaite, J.; Katelnikovas, A. Synthesis and Luminescence Properties of Green-to-Red Color-Tunable Upconverting K2Gd(PO4)(WO4):Yb3+,Tb3+,Eu3+ Phosphors. Crystals 2023, 13, 479. https://doi.org/10.3390/cryst13030479
Grigorjevaite J, Katelnikovas A. Synthesis and Luminescence Properties of Green-to-Red Color-Tunable Upconverting K2Gd(PO4)(WO4):Yb3+,Tb3+,Eu3+ Phosphors. Crystals. 2023; 13(3):479. https://doi.org/10.3390/cryst13030479
Chicago/Turabian StyleGrigorjevaite, Julija, and Arturas Katelnikovas. 2023. "Synthesis and Luminescence Properties of Green-to-Red Color-Tunable Upconverting K2Gd(PO4)(WO4):Yb3+,Tb3+,Eu3+ Phosphors" Crystals 13, no. 3: 479. https://doi.org/10.3390/cryst13030479
APA StyleGrigorjevaite, J., & Katelnikovas, A. (2023). Synthesis and Luminescence Properties of Green-to-Red Color-Tunable Upconverting K2Gd(PO4)(WO4):Yb3+,Tb3+,Eu3+ Phosphors. Crystals, 13(3), 479. https://doi.org/10.3390/cryst13030479







