Chirality as a Feature of the Crystal Structure of Lanthanide Ion Complexes—Some Simple Examples
Abstract
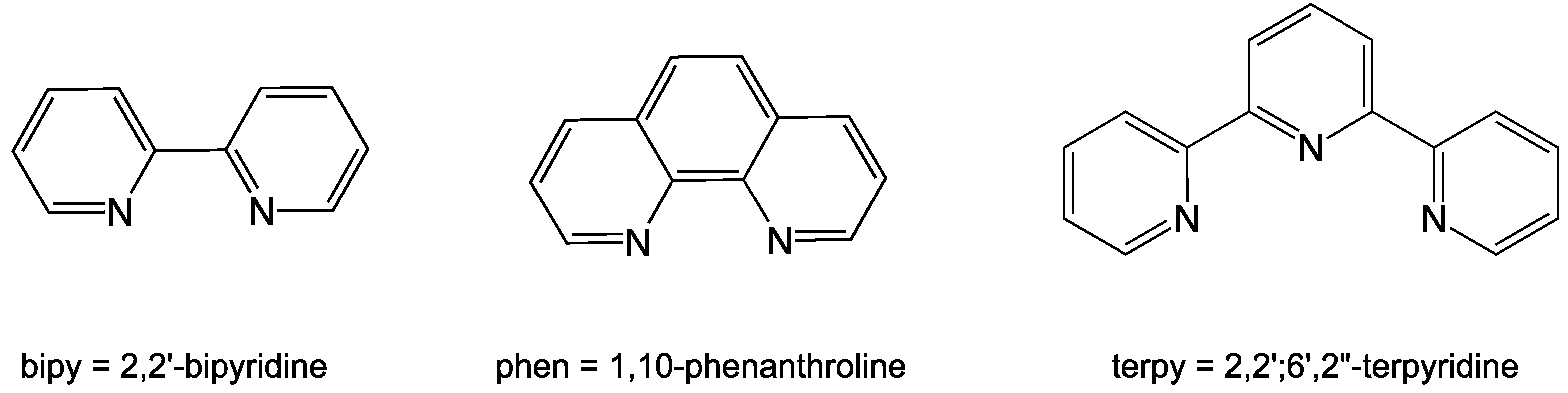
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Crystallography
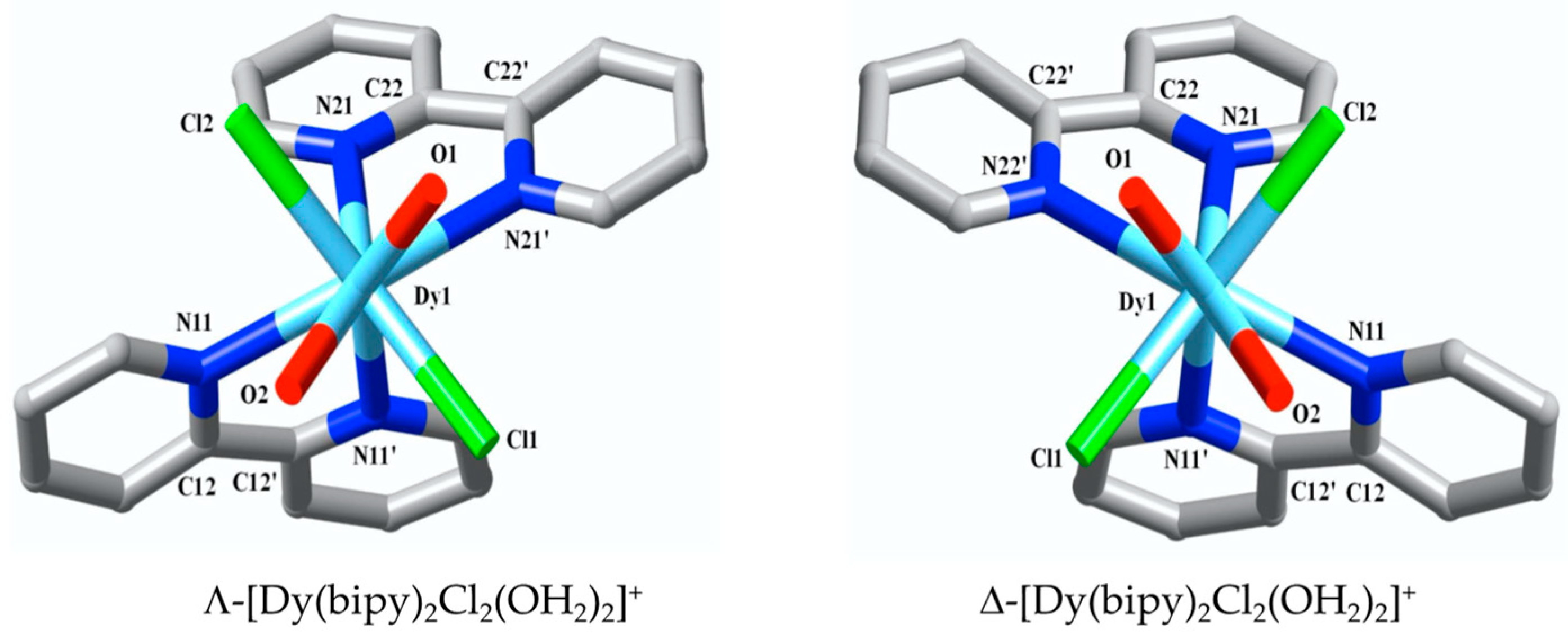
2.2.1. Crystal/Refinement Details for Complex 1: [Dy(bipy)2Cl2(OH2)2]Cl.H2O:
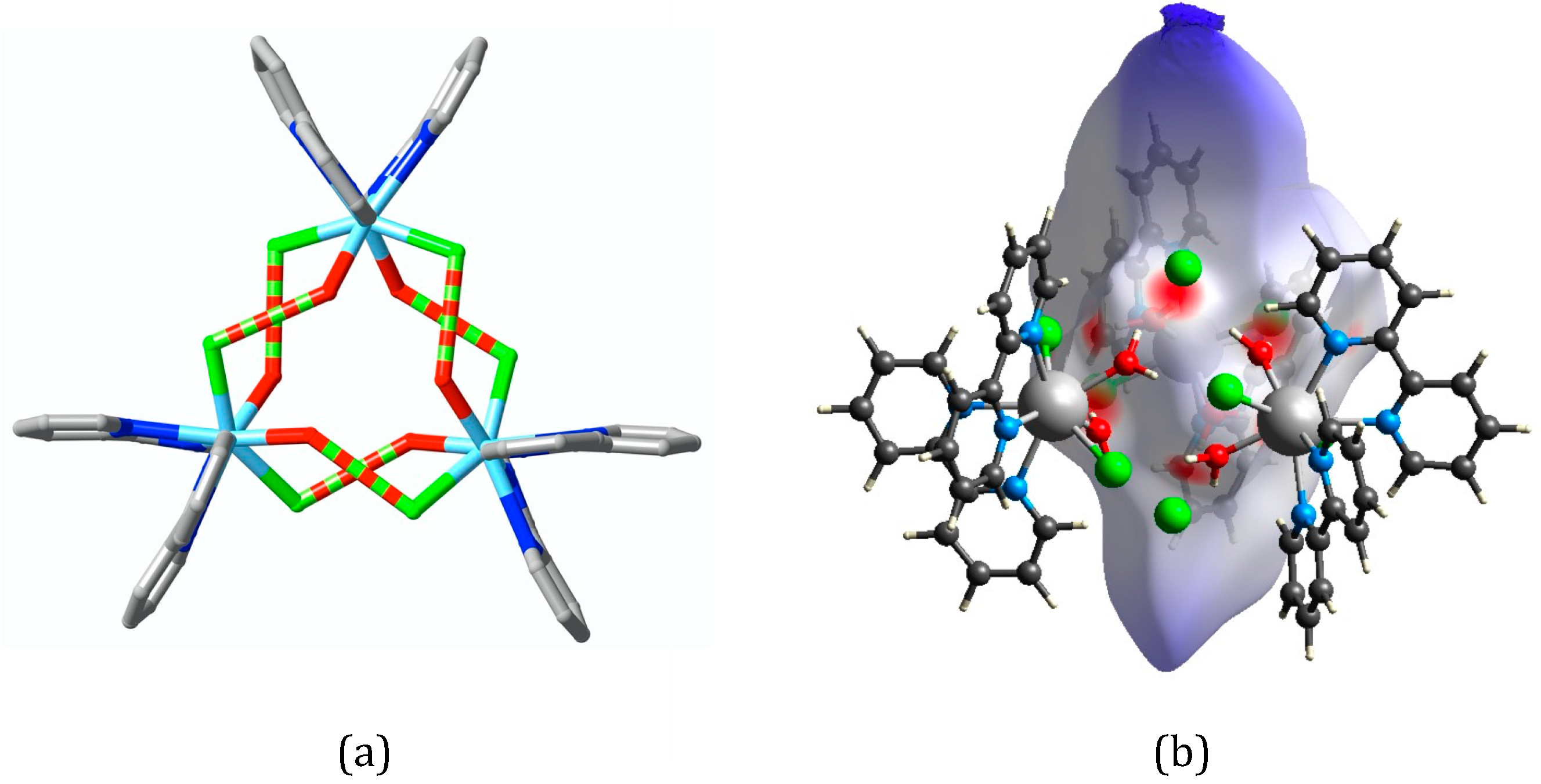
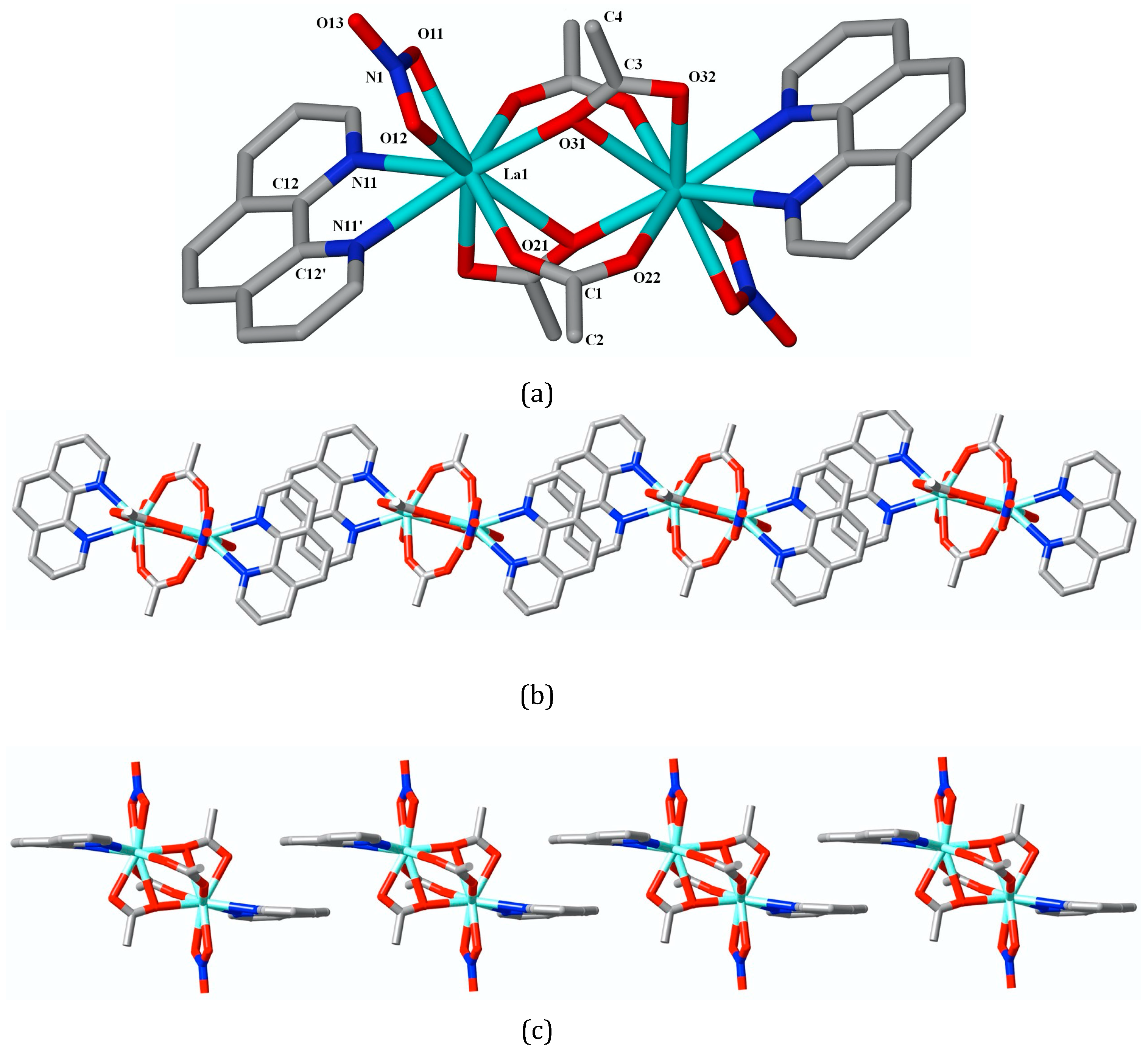
2.2.2. Crystal/Refinement Details for Complex 2: La2(phen)2(O2CCH3)4(NO3)2
2.2.3. Crystal/Refinement Details for Complex 3: Lu(terpy)(O2CCH3)3].NaNO3
2.2.4. Crystal/Refinement Details for Complex 4: Lu(phen)(O2CH)3(OH2)].H2O
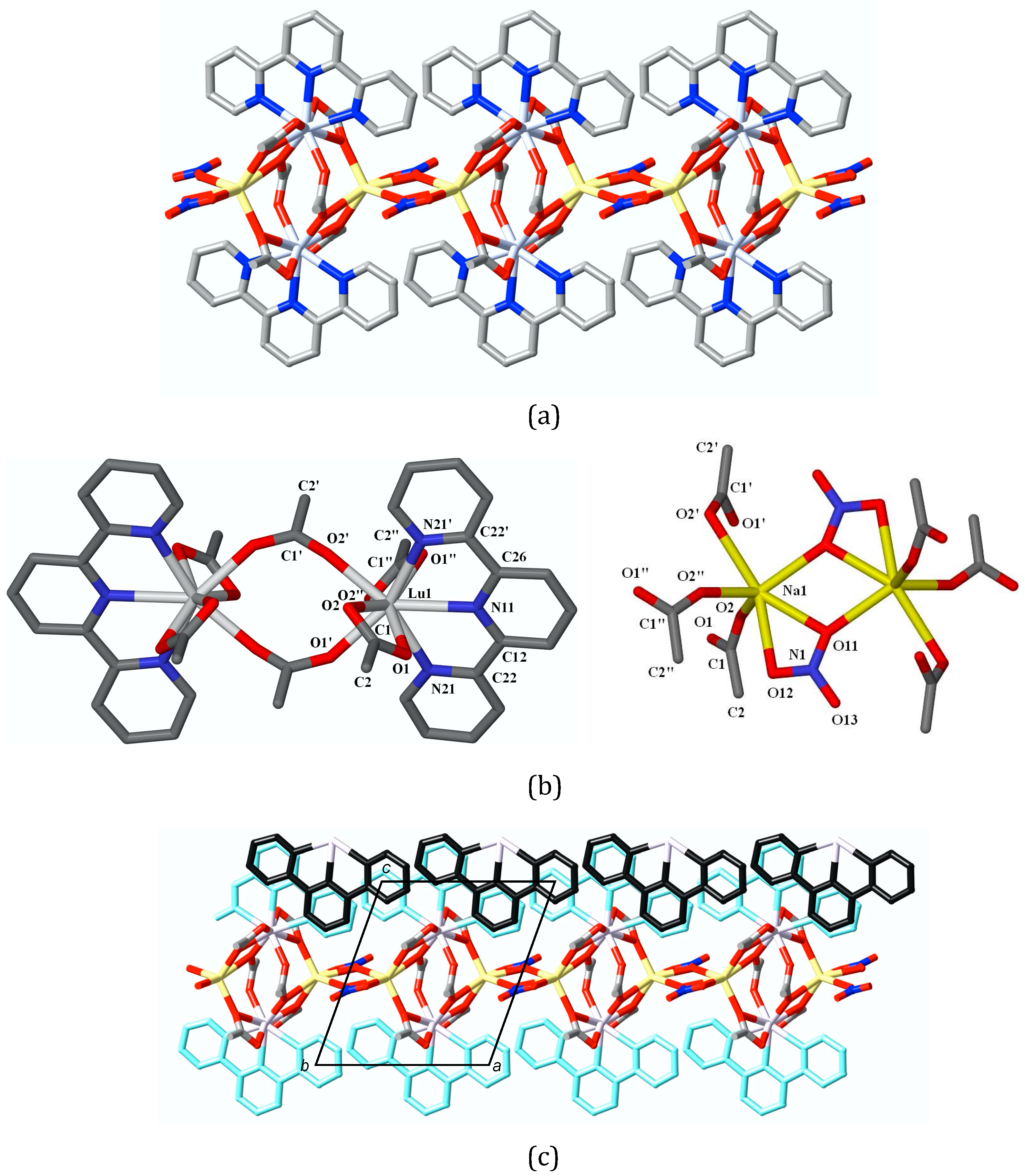
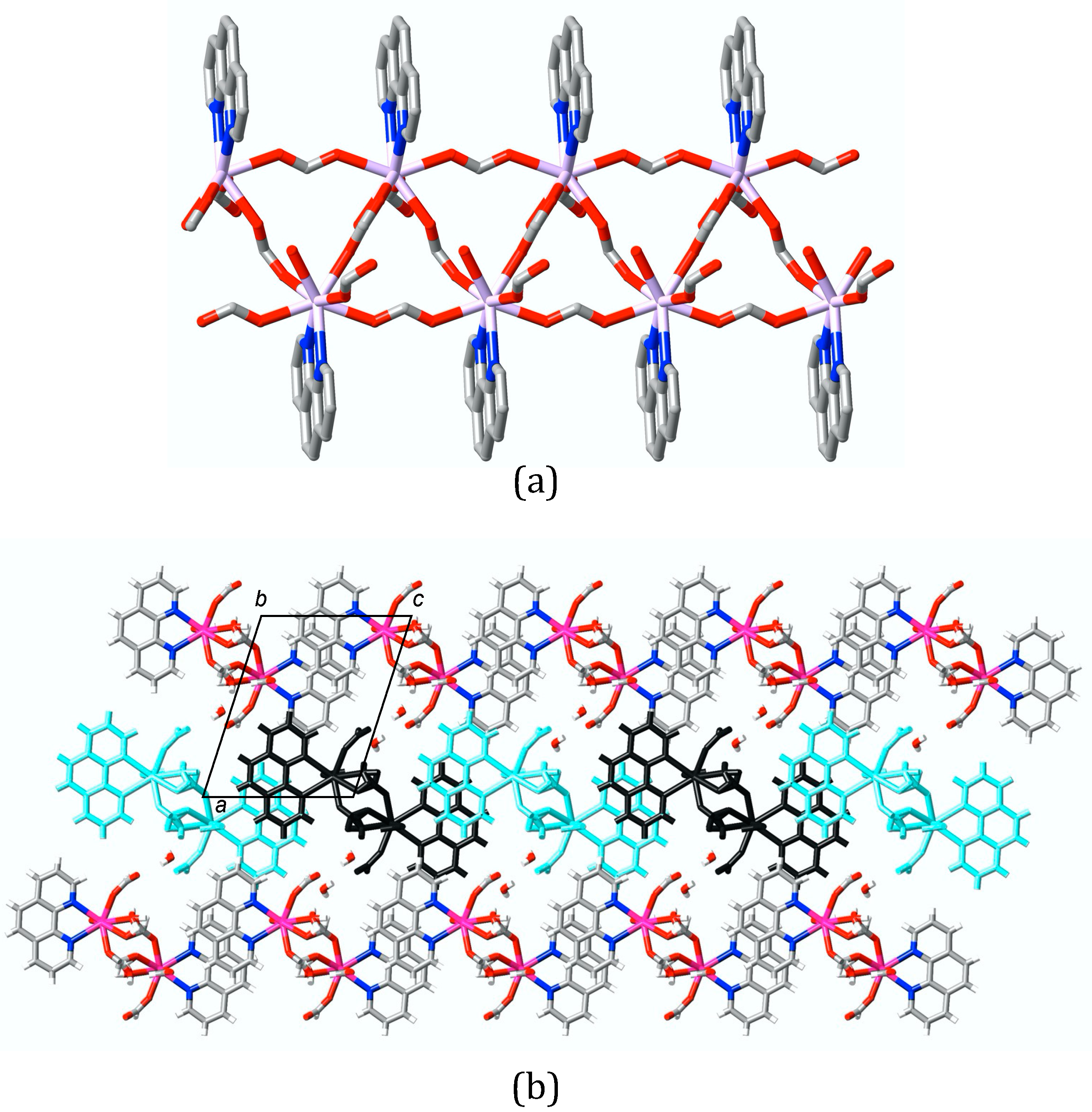
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, S.A. Upconverting Nanomaterials: Synthesis Applications and Perspectives; Altavilla, C., Ed.; Taylor and Francis: New York, NY, USA, 2016; pp. 3–18. [Google Scholar]
- Silva, F.T.; Lins, S.L.S.; Simas, A.M. Stereoisomerism in Lanthanide Complexes: Enumeration, Chirality, Identification, Random Coordination Ratios. Inorg. Chem. 2018, 57, 10557–10567. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, H.C. Chiral Lanthanide Complexes: Coordination Chemistry and Applications. Chem. Rev. 2002, 102, 1807–1850. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.F. Mechanistic Studies of Metal Aqua Ions: A Semi-historical Perspective. Helv. Chim. Acta 2005, 88, 523–545. [Google Scholar] [CrossRef]
- Wong, H.-Y.; Lo, W.-S.; Yim, K.-H.; Law, G.-L. Chirality and Chiroptics of Lanthanide Molecular and Supramolecular Assemblies. Chem 2019, 5, 3058–3095. [Google Scholar] [CrossRef]
- Parker, D.; Dickins, R.S.; Puschmann, H.; Crossland, C.; Howard, J.A.K. Being Excited by Lanthanide Coordination Complexes: Aqua Species, Chirality, Excited-State Chemistry, and Exchange Dynamics. Chem. Rev. 2002, 102, 1977–2010. [Google Scholar] [CrossRef] [PubMed]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Luminescent chiral lanthanide(III) complexes as potential molecular probes. Dalton Trans. 2009, 44, 9692–9707. [Google Scholar] [CrossRef]
- Zhao, Z.; Wong, M.; Mao, C.; Trieu, T.X.; Zhang, J.; Feng, P.; Bu, X. Site-Selective Crystallisation of Homochiral Camphorate Metal-Organic Frameworks for Lanthanide Separation. J. Am. Chem. Soc. 2014, 136, 12572–12575. [Google Scholar] [CrossRef]
- Zeng, M.; Ren, A.; Wu, W.; Zhao, Y.; Zhan, C.; Yao, J. Lanthanide MOFs for inducing molecular chirality of achiral stilbazolium with strong circularly polarized luminescence and efficient energy transfer for color tuning. Chem. Sci. 2020, 11, 9154–9161. [Google Scholar] [CrossRef]
- Nespomo, M.; Benahsene, A.H. Symmetry and Chirality in Crystals. J. Appl. Cryst. 2021, 54, 1594–1599. [Google Scholar] [CrossRef]
- Liu, C.-M.; Hao, X. Asymmetric Assembly of Chiral Lanthanide(III) Tetranuclear Cluster Complexes Using Achiral Mixed Ligands: Single-molecule Magnet Behavior and Magnetic Entropy Change. ACS Omega 2022, 7, 20229–20236. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Feng, Z.; Yang, J.; Shen, X.; Zhu, D. Unprecedented three-dimensional hydrogen-bonded hex topological chiral lanthanide–organic frameworks built from an achiral ligand. Acta Cryst. 2018, C74, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-W.; Zhu, J.; Song, H.-F.; Li, G.-M.; Yao, X.; Yan, P.F. Spontaneous Resolution of Racemic Salen-Type Ligand in the Construction of 3D Homochiral Lanthanide Frameworks. Cryst. Growth Des. 2014, 14, 5356–5360. [Google Scholar] [CrossRef]
- Mihalcea, I.; Zill, N.; Mereacre, V.; Anson, C.E.; Powell, A.K. Spontaneous Resolution in Homochiral Helical [Ln(nic)2(Hnic)(NO3)] Coordination Polymers Constructed from a Rigid Non-chiral Organic Ligand. Cryst. Growth Des. 2014, 14, 4729–4734. [Google Scholar] [CrossRef]
- Gao, M.-J.; Yang, P.; Cai, B.; Dai, J.-W.; Wu, J.-Z.; Yu, Y. Spontaneous resolution of lanthanide coordination polymers with 2-hydroxypyrimidine-4,6-dicarboxylic acid. CrystEngComm 2011, 14, 1264–1270. [Google Scholar] [CrossRef]
- Chen, H.-F.; Guo, G.-C.; Wang, M.-S.; Xu, G.; Zou, W.-Q.; Guo, S.-P.; Wu, M.-F.; Huang, J.-S. Spontaneous chiral resolution, nonlinear optical and luminescence of eight-coordinate lanthanide(III) complexes. Dalton Trans. 2009, 46, 10166–10168. [Google Scholar] [CrossRef]
- Kremer, C.; Torres, J.; Dominguez, S. Lanthanide complexes with oda, ida and nda: From discrete coordination compounds to supramolecular assemblies. J. Mol. Struct. 2008, 879, 130–149. [Google Scholar] [CrossRef]
- Masu, H.; Tominaga, M.; Katagiri, K.; Kato, T.; Azumaya, I. 2-D coordination network of a cyclic amide with a lanthanide metal cation and its columnar stacking. CrystEngComm 2006, 8, 578–580. [Google Scholar] [CrossRef]
- Brayshaw, P.A.; Bünzli, J.-C.G.; Froidevaux, P.; Harrowfield, J.M.; Kim, Y.; Sobolev, A.N. Synthetic, Structural, and Spectroscopic Studies on Solids Containing Tris(dipicolinato) Rare Earth Anions and Transition or Main Group Metal Cations. Inorg. Chem. 1995, 34, 2068–2076. [Google Scholar] [CrossRef]
- Albertsson, J. Structural Studies on the Rare Earth Carboxylates. 1. The Crystal and Molecular Structure of Na3[M(OCOCH2OCH2OCO)3].2NaClO4.6H2O, M = Nd, Gd and Yb. Acta Chem. Scand. 1968, 22, 1563–1578. [Google Scholar] [CrossRef]
- Kepert, C.J.; Lu, W.-M.; Semenova, L.I.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. XII. Solvated 1:1 Adducts of Some Lanthanoid(III) Carboxylates with 1,10-Phenanthroline and 2,2′:6′,2″-Terpyridine. Aust. J. Chem. 1999, 52, 481–496. [Google Scholar] [CrossRef]
- Semenova, L.I.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. XVIII. (Hydrated) Mononuclear 1:1 Adducts of Lanthanoid(III) Chlorides with 2,2′-Bipyridine. Aust. J. Chem. 1999, 52, 551–569. [Google Scholar] [CrossRef]
- Semenova, L.I.; White, A.H. Structural Systematics of Rare Earth Complexes. XIX. (Hydrated) 1:2 Mononuclear Adducts of Lanthanoid(III) Chlorides with 2,2′-Bipyridine and 1,10-Phenanthroline. Aust. J. Chem. 1999, 52, 571–600. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- McKenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 21.5; University of Western Australia: Perth, Australia, 2021. [Google Scholar]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Engineering grids of metal complexes: Development of the 2D M(terpy)2 embrace motif in crystals. CrystEngComm 2005, 7, 216–229. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Cotton, S.A.; Raithby, P.R. Systematics and surprises in lanthanide coordination chemistry. Coord. Chem. Rev. 2017, 340, 220–231. [Google Scholar] [CrossRef]
- Chan, E.J.; Harrowfield, J.M.; Skelton, B.W.; Sobolev, A.N.; White, A.H. Structure and Stereochemistry of Adducts of Tris(dipivaloylmethanide)europium(III), Eu(dpm)3, with Some Dipolar Aprotic Unidentate O-donors. Aust. J. Chem. 2020, 73, 455–461. [Google Scholar] [CrossRef]
- Lundberg, D.; Persson, I.; Eriksson, L.; D′Angelo, P.; De Panfilis, S. Structural Study of the N,N′-Dimethylpropyleneurea Solvated Lanthanoid(III) Ions in Solution and Solid State with an Analysis of the Ionic Radii of Lanthanoid(III) Ions. Inorg. Chem. 2010, 49, 4420–4432. [Google Scholar] [CrossRef] [PubMed]
- Riehl, J.P.; Richardson, F.S. Circularly Polarised Luminescence Spectroscopy. Chem. Rev. 1986, 86, 1–16. [Google Scholar] [CrossRef]
- Cotton, S.A.; Noy, O.E.; Liesenen, F.; Raithby, P.R. Unequivocal characterisation of a [Ln(terpy)(NO3)3·(H2O)] complex: The synthesis and structure of [M(terpy)(NO3)3·(H2O)] (M=Eu, Tb); a comparison with the structure of [Eu(bipy)2(NO3)3] and with other europium nitrate complexes {terpy=2,2′:6′,2″-terpyridyl; bipy=2,2′-bipyridyl}. Inorg. Chim. Acta 2003, 344, 37–42. [Google Scholar]
- Cotton, S.A.; Harrowfield, J.M.; Semenova, L.I.; Skelton, B.W.; Sobolev, A.N.; White, A.H. Allan White and Polypyridines: Extending the Lanthanide(III) Complex Series. Aust. J. Chem. 2020, 73, 434–446. [Google Scholar] [CrossRef]
- Mitcov, D.; Platunov, M.; Buch, C.D.; Reinholdt, A.; Dœssing, A.R.; Wilhelm, F.; Rogalev, A.; Pilikgos, S. Hard X-ray magnetochiral dichroism in a paramagnetic molecular 4f complex. Chem. Sci. 2020, 11, 8306–8311. [Google Scholar] [CrossRef]
- Zinna, F.; Di Bari, L. Lanthanide Circularly Polarized Luminescence: Bases and Applications. Chirality 2015, 27, 1–13. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, L.I.; Ling, I.; Sobolev, A.N. Chirality as a Feature of the Crystal Structure of Lanthanide Ion Complexes—Some Simple Examples. Crystals 2023, 13, 337. https://doi.org/10.3390/cryst13020337
Semenova LI, Ling I, Sobolev AN. Chirality as a Feature of the Crystal Structure of Lanthanide Ion Complexes—Some Simple Examples. Crystals. 2023; 13(2):337. https://doi.org/10.3390/cryst13020337
Chicago/Turabian StyleSemenova, Lioubov I., Irene Ling, and Alexandre N. Sobolev. 2023. "Chirality as a Feature of the Crystal Structure of Lanthanide Ion Complexes—Some Simple Examples" Crystals 13, no. 2: 337. https://doi.org/10.3390/cryst13020337
APA StyleSemenova, L. I., Ling, I., & Sobolev, A. N. (2023). Chirality as a Feature of the Crystal Structure of Lanthanide Ion Complexes—Some Simple Examples. Crystals, 13(2), 337. https://doi.org/10.3390/cryst13020337






