A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid
(This article belongs to the Section Inorganic Crystalline Materials)
Abstract
1. Overview
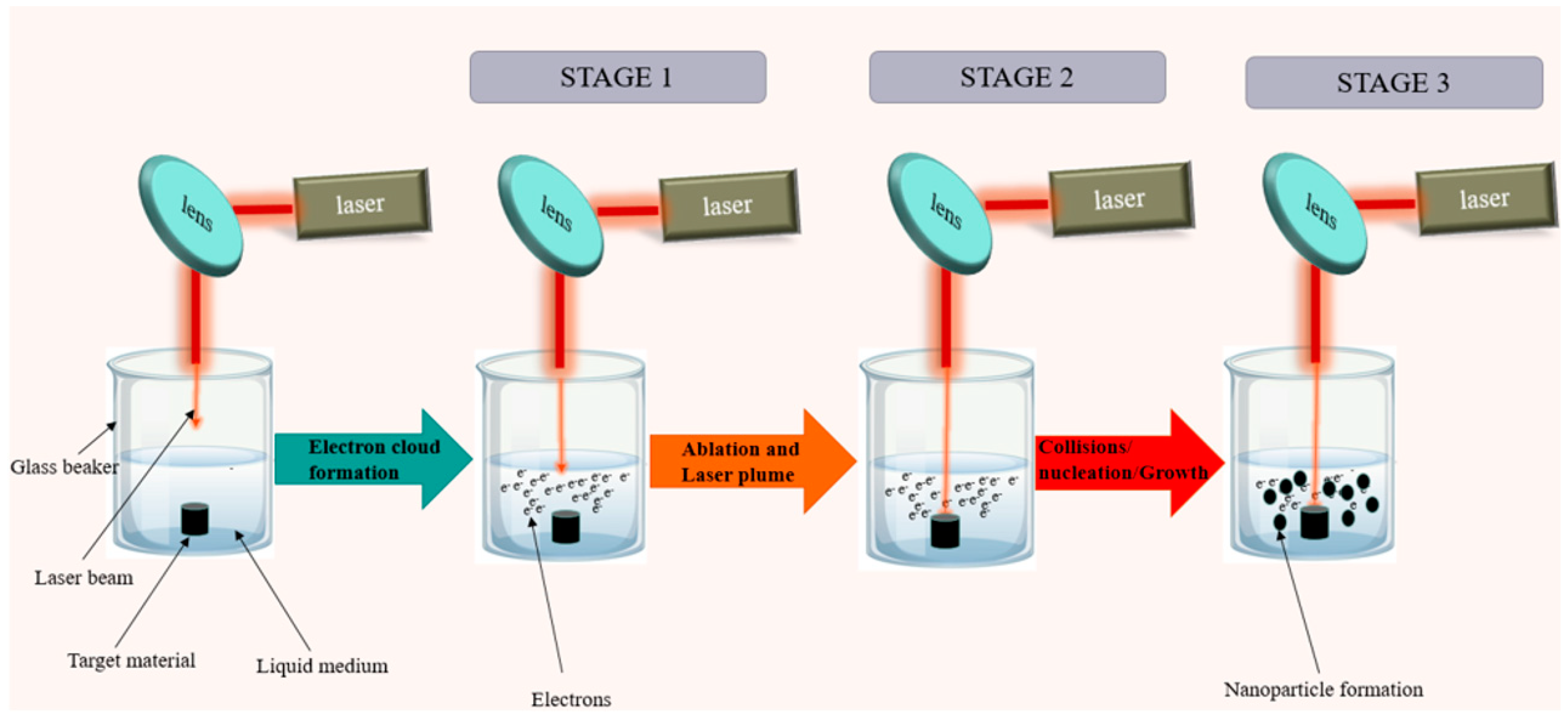
2. Modes of PLAL
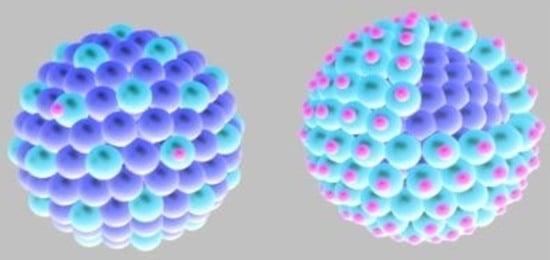
3. Bimetallic and Composite Nanoparticles
4. Effect of Liquid Medium
5. Effect of Laser Fluence
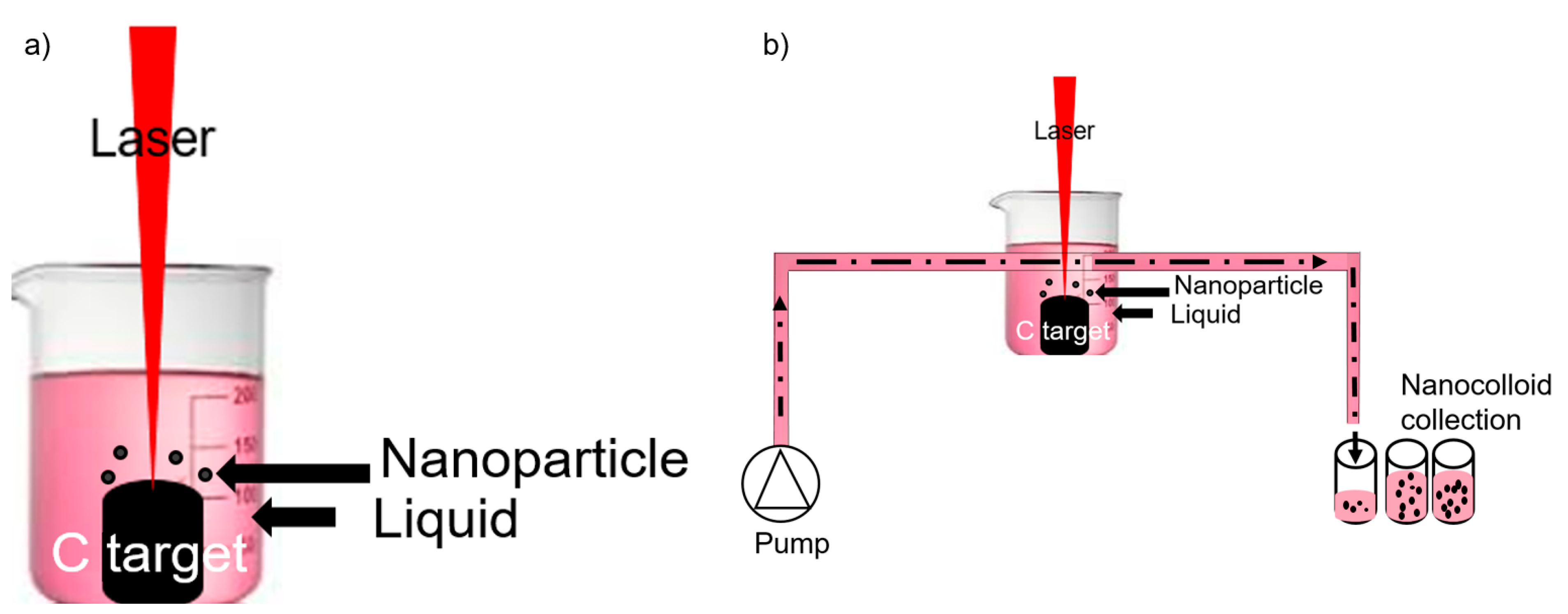
6. Effects of Ablation Time, Laser Pulse Width, and Repetition Rate
7. Effect of Laser Wavelength
8. Types of Ablation Targets
9. Nanoparticle Characterisation Techniques
9.1. Electron Microscopy
9.2. Ultraviolet–Visible Spectroscopy (UV-Vis)
9.3. Dynamic Light Scattering (DLS)
9.4. X-ray Photon Spectroscopy (XPS)
9.5. Fourier-Transform Infrared Spectroscopy (FTIR)
9.6. Four-Point Probe
10. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles engineering by pulsed laser ablation in liquids: Concepts and applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Wahyudiono; Kawai, S.; Mardis, M.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Bimetallic nanoparticle generation from Au − TiO2 film by pulsed laser ablation in an aqueous medium. Alex. Eng. J. 2021, 60, 2225–2234. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem.A Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef] [PubMed]
- Mardis, M.; Wahyudiono; Takada, N.; Kanda, H.; Goto, M. Formation of Au–carbon nanoparticles by laser ablation under pressurized CO2. Asia-Pac. J. Chem. Eng. 2018, 13, e2176. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Review of materials and fabrication methods for flexible nano and micro-scale physical and chemical property sensors. Appl. Sci. 2021, 11, 8563. [Google Scholar] [CrossRef]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Carbon Nanoparticles and Their Biomedical Applications. Appl. Sci. 2022, 12, 7865. [Google Scholar] [CrossRef]
- Nyabadza, A.; Kane, J.; Sreenilayam, S.; Vázquez, M.; Brabazon, D.; Sreenilayam, S.; Brabazon, D. Multi-Material Production of 4D Shape Memory Polymer Composites. In Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 879–894. [Google Scholar]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Li, M.; Chen, C.; Gadd, G.M. Nanoparticle and nanomineral production by fungi. Fungal Biol. Rev. 2022, 41, 31–44. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef]
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved solvothermal synthesis of γ-Fe2O3 magnetic nanoparticles for SiO2 coating. Nanomaterials 2021, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 14. [Google Scholar] [CrossRef]
- Cele, T. Preparation of Nanoparticles. Eng. Nanomater. Heal. Saf. 2020, 7, 389–406. [Google Scholar] [CrossRef]
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Ouyang, J.; Yang, H. Microwave-assisted synthesis and interfacial features of CdS/kaolinite nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 72–79. [Google Scholar] [CrossRef]
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.S.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D.; et al. Liquid Phase—Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Fitzpatrick, B.; Brabazon, D. Effect of liquid medium and laser processing parameters on the fabrication of carbon nanoparticles via pulsed laser ablation in liquid towards paper electronics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128151. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Magnesium Nanoparticle Synthesis from Powders via Pulsed Laser Ablation in Liquid for Nanocolloid Production. Appl. Sci. 2021, 11, 10974. [Google Scholar] [CrossRef]
- Amans, D.; Cai, W.; Barcikowski, S.; Info, A.; Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454. [Google Scholar] [CrossRef]
- Kanitz, A.; Kalus, M.R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Bagga, K.; McCann, R.; Wang, M.; Stalcup, A.; Vázquez, M.; Brabazon, D. Laser assisted synthesis of carbon nanoparticles with controlled viscosities for printing applications. J. Colloid Interface Sci. 2015, 447, 263–268. [Google Scholar] [CrossRef] [PubMed]
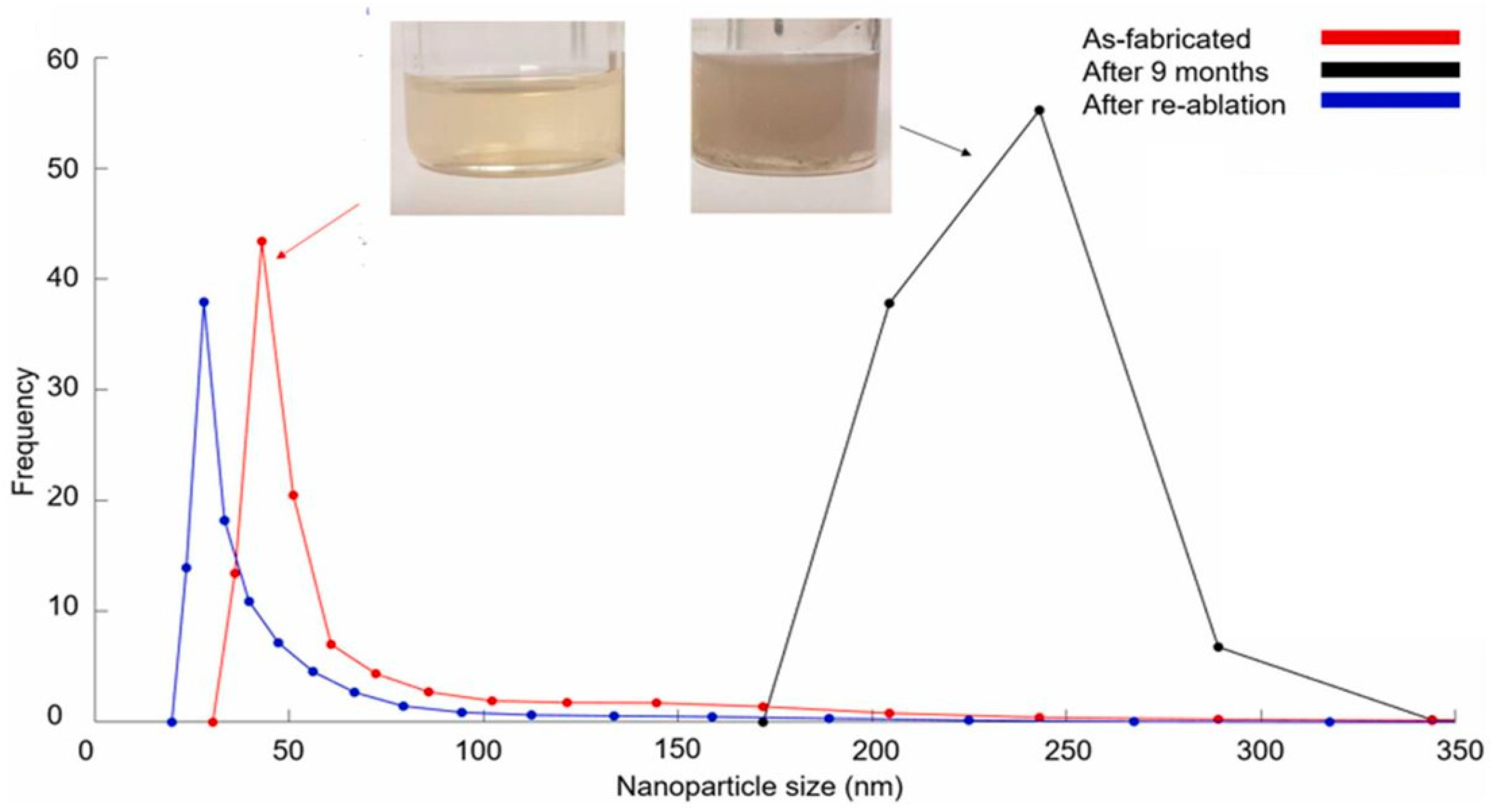
- Jasbi, N.E.; Dorranian, D. Effect of aging on the properties of TiO2 nanoparticle. J. Theor. Appl. Phys. 2016, 10, 157–161. [Google Scholar] [CrossRef]
- McCarthy, É.; Sreenilayam, S.P.; Ronan, O.; Ayub, H.; McCann, R.; McKeon, L.; Fleischer, K.; Nicolosi, V.; Brabazon, D. Silver nanocolloid generation using dynamic Laser Ablation Synthesis in Solution system and drop-casting. Nano-Struct. Nano-Objects 2022, 29, 100841. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Brabazon, D. Modelling of Pulsed Laser Ablation in Liquid via Monte Carlo techniques: The effect of laser parameters and liquid medium on the electron cloud. Solid State Sci. 2022, 133, 107003. [Google Scholar] [CrossRef]
- Povarnitsyn, M.E.; Itina, T.E.; Levashov, P.R.; Khishchenko, K.V. Mechanisms of nanoparticle formation by ultra-short laser ablation of metals in liquid environment. Phys. Chem. Chem. Phys. 2013, 15, 3108–3114. [Google Scholar] [CrossRef]
- Ibrahimkutty, S.; Wagener, P.; Menzel, A.; Plech, A.; Barcikowski, S. Nanoparticle formation in a cavitation bubble after pulsed laser ablation in liquid studied with high time resolution small angle X-ray scattering. Appl. Phys. Lett. 2012, 101, 4750250. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; De Giacomo, A. Plasma charging effect on the nanoparticles releasing from the cavitation bubble to the solution during nanosecond Pulsed Laser Ablation in Liquid. Appl. Surf. Sci. 2020, 515, 146031. [Google Scholar] [CrossRef]
- Taccogna, F.; Dell’Aglio, M.; Rutigliano, M.; Valenza, G.; De Giacomo, A. On the growth mechanism of nanoparticles in plasma during pulsed laser ablation in liquids. Plasma Sources Sci. Technol. 2017, 26, aa595b. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058. [Google Scholar] [CrossRef]
- Qayyum, H.; Ahmed, W.; Hussain, S.; Khan, G.A.; Rehman, Z.U.; Ullah, S.; Rahman, T.U.; Dogar, A.H. Laser synthesis of surfactant-free silver nanoparticles for toxic dyes degradation and SERS applications. Opt. Laser Technol. 2020, 129, 106313. [Google Scholar] [CrossRef]
- Mendivil Palma, M.I.; Krishnan, B.; Rodriguez, G.A.C.; Das Roy, T.K.; Avellaneda, D.A.; Shaji, S. Synthesis and Properties of Platinum Nanoparticles by Pulsed Laser Ablation in Liquid. J. Nanomater. 2016, 2016, 9651637. [Google Scholar] [CrossRef]
- Charde, R.P.; Van Devener, B.; Nigra, M.M. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts 2023, 13, 246. [Google Scholar] [CrossRef]
- Liu, K.; He, S.; Li, L.; Liu, Y.; Huang, Z.; Liu, T.; Wu, H.; Jiang, X.; Liu, K.; Tian, F. Spectroscopically clean Au nanoparticles for catalytic decomposition of hydrogen peroxide. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Priya, A.K.; Krishna, R.S.; Kumar, Y.G.; Rohini, P.; Dillibabu, S. Green synthesis of silver nanoparticles by pulsed laser ablation using citrus limetta juice extract for clad-modified fiber optic gas sensing application. Nanoeng. Fabr. Prop. Opt. Thin Film. Devices XVIII 2021, 11802, 184–193. [Google Scholar] [CrossRef]
- Urabe, A.A.; Nayef, U.M.; Kamel, R. Preparation and characterization of Pd nanoparticles via second harmonic Nd:YAG laser ablation and deposited on porous silicon for photo-conversion application. J. Opt. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Pitzalis, E.; Spiniello, R.; Ishak, R.; Muniz-Miranda, M. Production of palladium nanoparticles by pulsed laser ablation in water and their characterization. J. Phys. Chem. C 2011, 115, 5073–5083. [Google Scholar] [CrossRef]
- Piermatti, O. Green Synthesis of Pd Nanoparticles for Sustainable and Environmentally Benign Processes. Catalysts 2021, 11, 1258. [Google Scholar] [CrossRef]
- Peter, C.; Derible, A.; Becht, J.M.; Kiener, J.; Le Drian, C.; Parmentier, J.; Fierro, V.; Girleanu, M.; Ersen, O. Biosourced mesoporous carbon with embedded palladium nanoparticles by a one pot soft-template synthesis: Application to Suzuki reactions. J. Mater. Chem. A 2015, 3, 12297–12306. [Google Scholar] [CrossRef]
- Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Green Synthesis of Palladium Nanoparticles: Applications in Aryl Halide Cyanation and Hiyama Cross-Coupling Reaction Under Ligand Free Conditions. Catal. Lett. 2018, 148, 1562–1578. [Google Scholar] [CrossRef]
- Hegde, R.V.; Ghosh, A.; Jadhav, A.H.; Nizam, A.; Patil, S.A.; Peter, F.; Dateer, R.B. Biogenic synthesis of Pd-nanoparticles using Areca Nut Husk Extract: A greener approach to access α-keto imides and stilbenes. New J. Chem. 2021, 45, 16213–16222. [Google Scholar] [CrossRef]
- Sarmah, M.; Neog, A.B.; Boruah, P.K.; Das, M.R.; Bharali, P.; Bora, U. Effect of Substrates on Catalytic Activity of Biogenic Palladium Nanoparticles in C-C Cross-Coupling Reactions. ACS Omega 2019, 4, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Islami, M.R.; Tikdari, A.M. Green synthesis of Pd nanoparticles supported on modified Nonpareil almond shell using almond hull extract: A beneficial nanocatalyst for convenient reduction of organic dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 18111–18122. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Al-Antaki, A.H.M.; Luo, X.; Duan, X.; Lamb, R.N.; Hutchison, W.D.; Lawrance, W.; Raston, C.L. Continuous Flow Copper Laser Ablation Synthesis of Copper(I and II) Oxide Nanoparticles in Water. ACS Omega 2019, 4, 13577–13584. [Google Scholar] [CrossRef]
- Altuwirqi, R.M.; Albakri, A.S.; Al-Jawhari, H.; Ganash, E.A. Green synthesis of copper oxide nanoparticles by pulsed laser ablation in spinach leaves extract. Optik. 2020, 219, 165280. [Google Scholar] [CrossRef]
- Kalus, M.-R.R.; Lanyumba, R.; Barcikowski, S.; Gökce, B. Discrimination of ablation, shielding, and interface layer effects on the steady-state formation of persistent bubbles under liquid flow conditions during laser synthesis of colloids. J. Flow Chem. 2021, 11, 773–792. [Google Scholar] [CrossRef]
- Streubel, R.; Bendt, G.; Gökce, B. Pilot-scale synthesis of metal nanoparticles by high-speed pulsed laser ablation in liquids. Nanotechnology 2016, 27, 205602. [Google Scholar] [CrossRef]
- Barcikowski, S.; Meńndez-Manjón, A.; Chichkov, B.; Brikas, M.; Račiukaitis, G. Generation of nanoparticle colloids by picosecond and femtosecond laser ablations in liquid flow. Appl. Phys. Lett. 2007, 91, 083113. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Wang, C.X.; Yang, G.W. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 2017, 87, 140–220. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Yang, G. Fabrication of One-Dimensional chain of iron-based bimetallic alloying nanoparticles with unique magnetizations. Cryst. Growth Des. 2014, 14, 5847–5855. [Google Scholar] [CrossRef]
- Ahmadinejad, E.; Mahdieh, M.H.; Hossein Mahdieh, M. Laser-assisted synthesis of Ag–Cu alloy nanoparticles with tunable surface plasmon resonance frequency in presence of external electric field. J. Laser Appl. 2021, 34, 012004. [Google Scholar] [CrossRef]
- Sapkota, D.; Li, Y.; Musaev, O.R.; Wrobel, J.M.; Kruger, M.B. Effect of electric fields on tin nanoparticles prepared by laser ablation in water. J. Laser Appl. 2017, 29, 012002. [Google Scholar] [CrossRef]
- Kanakkillam, S.S.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Surfactant free stable cobalt oxide nanocolloid in water by pulsed laser fragmentation and its thin films for visible light photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124657. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Brabazon, D. Magnesium nanoparticle synthesis from powders via LASIS—Effects of liquid medium, laser pulse width and ageing on nanoparticle size, concentration, stability and electrical properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129651. [Google Scholar] [CrossRef]
- Roy, A.; Datta, S.; Luthra, R.; Khan, M.A.; Gacem, A.; Hasan, M.A.; Yadav, K.K.; Ahn, Y.; Jeon, B.-H. Green synthesis of metalloid nanoparticles and its biological applications: A review. Front. Chem. 2022, 10, 994724. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ayoub, M.H.; Khan, A.M.; Waseem, A.; Yasir, M.; Khan, M.S.; Bajwa, T.M.; Shaikh, A.J. Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129057. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Mostafa, A.M.; Ahmed, H.A. Effect of liquid media and laser energy on the preparation of Ag nanoparticles and their nanocomposites with Au nanoparticles via laser ablation for optoelectronic applications. Optik 2021, 241, 167217. [Google Scholar] [CrossRef]
- Messina, G.C.; Sinatra, M.G.; Bonanni, V.; Brescia, R.; Alabastri, A.; Pineider, F.; Campo, G.; Sangregorio, C.; Li-Destri, G.; Sfuncia, G.; et al. Tuning the Composition of Alloy Nanoparticles Through Laser Mixing: The Role of Surface Plasmon Resonance. J. Phys. Chem. C 2016, 120, 12810–12818. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, srep23352. [Google Scholar] [CrossRef]
- Elsayed, K.A.; Alomari, M.; Drmosh, Q.A.; Alheshibri, M.; Al Baroot, A.; Kayed, T.S.; Manda, A.A.; Al-Alotaibi, A.L. Fabrication of ZnO-Ag bimetallic nanoparticles by laser ablation for anticancer activity. Alex. Eng. J. 2022, 61, 1449–1457. [Google Scholar] [CrossRef]
- Censabella, M.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.G.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, S.J.; Koutavarapu, R.; Kim, S.K.; Choi, H.C.; Choi, M.Y. Enhanced Catalytic Dechlorination of 1,2-Dichlorobenzene Using Ni/Pd Bimetallic Nanoparticles Prepared by a Pulsed Laser Ablation in Liquid. Catalysts 2018, 8, 390. [Google Scholar] [CrossRef]
- Ali, I.; Pan, Y.; Jamil, Y.; Shah, A.A.; Amir, M.; Al Islam, S.; Fazal, Y.; Chen, J.; Shen, Z. Comparison of copper-based Cu-Ni and Cu-Fe nanoparticles synthesized via laser ablation for magnetic hyperthermia and antibacterial applications. Phys. B Condens. Matter 2023, 650, 414503. [Google Scholar] [CrossRef]
- Jwied, D.H.; Nayef, U.M.; Mutlak, F.A.H. Synthesis of C:Se (core:shell) nanoparticles via laser ablation on porous silicon for photodetector application. Optik 2021, 231, 166493. [Google Scholar] [CrossRef]
- Rashid, T.M.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A.H. Synthesis and characterization of Au:ZnO (core:shell) nanoparticles via laser ablation. Optik 2021, 244, 167569. [Google Scholar] [CrossRef]
- Dahiya, U.R.; Singh, S.; Garg, C.K.; Rai, A.; Kalyanasundaram, D. Modified Surface Composition and Biocompatibility of Core-Shell Nitinol Nanoparticles Fabricated via Laser Ablation of Differently Passivized Targets. Front. Mater. 2022, 9, 212. [Google Scholar] [CrossRef]
- Monu, M.C.C.; Ekoi, E.J.; Hughes, C.; Kumar, S.S.; Brabazon, D. Resultant physical properties of as-built nitinol processed at specific volumetric energy densities and correlation with in-situ melt pool temperatures. J. Mater. Res. Technol. 2022, 21, 2757–2777. [Google Scholar] [CrossRef]
- Chekotu, J.C.; Kinahan, D.; Goodall, R.; Brabazon, D. Influence of Structural Porosity and Martensite Evolution on Mechanical Characteristics of Nitinol via In-Silico Finite Element Approach. Materials 2022, 15, 5365. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C 2021, 120, 111717. [Google Scholar] [CrossRef]
- Madalena Santos, P.; Rangel-Yagui, C.O.; Janorkar, A.V.; Luo, L.; Sargioti, N.; Levingstone, T.J.; O’Cearbhaill, E.D.; McCarthy, H.O.; Dunne, N.J. Metallic Microneedles for Transdermal Drug Delivery: Applications, Fabrication Techniques and the Effect of Geometrical Characteristics. Bioengineering 2022, 10, 24. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of alloy nanoparticles by laser ablation of Au/Fe multilayer films in liquid environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Agnoli, S.; Granozzi, G.; Meneghetti, M.; Campo, G.; Bonanni, V.; Pineider, F.; Sangregorio, C.; Ghigna, P.; et al. Laser generation of iron-doped silver nanotruffles with magnetic and plasmonic properties. Nano Res. 2015, 8, 4007–4023. [Google Scholar] [CrossRef]
- Vinod, M.; Gopchandran, K.G. Ag@Au core–shell nanoparticles synthesized by pulsed laser ablation in water: Effect of plasmon coupling and their SERS performance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 913–919. [Google Scholar] [CrossRef]
- Abed, M.A.; Mutlak, F.A.H.; Ahmed, A.F.; Nayef, U.M.; Abdulridha, S.K.; Jabir, M.S. Synthesis of Ag/Au (core/shell) nanoparticles by laser ablation in liquid and study of their toxicity on blood human components. J. Phys. Conf. Ser. 2021, 1795, 012013. [Google Scholar] [CrossRef]
- Riedel, R.; Mahr, N.; Yao, C.; Wu, A.; Yang, F.; Hampp, N. Synthesis of gold–silica core–shell nanoparticles by pulsed laser ablation in liquid and their physico-chemical properties towards photothermal cancer therapy. Nanoscale 2020, 12, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Serkov, A.A.; Barmina, E.V.; Simakin, A.V.; Kuzmin, P.G.; Voronov, V.V.; Shafeev, G.A. Generation of core–shell nanoparticles Al@Ti by laser ablation in liquid for hydrogen storage. Appl. Surf. Sci. 2015, 348, 71–74. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Izaak, T.I.; Kharlamova, T.S.; Martynova, D.O.; Lapin, I.N.; Svetlichnyi, V.A. Ag/SiOx nanocomposite powders synthesized from colloids obtained by pulsed laser ablation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 80–88. [Google Scholar] [CrossRef]
- Hassan, M.; Gondal, M.A.; Cevik, E.; Dastageer, M.A.; Baig, U.; Moqbel, R.A.; Qahtan, T.F.; Bozkurt, A.; Al Abass, N. Laser assisted anchoring of cadmium sulfide nanospheres into tungsten oxide nanosheets for enhanced photocatalytic and electrochemical energy storage applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126318. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Mahdi, R.; Kadhim, A. The effect of laser energy on the properties of carbon nanotube—Iron oxide nanoparticles composite prepared via pulsed laser ablation in liquid. Mater. Res. Express 2018, 5, 105004. [Google Scholar] [CrossRef]
- Alamro, F.S.; Mostafa, A.M.; Abu Al-Ola, K.A.; Ahmed, H.A.; Toghan, A. Synthesis of Ag Nanoparticles-Decorated CNTs via Laser Ablation Method for the Enhancement the Photocatalytic Removal of Naphthalene from Water. Nanomaterials 2021, 11, 2142. [Google Scholar] [CrossRef]
- Al Baroot, A.; Alheshibri, M.; Drmosh, Q.A.; Akhtar, S.; Kotb, E.; Elsayed, K.A. A novel approach for fabrication ZnO/CuO nanocomposite via laser ablation in liquid and its antibacterial activity. Arab. J. Chem. 2022, 15, 103606. [Google Scholar] [CrossRef]
- Xu, X.; Gao, L.; Duan, G. The Fabrication of Au@C Core/Shell Nanoparticles by Laser Ablation in Solutions and Their Enhancements to a Gas Sensor. Micromachines 2018, 9, 278. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- García Guillén, G.; Zuñiga Ibarra, V.A.; Mendivil Palma, M.I.; Krishnan, B.; Avellaneda Avellaneda, D.; Shaji, S. Effects of Liquid Medium and Ablation Wavelength on the Properties of Cadmium Sulfide Nanoparticles Formed by Pulsed-Laser Ablation. ChemPhysChem 2017, 18, 1035–1046. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Zhang, H.; Xu, X.; Zeng, H. Size and structure control of Si nanoparticles by laser ablation in different liquid media and further centrifugation classification. J. Phys. Chem. C 2009, 113, 19091–19095. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]
- Kanitz, A.; Hoppius, J.S.; Gurevich, E.L.; Ostendorf, A. Influence of the Liquid on Femtosecond Laser Ablation of Iron. Phys. Procedia 2016, 83, 114–122. [Google Scholar] [CrossRef]
- Baladi, A.; Sarraf Mamoory, R. Investigation of different liquid media and ablation times on pulsed laser ablation synthesis of aluminum nanoparticles. Appl. Surf. Sci. 2010, 256, 7559–7564. [Google Scholar] [CrossRef]
- Goncharova, D.A.; Kharlamova, T.S.; Lapin, I.N.; Svetlichnyi, V.A. Chemical and Morphological Evolution of Copper Nanoparticles Obtained by Pulsed Laser Ablation in Liquid. J. Phys. Chem. C 2019, 123, 21731–21742. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; de Grilo, J.P.F.; do Nascimento, R.M.; Silva, F.S. Effects of laser fluence and liquid media on preparation of small Ag nanoparticles by laser ablation in liquid. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Svetlichnyi, V.A.; Goncharov, D.A.; Lapin, I.N.; Shabalina, A.V. Influence of the Solvent on the Structure and Morphology of Nanoparticles Fabricated by Laser Ablation of Bulk Magnesium Targets. Russ. Phys. J. 2018, 61, 1047–1053. [Google Scholar] [CrossRef]
- Zhou, R.; Yin, Y.; Long, D.; Cui, J.; Yan, H.; Liu, W.; Pan, J.H. PVP-assisted laser ablation growth of Ag nanocubes anchored on reduced graphene oxide (rGO) for efficient photocatalytic CO2 reduction. Prog. Nat. Sci. Mater. Int. 2019, 29, 660–666. [Google Scholar] [CrossRef]
- Li, Y.; Musaev, O.R.; Wrobel, J.M.; Kruger, M.B. Laser ablation in liquids of germanium in externally applied electric fields. J. Laser Appl. 2016, 28, 022004. [Google Scholar] [CrossRef]
- Gondal, M.A.; Qahtan, T.F.; Dastageer, M.A.; Saleh, T.A.; Maganda, Y.W.; Anjum, D.H. Effects of oxidizing medium on the composition, morphology and optical properties of copper oxide nanoparticles produced by pulsed laser ablation. Appl. Surf. Sci. 2013, 286, 149–155. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Optical and structural properties of Bi-based nanoparticles prepared via pulsed Nd:YAG laser ablation in organic liquids. Appl. Phys. A 2018, 124, 1–7. [Google Scholar] [CrossRef]
- Yehia, S.A.; Carpen, L.G.; Stokker-Cheregi, F.; Poroșnicu, C.; Sătulu, V.; Staicu, C.; Butoi, B.; Lungu, I.; Virot, F.; Grisolia, C.; et al. Laser ablation of a solid target in liquid medium for beryllium nanoparticles synthesis. Nucl. Mater. Energy 2022, 31, 101160. [Google Scholar] [CrossRef]
- Balati, A.; Tek, S.; Nash, K.; Shipley, H. Nanoarchitecture of TiO2 microspheres with expanded lattice interlayers and its heterojunction to the laser modified black TiO2 using pulsed laser ablation in liquid with improved photocatalytic performance under visible light irradiation. J. Colloid Interface Sci. 2019, 541, 234–248. [Google Scholar] [CrossRef]
- Hamad, A.H. Nanosecond laser generation of silver nanoparticles in ice water. Chem. Phys. Lett. 2020, 755, 137782. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Ibrahium, H.A.; Awwad, N.S.; Menazea, A.A. Gold nanoparticles doped Polyvinyl Alcohol/Chitosan blend via laser ablation for electrical conductivity enhancement. J. Mol. Struct. 2020, 1221, 128814. [Google Scholar] [CrossRef]
- Lau, M.; Waag, F.; Barcikowski, S. Direct Integration of Laser-Generated Nanoparticles into Transparent Nail Polish: The Plasmonic “Goldfinger”. Ind. Eng. Chem. Res. 2017, 56, 3291–3296. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Saraeva, I.N.; Lednev, V.N.; Pershin, S.M.; Rudenko, A.A.; Ionin, A.A. Single-shot femtosecond laser ablation of gold surface in air and isopropyl alcohol. Appl. Phys. Lett. 2018, 112, 203101. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mousa, A.M.; Khashan, K.S.; Mohsin, M.H.; Hamid, M.K. Synthesis of PbI2 nanoparticles by laser ablation in methanol. J. Mater. Sci. Mater. Electron. 2016, 27, 10696–10700. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Fang, Y.; Fan, Z.; Ma, G.; Liu, Y.; Zhao, X. Synthesis of polyynes by intense femtosecond laser irradiation of SWCNTs suspended in methanol. Chem. Phys. Lett. 2017, 682, 96–100. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.P.; Kalai Priya, A.; Rohini, P.; Anandhan, S.V.; Krishnan, U.M.; Kalyanavalli, V.; Shukla, S.; Sastikumar, D. Synthesis of water-soluble fluorescent carbon nanoparticles (CNPs) from nanosecond pulsed laser ablation in ethanol. Opt. Laser Technol. 2021, 135, 106717. [Google Scholar] [CrossRef]
- Johny, J.; Sepulveda-Guzman, S.; Krishnan, B.; Avellaneda, D.; Shaji, S. Facile and fast synthesis of SnS 2 nanoparticles by pulsed laser ablation in liquid. Appl. Surf. Sci. 2018, 435, 1285–1295. [Google Scholar] [CrossRef]
- De Bonis, A.; Curcio, M.; Santagata, A.; Galasso, A.; Teghil, R. Transition Metal Carbide Core/Shell Nanoparticles by Ultra-Short Laser Ablation in Liquid. Nanomaterials 2020, 10, 145. [Google Scholar] [CrossRef]
- Scardaci, V.; Condorelli, M.; Barcellona, M.; Salemi, L.; Pulvirenti, M.; Fragalà, M.E.; Compagnini, G. Fast One-Step Synthesis of Anisotropic Silver Nanoparticles. Appl. Sci. 2021, 11, 8949. [Google Scholar] [CrossRef]
- Schille, J.; Schneider, L.; Lickschat, P.; Loeschner, U.; Ebert, R.; Exner, H. High-pulse repetition frequency ultrashort pulse laser processing of copper. J. Laser Appl. 2015, 27, S28007. [Google Scholar] [CrossRef]
- Al-Mamun, S.A.; Nakajima, R.; Ishigaki, T. Effect of liquid level and laser power on the formation of spherical alumina nanoparticles by nanosecond laser ablation of alumina target. Thin Solid Film. 2012, 523, 46–51. [Google Scholar] [CrossRef]
- Bärsch, N.; Jakobi, J.; Weiler, S.; Barcikowski, S. Pure colloidal metal and ceramic nanoparticles from high-power picosecond laser ablation in water and acetone. Nanotechnology 2009, 20, 445603. [Google Scholar] [CrossRef]
- Riabinina, D.; Chaker, M.; Margot, J. Dependence of gold nanoparticle production on pulse duration by laser ablation in liquid media. Nanotechnology 2012, 23, 135603. [Google Scholar] [CrossRef]
- Hahn, A.; Barcikowski, S.; Chichkov, B.N. Influences on nanoparticle production during pulsed laser ablation. J. Laser Micro Nanoeng. 2007, 3, 73–77. [Google Scholar] [CrossRef]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Hennig, G. Surface structuring with ultra-short laser pulses: Basics, limitations and needs for high throughput. Phys. Procedia 2014, 56, 1047–1058. [Google Scholar] [CrossRef]
- Leitz, K.H.; Redlingshöer, B.; Reg, Y.; Otto, A.; Schmidt, M. Metal ablation with short and ultrashort laser pulses. Phys. Procedia 2011, 12, 230–238. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. The effect of laser fluence for enhancing the antibacterial activity of NiO nanoparticles by pulsed laser ablation in liquid media. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100382. [Google Scholar] [CrossRef]
- Shih, C.Y.; Shugaev, M.V.; Wu, C.; Zhigilei, L.V. The effect of pulse duration on nanoparticle generation in pulsed laser ablation in liquids: Insights from large-scale atomistic simulations. Phys. Chem. Chem. Phys. 2020, 22, 7077–7099. [Google Scholar] [CrossRef]
- Lam, J.; Lombard, J.; Dujardin, C.; Ledoux, G.; Merabia, S.; Amans, D. Dynamical study of bubble expansion following laser ablation in liquids. Appl. Phys. Lett. 2016, 108. [Google Scholar] [CrossRef]
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.D.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Tomko, J.; Naddeo, J.J.; Jimenez, R.; Tan, Y.; Steiner, M.; Fitz-Gerald, J.M.; Bubb, D.M.; O’Malley, S.M. Size and polydispersity trends found in gold nanoparticles synthesized by laser ablation in liquids. Phys. Chem. Chem. Phys. 2015, 17, 16327–16333. [Google Scholar] [CrossRef]
- Shih, C.Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, A.; Galasso, A.; Santagata, A.; Teghil, R. Laser ablation of GaAs in liquid: The role of laser pulse duration. J. Phys. D Appl. Phys. 2015, 49, 035301. [Google Scholar] [CrossRef]
- Jeon, J.W.; Yoon, S.; Choi, H.W.; Kim, J.; Farson, D.; Cho, S.H. The effect of laser pulse widths on laser-Ag nanoparticle interaction: Femto- to nanosecond lasers. Appl. Sci. 2018, 8, 112. [Google Scholar] [CrossRef]
- Saraeva, I.N.; Nastulyavichus, A.A.; Kudryashov, S.I.; Rudenko, A.A.; Zayarny, D.A.; Ionin, A.A.; Klevkov, Y.V.; Zhilnikova, M.I.; Simakin, A. V The effect of laser pulsewidth on the selenium nanoparticles mass yield. Laser Phys. Lett. 2019, 16, 066004. [Google Scholar] [CrossRef]
- Zhang, K.; Ivanov, D.S.; Ganeev, R.A.; Boltaev, G.S.; Krishnendu, P.S.; Singh, S.C.; Garcia, M.E.; Zavestovskaya, I.N.; Guo, C. Pulse duration and wavelength effects of laser ablation on the oxidation, hydrolysis, and aging of aluminum nanoparticles in water. Nanomaterials 2019, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Kohsakowski, S.; Santagata, A.; Dell’Aglio, M.; de Giacomo, A.; Barcikowski, S.; Wagener, P.; Gökce, B. High productive and continuous nanoparticle fabrication by laser ablation of a wire-target in a liquid jet. Appl. Surf. Sci. 2017, 403, 487–499. [Google Scholar] [CrossRef]
- Baladi, A.; Mamoory, R.S. Effect of Laser Wavelength and Ablation Time on Pulsed Laser Ablation Synthesis of Al Nanoparticles in Ethanol. Int. J. Mod. Phys. Conf. Ser. 2012, 5, 58–65. [Google Scholar] [CrossRef]
- Smejkal, P.; Pfleger, J.; Vlcková, B.; Dammer, O. Laser ablation of silver in aqueous ambient: Effect of laser pulse wavelength and energy on efficiency of the process. J. Phys. Conf. Ser. 2007, 59, 185–188. [Google Scholar] [CrossRef]
- Tsuji, T.; Iryo, K.; Nishimura, Y.; Tsuji, M. Preparation of metal colloids by a laser ablation technique in solution: Influence of laser wavelength on the ablation efficiency (II). J. Photochem. Photobiol. A Chem. 2001, 145, 201–207. [Google Scholar] [CrossRef]
- Intartaglia, R.; Bagga, K.; Brandi, F. Study on the productivity of silicon nanoparticles by picosecond laser ablation in water: Towards gram per hour yield. Opt. Express 2014, 22, 3117. [Google Scholar] [CrossRef]
- Hoffman, J.; Chrzanowska, J.; Kucharski, S.; Moscicki, T.; Mihailescu, I.N.; Ristoscu, C.; Szymanski, Z. The effect of laser wavelength on the ablation rate of carbon. Appl. Phys. A Mater. Sci. Process. 2014, 117, 395–400. [Google Scholar] [CrossRef]
- Kim, J.; Amaranatha Reddy, D.; Ma, R.; Kim, T.K. The influence of laser wavelength and fluence on palladium nanoparticles produced by pulsed laser ablation in deionized water. Solid State Sci. 2014, 37, 96–102. [Google Scholar] [CrossRef]
- Schwenke, A.; Wagener, P.; Nolte, S.; Barcikowski, S. Influence of processing time on nanoparticle generation during picosecond-pulsed fundamental and second harmonic laser ablation of metals in tetrahydrofuran. Appl. Phys. A Mater. Sci. Process. 2011, 104, 77–82. [Google Scholar] [CrossRef]
- Waag, F.; Barcikowski, S. Laser Synthesis of Metallic and Oxidic Transition Metal, Multi-element Nanoparticles for Catalytic Applications. In Duepublico; Universität Duisburg-Essen: North Rhine-Westphalia, Germany, 2019; pp. 7–200. [Google Scholar]
- Semerok, A.; Chaléard, C.; Detalle, V.; Lacour, J.L.; Mauchien, P.; Meynadier, P.; Nouvellon, C.; Sallé, B.; Palianov, P.; Perdrix, M.; et al. Experimental investigations of laser ablation efficiency of pure metals with femto, pico and nanosecond pulses. Appl. Surf. Sci. 1999, 138, 311–314. [Google Scholar] [CrossRef]
- Letzel, A.; Santoro, M.; Frohleiks, J.; Ziefuß, A.R.; Reich, S.; Plech, A.; Fazio, E.; Neri, F.; Barcikowski, S.; Gökce, B. How the re-irradiation of a single ablation spot affects cavitation bubble dynamics and nanoparticles properties in laser ablation in liquids. Appl. Surf. Sci. 2019, 473, 828–837. [Google Scholar] [CrossRef]
- Sreenilayam, S.P.; McCarthy, É.; McKeon, L.; Ronan, O.; McCann, R.; Fleischer, K.; Freeland, B.; Nicolosi, V.; Brabazon, D. Additive-free silver nanoparticle ink development using flow-based Laser Ablation Synthesis in Solution and Aerosol Jet printing. Chem. Eng. J. 2022, 449, 137817. [Google Scholar] [CrossRef]
- Rehbock, C.; Jakobi, J.; Gamrad, L.; van der Meer, S.; Tiedemann, D.; Taylor, U.; Kues, W.; Rath, D.; Barcikowski, S. Current state of laser synthesis of metal and alloy nanoparticles as ligand-free reference materials for nano-toxicological assays. Beilstein J. Nanotechnol. 2014, 5, 1523–1541. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Zhu, G.; Wang, T.; Yu, D.; Lu, Y.; Yu, H. One-step synthesis of copper-platinum nanoparticles modified electrode for non-enzymatic salivary glucose detection. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130672. [Google Scholar] [CrossRef]
- Dobrucka, R. Synthesis of MgO Nanoparticles Using Artemisia abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 547–555. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-pot green synthesis of magnesium oxide nanoparticles using Penicillium chrysogenum melanin pigment and gamma rays with antimicrobial activity against multidrug-resistant microbes. Adv. Powder Technol. 2018, 29, 2616–2625. [Google Scholar] [CrossRef]


| Materials | Liquid Medium | Alloy/Core–Shell | Ref. |
|---|---|---|---|
| Ag-Au | DI water, chloroauric acid | core–shell | [64] |
| ZnO-Ag | DI water | core–shell | [67] |
| Au-TiO2 | DI water | core–shell | [2] |
| Pt-Pd | DI Water | core–shell and alloy | [68] |
| Ni-Pd | DI water, methanol | core–shell | [69] |
| Au-Fe | DI water, ethanol | alloy | [78] |
| Ag-Fe | DI water | alloy | [79] |
| C-Se | Ethanol | core–shell | [71] |
| Au-ZnO | DI water | core–shell | [72] |
| Ag-Au | DI water | core–shell | [80] |
| Ag-Au | DI water | core–shell | [81] |
| Au-Si | DI water | core–shell | [82] |
| Ni-Ti | DI water | core–shell | [73] |
| Al-Ti | IPA | core–shell | [83] |
| Ti-N | Acetone | core–shell | [76] |
| Ag-Si | DI water | core–shell | [84] |
| WO3-CdS | DI water | alloy | [85] |
| Ag-Cu | DI water | alloy | [57] |
| Cu-Ni | DI water | alloy | [70] |
| Cu-Fe | DI water | alloy | [70] |
| Au-Ni | DI water | alloy | [65] |
| Au-Fe | DI water, acetone, methyl methacrylate | core–shell | [66] |
| Fe-C | DI water | alloy | [86] |
| Ag-C | Nitric acid, sulfuric acid | alloy | [87] |
| ZnO-CuO | DI water | alloy | [88] |
| Au-C | Ethanol, toluene | core–shell | [89] |
| Material | Liquid Media | Flow/Batch Mode | Ref. |
|---|---|---|---|
| Au-Fe | Acetone, methyl methacrylate, DI water | Batch | [66] |
| Au-Fe | Ethanol, DI water | Batch | [78] |
| Ni-Pd | Methanol, DI water | Batch | [69] |
| Mg | IPA, DI water | Batch | [60] |
| C | Ethanol, DI water, medical liquid | Batch | [22] |
| Al | Ethanol, acetone, and ethylene glycol | Flow | [97] |
| Cu | Ethyl alcohol, DI water, H2O2, NaOH | Flow | [98] |
| Ag | Ethanol, acetone, DI water | Flow | [99] |
| Cu | Spinach extract, DI water | Flow | [51] |
| Mg | Ethyl alcohol, ethyl acetate, hexane, DI water | Flow | [100] |
| Ag | Tetrahydrofuran, dimethylformamide, DI water | Batch | [95] |
| Ag | Polyvinylpyrrolidone solution, DI water | Batch | [101] |
| Ge | Ethanol, DI water | Batch | [102] |
| Cu | H2O2, DI water | Batch | [103] |
| Bi | Ethanol, ethanol, methyl ethyl ketone | Batch | [104] |
| Au-C | Toluene, ethanol | Flow | [89] |
| Ag-Au | Chloroauric acid, DI water | Batch | [64] |
| Be | Acetone, heavy water | Batch | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. https://doi.org/10.3390/cryst13020253
Nyabadza A, Vazquez M, Brabazon D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals. 2023; 13(2):253. https://doi.org/10.3390/cryst13020253
Chicago/Turabian StyleNyabadza, Anesu, Mercedes Vazquez, and Dermot Brabazon. 2023. "A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid" Crystals 13, no. 2: 253. https://doi.org/10.3390/cryst13020253
APA StyleNyabadza, A., Vazquez, M., & Brabazon, D. (2023). A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals, 13(2), 253. https://doi.org/10.3390/cryst13020253