Matere Bonds in Technetium Compounds: CSD Survey and Theoretical Considerations
Abstract
1. Introduction
2. Methods
3. Results and Discussion
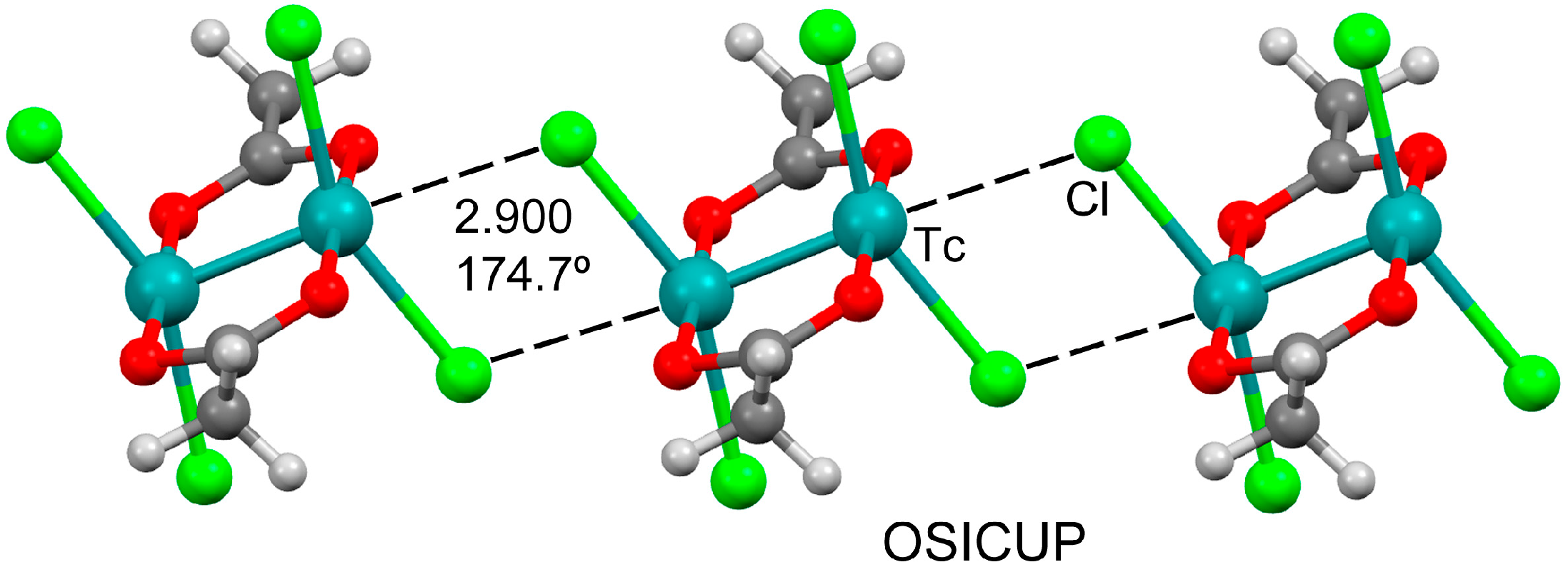
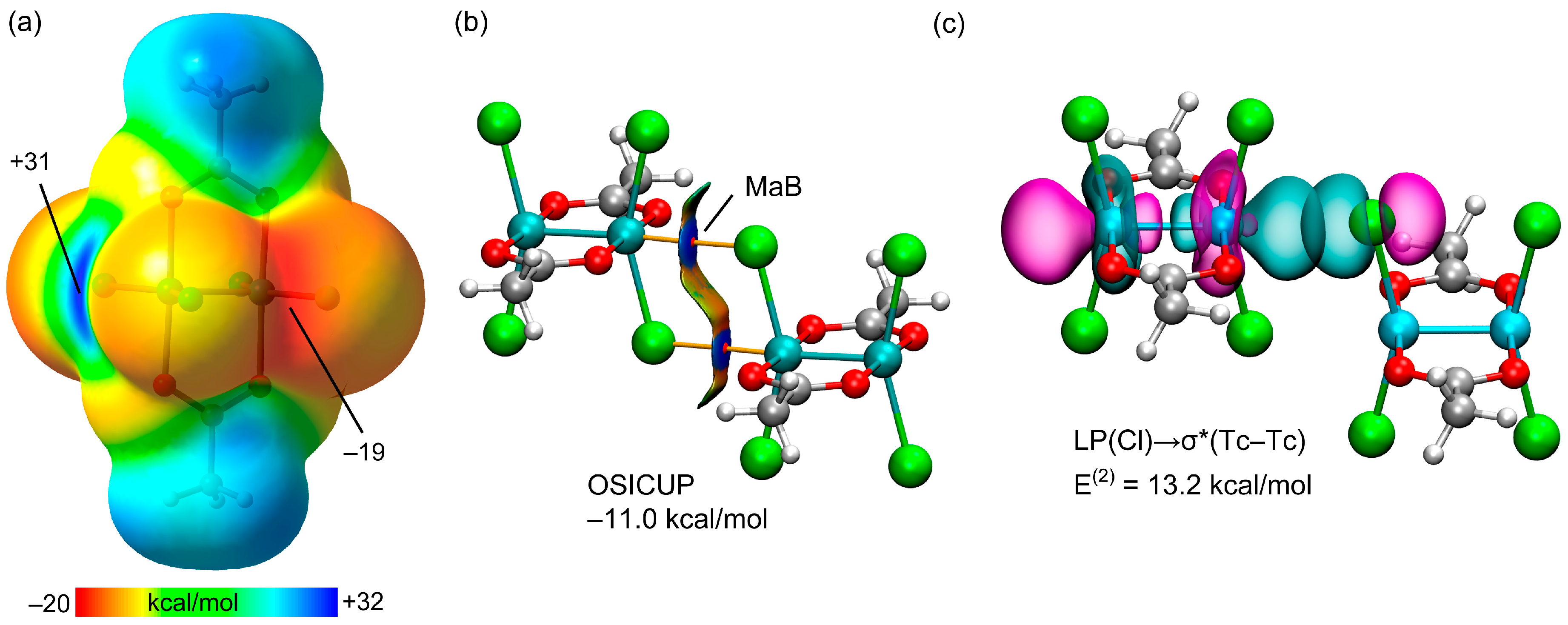
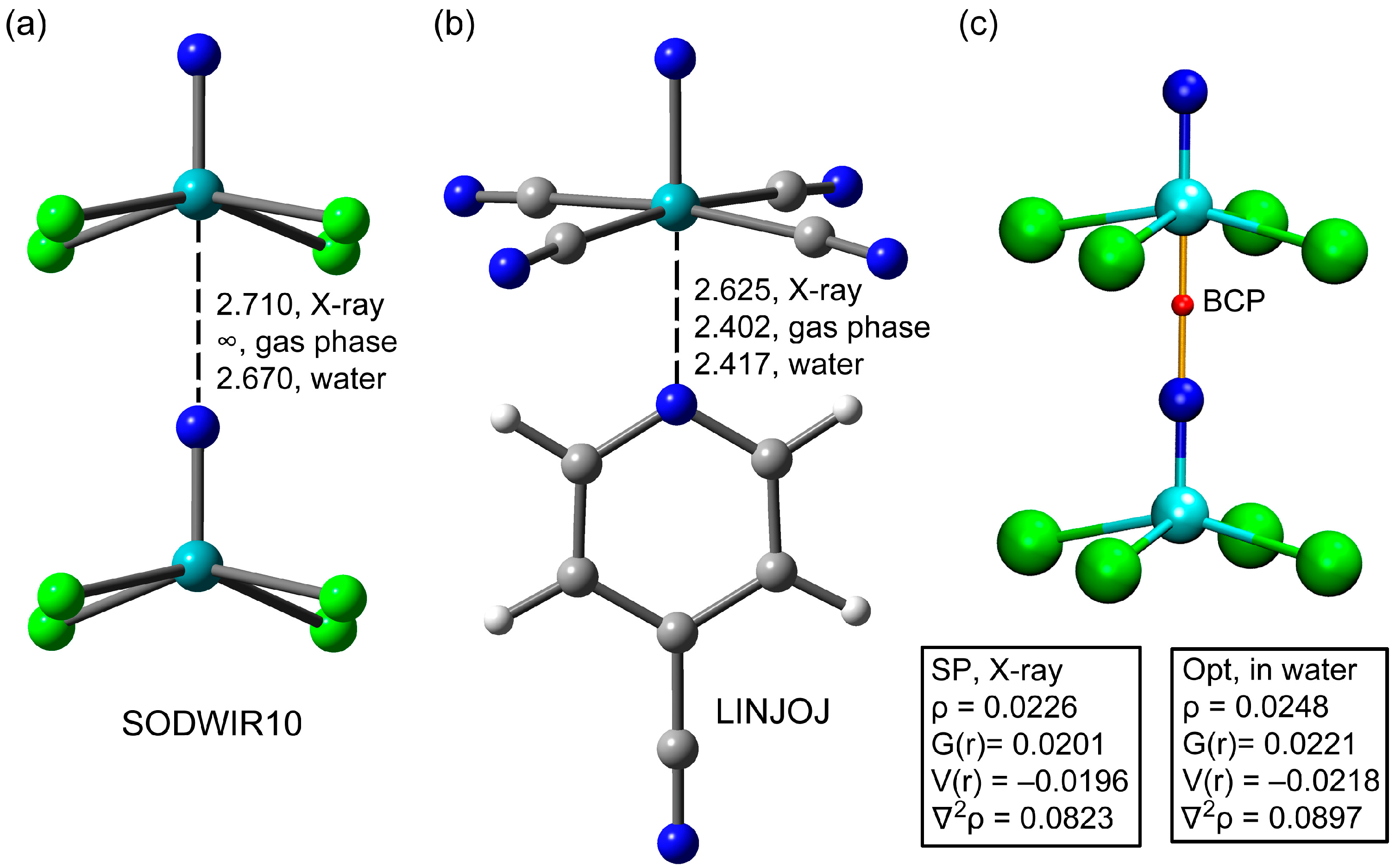
3.1. Anion⋯Anion Matere Bonds
3.2. Anion⋯Cation Matere Bonds
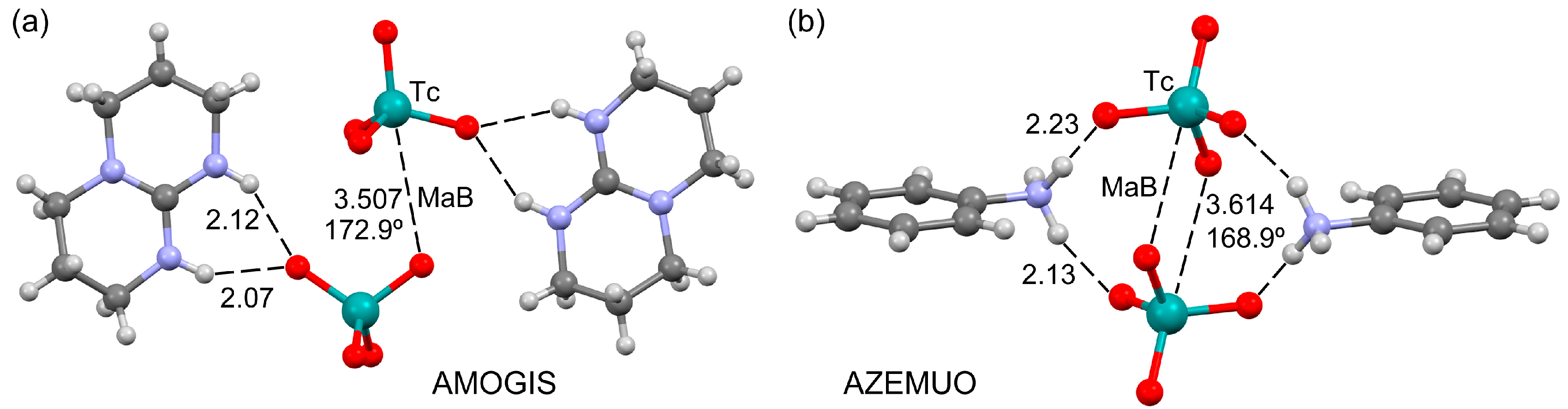
3.3. Anion⋯Lewis Base Matere Bond
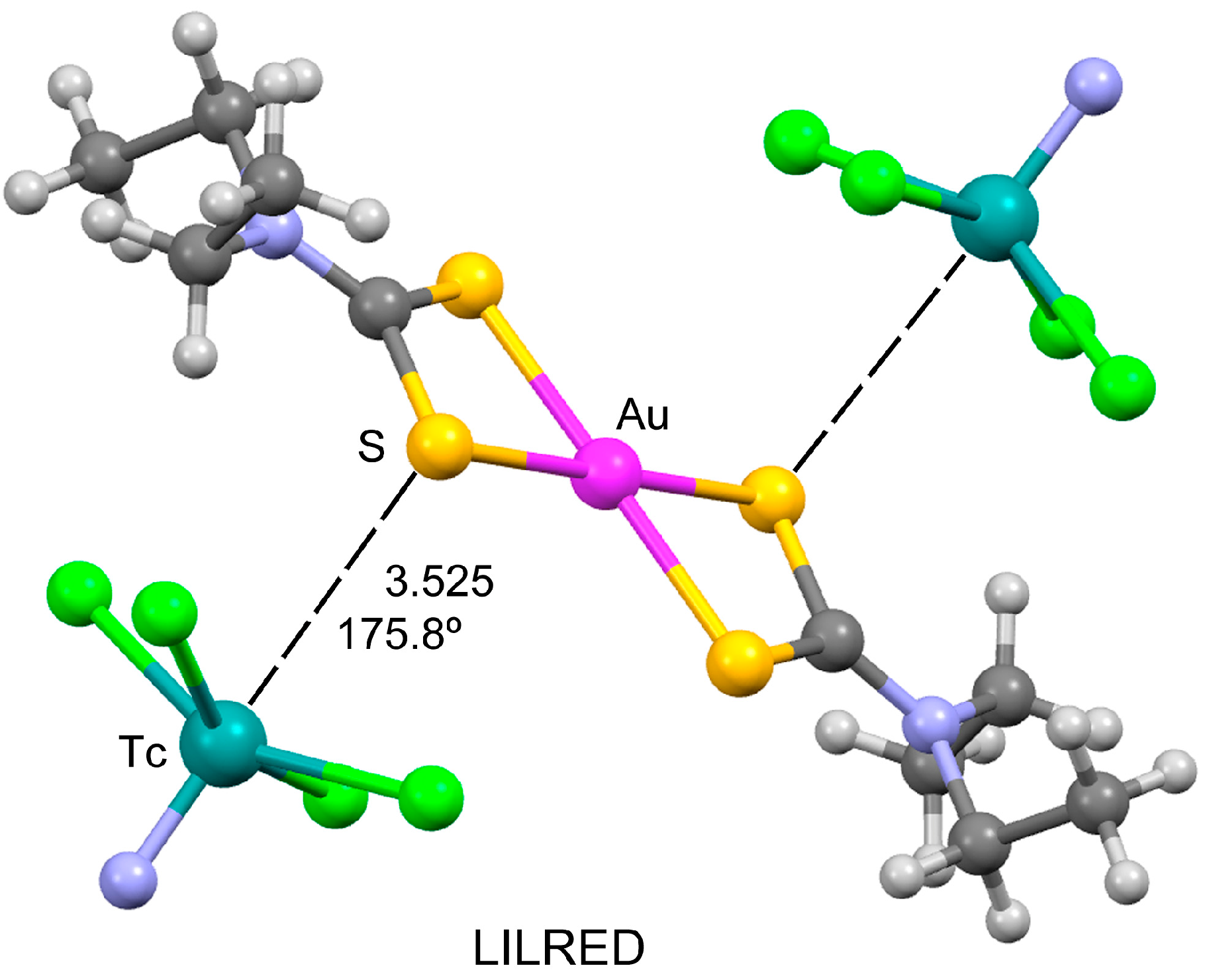
3.4. Lewis Acid⋯Lewis Base Matere Bonds
3.5. Optimized Matere Dimers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanov, D.M.; Bokach, N.B.; Kukushkin, V.Y.; Frontera, A. Metal Centers as Nucleophiles: Oxymoron of Halogen Bond-Involving Crystal Engineering. Chem. Eur. J. 2022, 28, e202103173. [Google Scholar] [PubMed]
- Blasi, D.; Nicolai, V.; Gomila, R.M.; Mercandelli, P.; Frontera, A.; Carlucci, L. Unprecedented {dz2-CuIIO4}⋯π-hole interactions: The case of a cocrystal of a Cu (II) bis-beta-diketonate complex. Chem. Commun. 2022, 58, 9524–9527. [Google Scholar] [CrossRef] [PubMed]
- Andreo, L.; Gomila, R.M.; Priola, E.; Giordana, A.; Pantaleone, S.; Diana, E.; Mahmoudi, G.; Frontera, A. Anion⋯Anion [AuI4]−⋯[AuI2]− Complex Trapped in the Solid State by Tetramethylammonium Cations. Cryst. Growth Des. 2022, 22, 6539–6544. [Google Scholar] [CrossRef] [PubMed]
- Gomila, R.M.; Bauzá, A.; Mooibroek, T.J.; Frontera, A. Spodium bonding in five coordinated Zn(II): A new player in crystal engineering? CrystEngComm 2021, 23, 3084–3093. [Google Scholar] [CrossRef]
- Bauzá, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487. [Google Scholar] [CrossRef]
- Biswal, H.S.; Sahu, A.K.; Frontera, A.; Bauzá, A. Spodium Bonds in Biological Systems: Expanding the Role of Zn in Protein Structure and Function. J. Chem. Inf. Model. 2021, 61, 3945–3954. [Google Scholar] [CrossRef]
- Stenlid, J.H.; Johansson, A.J.; Brinck, T. σ-Holes and σ-lumps direct the Lewis basic and acidic interactions of noble metal nanoparticles: Introducing regium bonds. Phys. Chem. Chem. Phys. 2018, 20, 2676–2692. [Google Scholar] [CrossRef]
- Stenlid, J.H.; Brinck, T. Extending the σ-Hole Concept to Metals: An Electrostatic Interpretation of the Effects of Nanostructure in Gold and Platinum Catalysis. J. Am. Chem. Soc. 2017, 139, 11012–11015. [Google Scholar] [CrossRef]
- Legon, A.C.; Walker, N.R. What’s in a name? ‘Coinage-metal’ non-covalent bonds and their definition. Phys. Chem. Chem. Phys. 2018, 20, 19332–19338. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Calabrese, M.; Terraneo, G.; Bordignon, S.; Frontera, A.; Resnati, G. Molecular Electrostatic Potential and Noncovalent Interactions in Derivatives of Group 8 Elements. Angew. Chem. Int. Ed. 2021, 60, 20723–20727. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Terraneo, G.; Frontera, A.; Resnati, G. Anion⋅⋅⋅Anion Interactions Involving σ-Holes of Perrhenate, Pertechnetate and Permanganate Anions. ChemPhysChem 2021, 22, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Noncovalent Interactions Involving Group 6 in Biological Systems: The Case of Molybdopterin and Tungstopterin Cofactors. Chem. Eur. J. 2022, 28, e202201660. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Pizzi, A.; Daolio, A.; Frontera, A.; Resnati, G. σ-Hole interactions in organometallic catalysts: The case of methyltrioxorhenium(VII). Dalton Trans. 2023, 52. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Matere Bonds vs. Multivalent Halogen and Chalcogen Bonds: Three Case Studies. Molecules 2022, 27, 6597. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Leibnitz, P.; Reck, G.; Pietzsch, H.-J.; Spies, H. Structures of technetium and rhenium complexes. Forschungszent. Rossendorf. 2001, 311, 34. [Google Scholar]
- Maruk, A.Y.; Grigor’ev, M.S.; German, K.E. Synthesis and crystal structure of anilinium pertechnetate and perrhenate at low and room temperatures. Koordinatsionnaia. Khimiia. 2010, 36, 381. [Google Scholar]
- Baldas, J.; Boas, J.F.; Colmanet, S.F.; Rae, A.D.; Williams, G.A. Preparation and ESR studies of salts of crown ether complex cations and nitridotechnetium(VI) anions: Structures of the twinned ‘infinite sandwich’ [Cs(18-crown-6)][TcNCl4] complex and of [Rb(15-crown-5)2] [TcNCl4(OH2)]. Proceed. Royal Soc. Lond. Ser. A 1993, 442, 437. [Google Scholar]
- Gomila, R.M.; Frontera, A. Crystallographic and Theoretical Study of Osme Bonds in Nitrido-Osmium(VI) Complexes. Inorganics 2022, 10, 133. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Abram, U. [Au(Et2dtc)2][TcNCl4]—Darstellung und Struktur. ZAAC 2000, 629, 619–620. [Google Scholar] [CrossRef]
- Ikeda, H.; Ito, A.; Sakuda, E.; Kitamura, N.; Takayama, T.; Sekine, T.; Shinohara, A.; Yoshimura, T. Excited-State Characteristics of Tetracyanidonitridorhenium(V) and -technetium(V) Complexes with N-Heteroaromatic Ligands. Inorg. Chem. 2013, 52, 6319. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Abram, U. Rhenium and technetium complexes with tridentate S,N,O ligands derived from benzoylhydrazine. Polyhedron 2009, 28, 3945–3952. [Google Scholar] [CrossRef]
- Linder, K.E.; Chan, Y.-W.; Cyr, J.E.; Malley, M.F.; Nowotnik, D.P.; Nunn, A.D. TcO(PnAO-1-(2-nitroimidazole)) [BMS-181321], a new technetium-containing nitroimidazole complex for imaging hypoxia: Synthesis, characterization, and xanthine oxidase-catalyzed reduction. J. Med. Chem. 1994, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Poineau, F.; Johnstone, E.V.; Weck, P.F.; Kim, E.; Forster, P.M.; Scott, B.L.; Sattelberger, A.P.; Czerwinski, K.R. Synthesis and Structure of Technetium Trichloride. J. Am. Chem. Soc. 2010, 132, 15864–15865. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, F.; Esterhuysen, C.; Dillen, J. Electrostatic surface potential analysis of the ion in the gas phase, the condensed phase and a novel extrapolation to the solid state. Comput. Theor. Chem. 2016, 1090, 225–233. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguera, S.; Gomila, R.M.; Bauzá, A.; Frontera, A. Matere Bonds in Technetium Compounds: CSD Survey and Theoretical Considerations. Crystals 2023, 13, 187. https://doi.org/10.3390/cryst13020187
Burguera S, Gomila RM, Bauzá A, Frontera A. Matere Bonds in Technetium Compounds: CSD Survey and Theoretical Considerations. Crystals. 2023; 13(2):187. https://doi.org/10.3390/cryst13020187
Chicago/Turabian StyleBurguera, Sergi, Rosa M. Gomila, Antonio Bauzá, and Antonio Frontera. 2023. "Matere Bonds in Technetium Compounds: CSD Survey and Theoretical Considerations" Crystals 13, no. 2: 187. https://doi.org/10.3390/cryst13020187
APA StyleBurguera, S., Gomila, R. M., Bauzá, A., & Frontera, A. (2023). Matere Bonds in Technetium Compounds: CSD Survey and Theoretical Considerations. Crystals, 13(2), 187. https://doi.org/10.3390/cryst13020187







