Solid State Structure and Hydrogen Bonding of Some Cyclic NH Carboximides
Abstract
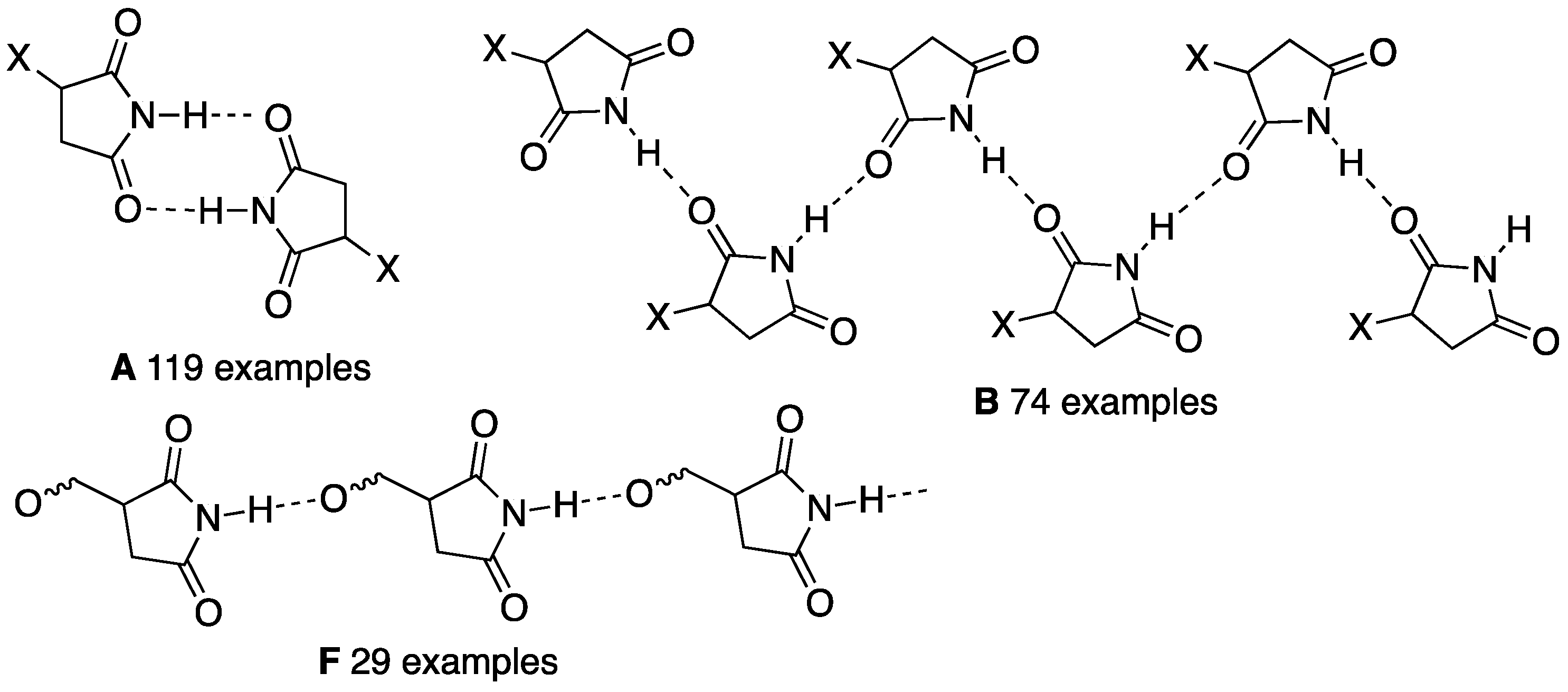
1. Introduction
2. Materials and Methods
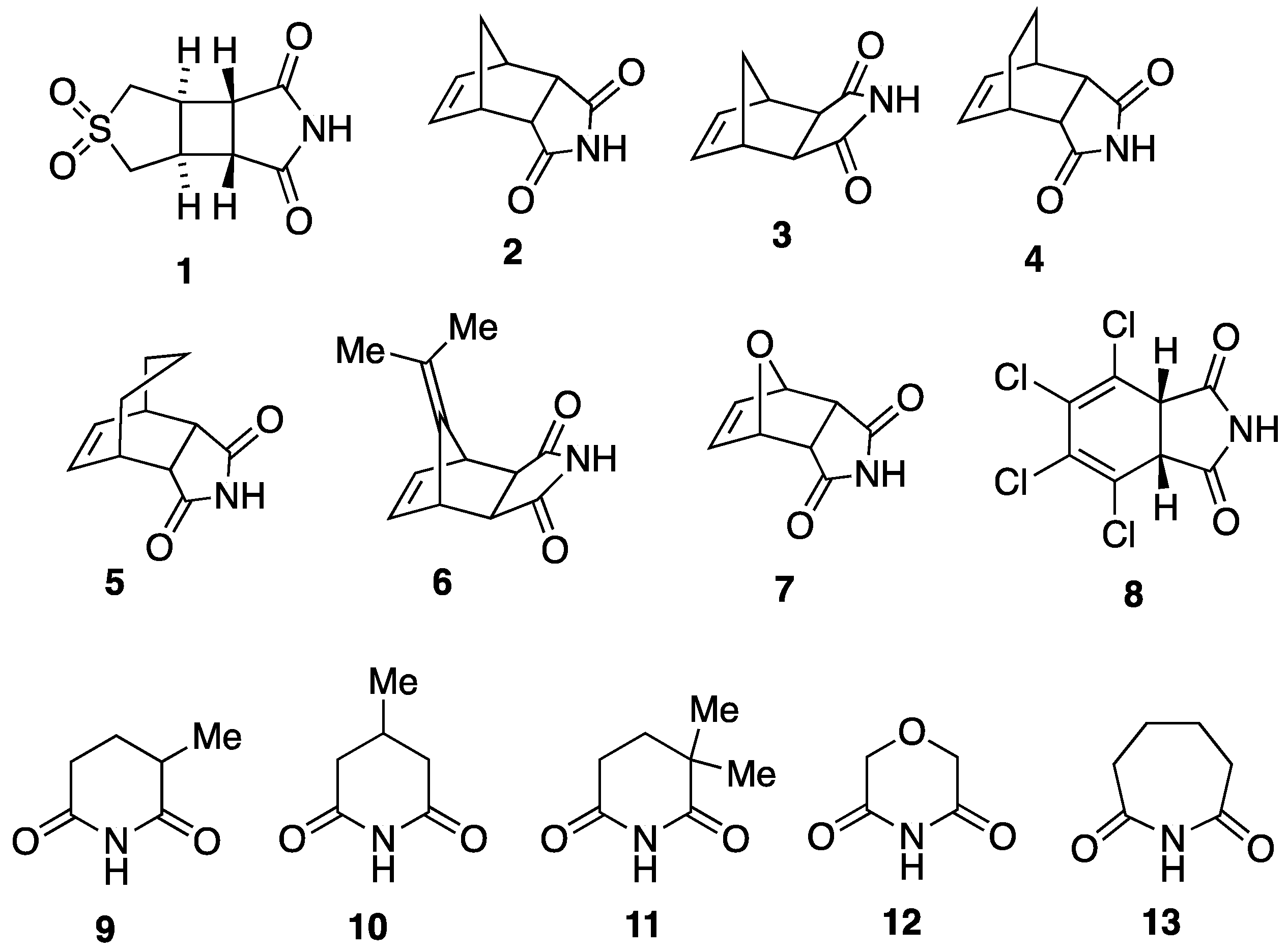
2.1. Synthesis of Imides 1–13
2.2. Crystallography
3. Results and Discussion
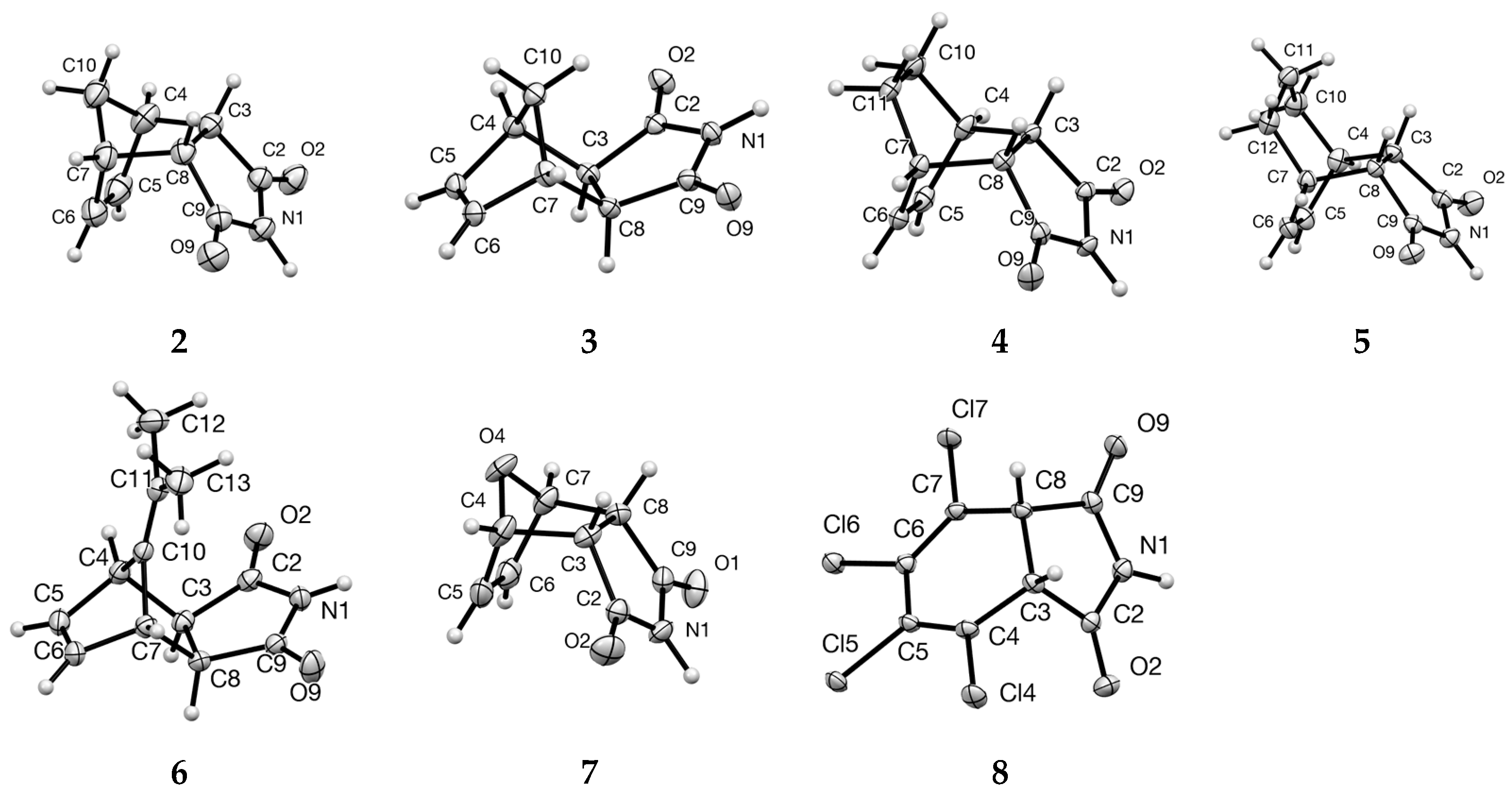
3.1. Cyclobutane-Fused Succimimide 1
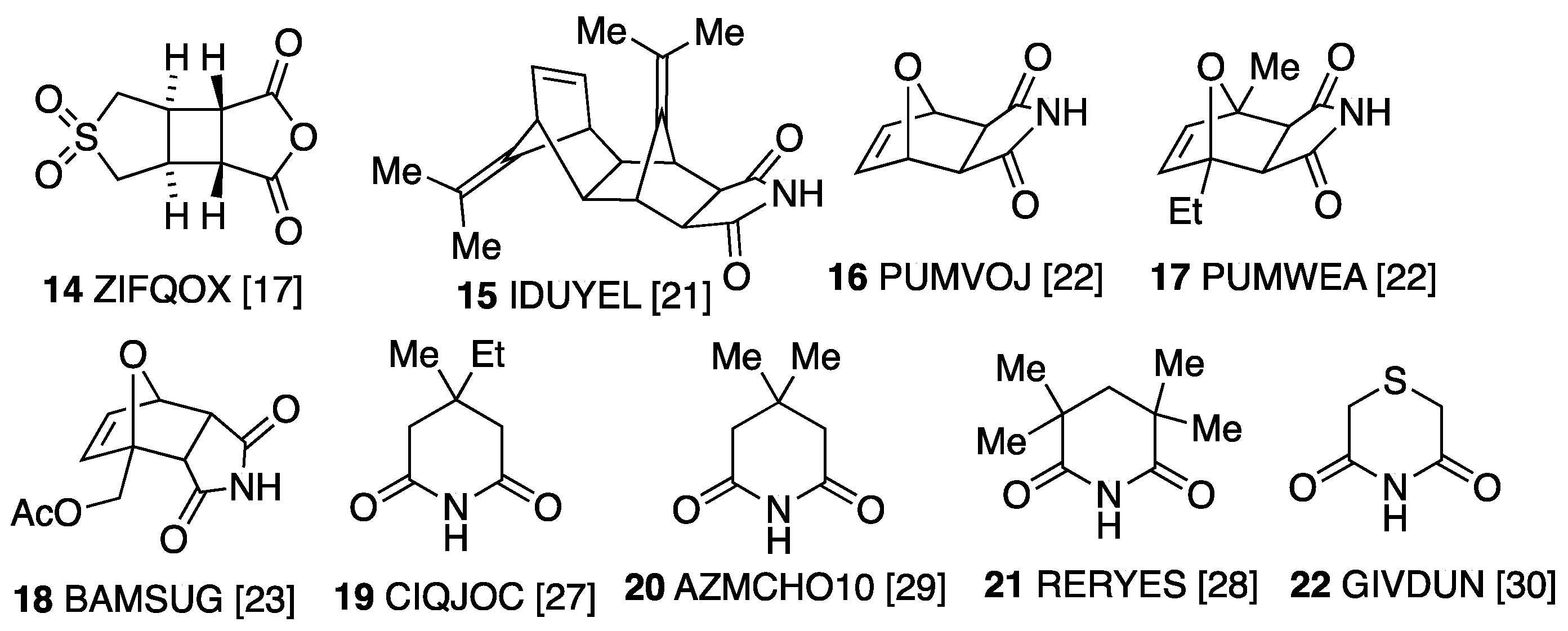
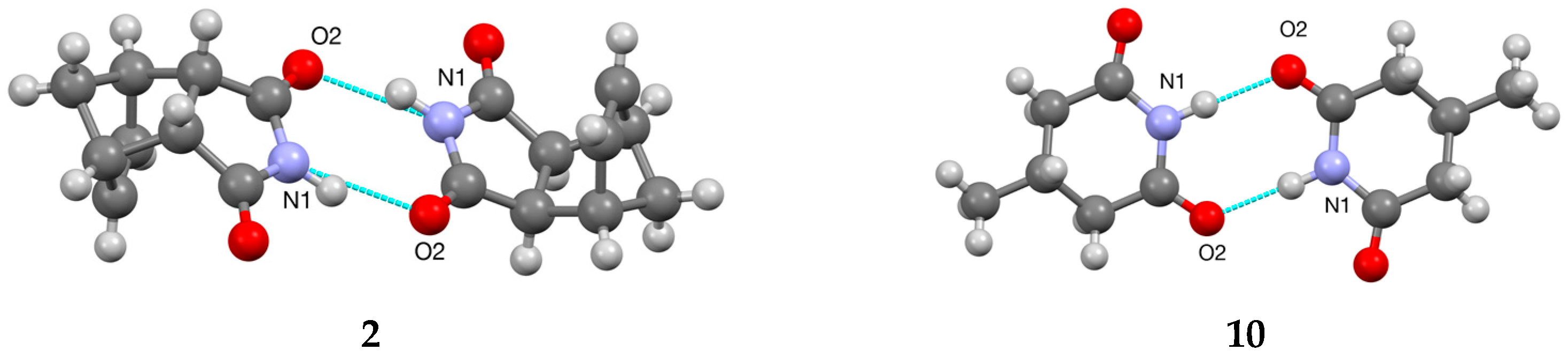
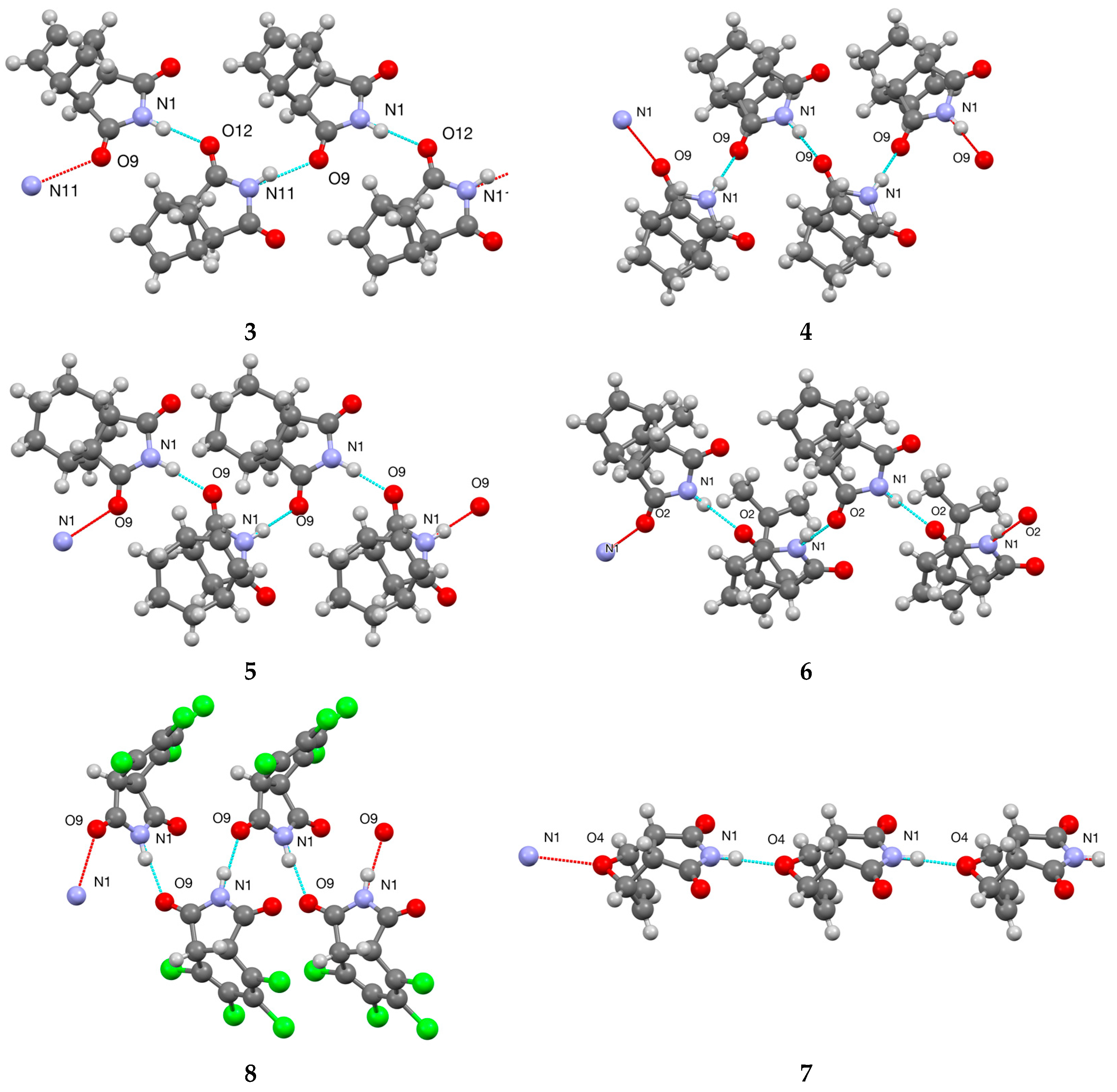
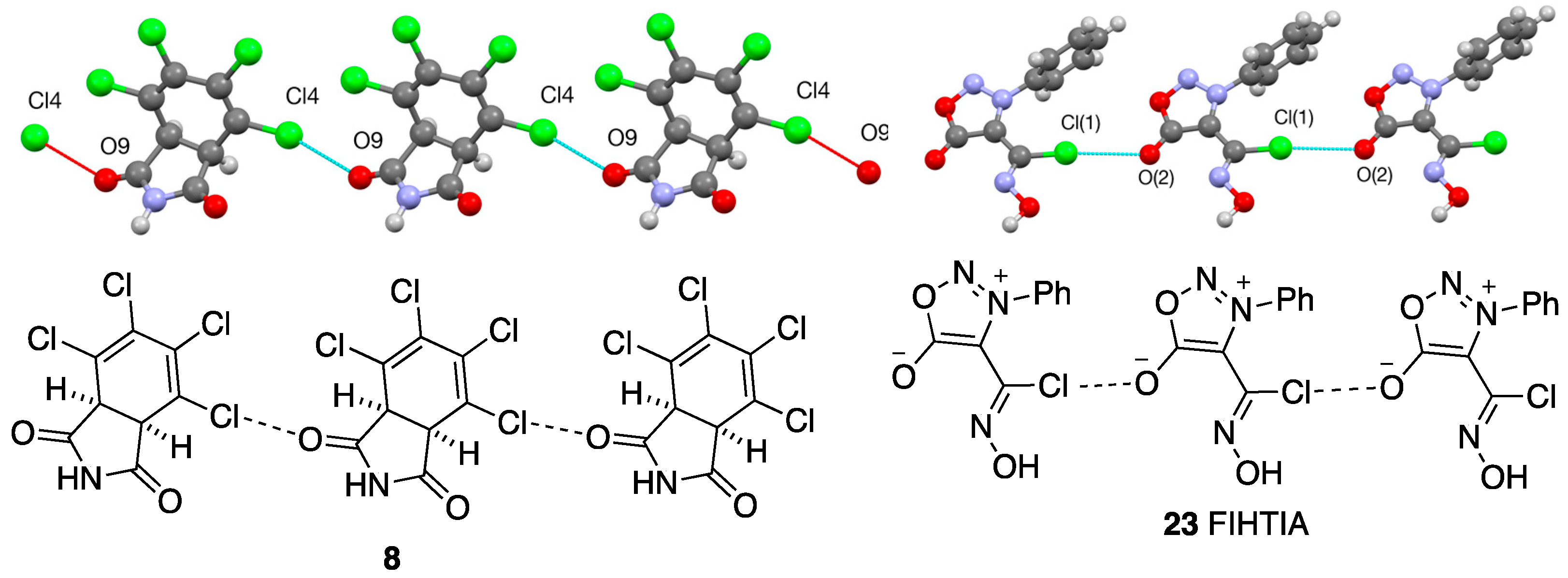
3.2. Bi- and Tricyclic Diels–Alder Adducts of Maleimide 2–8
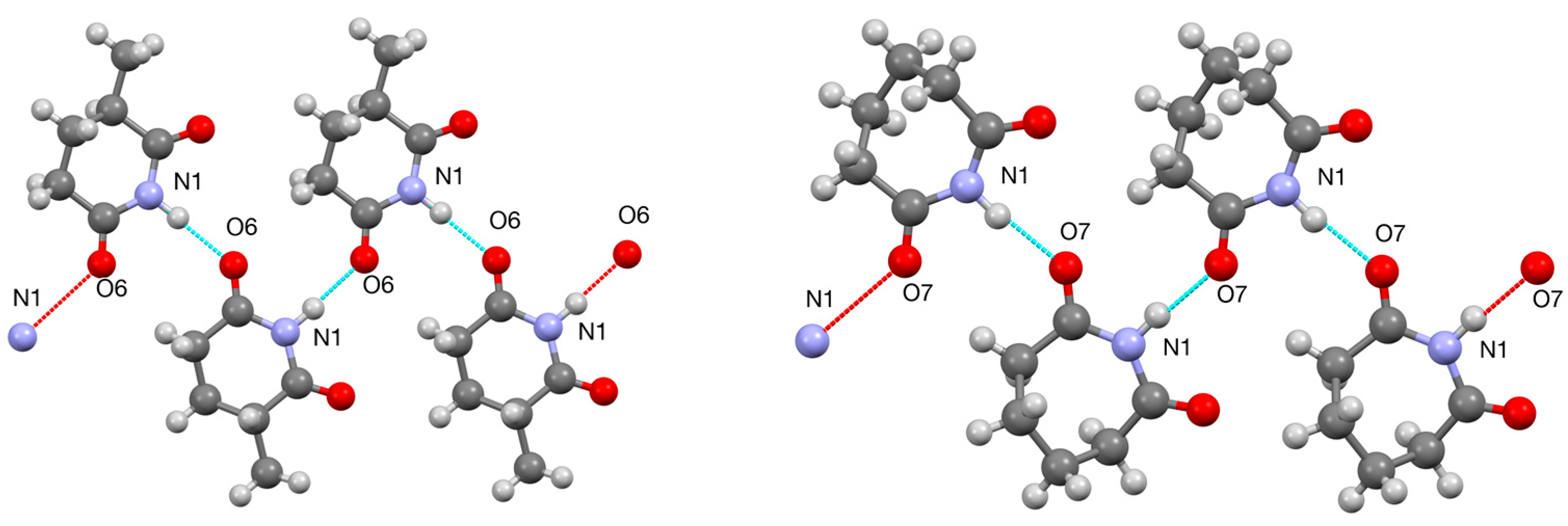
3.3. Ring-Methylated Glutarimides 9–11 and Morpholine Analogue 12
3.4. Adipimide 13
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aitken, R.A.; Sonecha, D.K. The solid state structures of cyclic NH carboximides. Crystals 2020, 10, 606. [Google Scholar] [CrossRef]
- Shaikhrazieva, V.S.; Tolstikov, G.A.; Enikeev, R.S. Photoinitiated addition of maleic anhydride and its derivatives to 3-sulfolene. Zh. Org. Khim 1972, 8, 377–382. [Google Scholar]
- Michaelis, S.; Blechert, S. Ring-opening cross-metathesis (ROCM) as a novel tool for the ligation of peptides. Chem. Eur. J. 2007, 13, 2358–2368. [Google Scholar] [CrossRef]
- Fujimoto, M.; Okabe, K. Nouvelle méthode de synthèse des dérivés d’ethano-4,7-polyhydroisoindoline. Chem. Pharm. Bull. 1962, 10, 714–719. [Google Scholar] [CrossRef]
- Abou-Gharbia, M.; Patel, U.R.; Webb, M.B.; Moyer, J.A.; Andree, T.H.; Muth, E.A. Polycyclic aryl- and heteroarylpiperazinyl imides as 5-HT1A receptor ligands and potential anxiolytic agents: Synthesis and structure–activity relationship studies. J. Med. Chem. 1988, 31, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Poos, G.I.; Lehman, M.M.; Landis, E.B.; Rosenau, J.D. Bicyclic bases. IV. Aryl substituted bridged hydroisoindolines. J. Med. Chem. 1962, 5, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.; Fayter, A.E.R.; Houston, J.E.; Evans, R.C.; Gibson, M.I. Facially amphipathic glycopolymers inhibit ice recrystallzation. J. Am. Chem. Soc. 2018, 140, 5682–5685. [Google Scholar] [CrossRef]
- Kwart, H.; Burchuk, I. Isomerism and adduct stability in the Diels-Alder reaction. I. The adducts of furan and maleimide. J. Am. Chem. Soc. 1952, 74, 3094–3097. [Google Scholar] [CrossRef]
- Raasch, M.S. Annelations with tetrachlorothiophene 1,1-dioxide. J. Org. Chem. 1980, 45, 856–867. [Google Scholar] [CrossRef]
- Kreituss, I.; Chen, K.-Y.; Eitel, S.H.; Adam, J.-M.; Wuitschik, G.; Fettes, A.; Bode, J.W. A robust, recyclable resin for decagram scale resolution of (±)-mefloquine and other chiral N-heterocycles. Angew. Chem. Int. Ed. 2016, 55, 1553–1556. [Google Scholar] [CrossRef]
- Aitken, R.A.; Farrell, D.M.M.; Kirton, E.H.M. Synthesis and pyrolysis of tetrahydro-1,4-oxazine-3,5-diones and tetrahydro-1,4-thiazine-3,5-diones. Chem. Heterocycl. Compd. 2001, 37, 1526–1531. [Google Scholar] [CrossRef]
- Sircar, S.S.G. The influence of groups and associated rings on the stability of certain heterocyclic ring systems. Part 1. The substituted glutarimides. J. Chem. Soc. 1927, 600–605. [Google Scholar] [CrossRef]
- Dabrowski, Z.; Cybulski, J. Structure and stereochemistry of lactams and cyclic imides. V. Mass spectrometry of substituted glutarimides. Bull. Acad. Pol. Sci.-Chim. 1981, 29, 11–16. [Google Scholar]
- Mizuno, H.; Manabe, A. Pyrimidine Compounds and Pests Controlling Composition Containing the Same. WO2004/99160 A1, 18 November 2004. [Google Scholar]
- Alekseeva, O.; Konstantinova, M.; Rasumovskii, S. Efficient method of cyclic imides synthesis under ozone influence by the example of ε-caprolactam oxidation reaction. Heteroatom Chem. 2008, 19, 661–666. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Chen, T.-G.; Barton, L.M.; Lin, Y.; Tsien, J.; Kossler, D.; Bastida, I.; Asai, S.; Bi, C.; Chen, J.S.; Shan, M.; et al. Building C(sp3)-rich complexity by combining cycloaddition and C–C cross-coupling reactions. Nature 2018, 560, 350–354. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Morgan, M.S.; Tipson, R.S.; Lowy, A.; Baldwin, W.E. Some derivatives of cis-3,6-endomethylene-Δ4-tetrahydrophthalic acid. J. Am. Chem. Soc. 1944, 66, 404–407. [Google Scholar] [CrossRef]
- Hong, B.-C.; Shr, Y.-J.; Liao, J.-H. Unprecedented microwave effects on the cycloaddition of fulvenes. A new approach to the construction of polycyclic ring systems. Org. Lett. 2002, 4, 663–666. [Google Scholar] [CrossRef]
- Struga, M.; Miroslaw, B.; Pakosinska-Parys, M.; Drzewiecka, A.; Borowski, P.; Kossakowski, J.; Koziol, A.E. Synthesis, characterization and supramolecular synthons in crystals of new derivatives of 10-oxa-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. J. Mol. Struct. 2010, 965, 23–30. [Google Scholar] [CrossRef]
- Bennett, G.D.; Spengler, A.O.; Wheeler, K.A. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, 2016; BAMSUG. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-H.; Yeh, M.-Y.; Lee, M.-J.; Su, Y.-S. Efficient syntheses of 3-(3-arylsydnon-4-yl)triazole derivatives. Synthesis 2004, 2877–2885. [Google Scholar] [CrossRef]
- Petersen, C.S. The crystal structure of glutarimide. Acta Chem. Scand. 1971, 25, 379. [Google Scholar] [CrossRef]
- Tutughamiarso, M.; Bolte, M. 4-Ethyl-4-methyl piperidine-2,6-dione. Acta Crystallogr. Sect. E 2007, 63, o4743. [Google Scholar] [CrossRef]
- Maurin, J.K.; Czarnocki, Z.; Paluchowska, B.; Winnicka-Maurin, M. Conformation-related reaction efficiency of glutarimides with phenyllithium. Structures of 3,3,5,5-tetramethylglutarimide and 2-hydroxy-2-phenyl-3,3,5,5-tetramethyl-6-piperidone. X-ray and theoretical study. Acta Crystallogr. Sect. B 1997, 53, 719–725. [Google Scholar] [CrossRef]
- Bocelli, G.; Grenier-Loustalot, M.F. The structure of 4,4-dimethylazacyclohexane-2,6-dione (4,4-dimethyl-2,6-piperidinedione). Acta Crystallogr. Sect. B 1981, 37, 1302–1304. [Google Scholar] [CrossRef]
- Aitken, R.A.; Slawin, A.M.Z.; Yeh, P.-P. Tetrahydro-1,4-thiazine-3,5-dione. Molbank 2018, 2018, M1036. [Google Scholar] [CrossRef]



| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| CCDC deposit no. | 2225715 | 2225716 | 2225713 | 2225707 |
| Empirical formula | C8H9NO4S | C9H9NO2 | C9H9NO2 | C10H11NO2 |
| Crystal system | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | P21/n (No. 14) | P21/c (No. 14) | P21/c (No. 14) | P21/c (No. 14) |
| Temperature (K) | 93 | 173 | 93 | 125 |
| Crystal form | colourless prism | colourless prism | colourless prism | colourless prism |
| Size (mm) | 0.10 × 0.10 × 0.03 | 0.12 × 0.03 × 0.03 | 0.20 × 0.10 × 0.10 | 0.12 × 0.05 × 0.02 |
| Unit cell | a = 6.0139 (3) | a = 11.1131 (3) | a = 8.394 (2) Å | a = 10.3980 (2) |
| dimensions (Å, °) | b = 14.2212 (6) | b = 6.07791 (12) | b = 16.964 (5) | b = 6.53350 (10) |
| c = 10.2142 (5) | c = 12.2001 (3) | c = 10.681 (3) | c = 12.8770 (2) | |
| β = 100.804 (5) | β = 100.690 (3) | β = 90.818 (7) | β = 105.469 (2) | |
| Volume (Å3) | 858.08 (7) | 770.90 (4) | 1520.8 (7) | 843.11 (3) |
| Z | 4 | 4 | 8 | 4 |
| Dc (g cm−3) | 1.666 | 1.406 | 1.425 | 1.396 |
| Absorption coefficient | 0.363 mm−1 | 0.830 mm−1 | 0.102 mm−1 | 0.802 mm−1 |
| Radiation type, wavelength | Mo Kα, 0.71073 Å | Cu Kα, 1.54184 Å | Mo Kα, 0.71075 Å | Cu Kα, 1.54184 Å |
| F(000) | 448.00 | 344.00 | 688.00 | 376.00 |
| θ range | 2.484–28.235° | 4.253–68.319° | 2.253–25.355° | 4.412–75.133° |
| Limiting indices | –7 ≤ h ≤ 7, –18 ≤ k ≤ 18, –12 ≤ l ≤ 13 | –13 ≤ h ≤ 11, –6 ≤ k ≤ 7, –14 ≤ l ≤ 14 | –10 ≤ h ≤ 8, –14 ≤ k ≤ 20, –12 ≤ l ≤ 12 | –12 ≤ h ≤ 12, –8 ≤ k ≤ 8, –16 ≤ l ≤ 15 |
| Reflns collected/unique | 12460/1899 | 7229/1409 | 7224/2745 | 9087/1705 |
| Rint | 0.0799 | 0.0216 | 0.0281 | 0.0159 |
| Data/restraints/parameters | 1899/1/131 | 1409/1/114 | 2745/2/226 | 1705/1/122 |
| Data with I > 2σ (I) | 1659 | 1385 | 2584 | 1672 |
| Goodness of fit on F2 | 1.175 | 1.068 | 1.048 | 1.094 |
| R1, wR2 (data I > 2σ (I)) | 0.0505, 0.1563 | 0.0781, 0.1697 | 0.0393, 0.1062 | 0.0389, 0.1039 |
| R1, wR2 (all data) | 0.0552, 0.1584 | 0.0783, 0.1703 | 0.0410, 0.1075 | 0.0394, 0.1044 |
| Largest diff. peak/hole (e Å2) | 0.77 and −0.55 | 0.42 and −0.68 | 0.48 and −0.26 | 0.28 and −0.24 |
| Compound | 5 | 6 | 7 | 8 |
|---|---|---|---|---|
| CCDC deposit no. | 2225709 | 2225714 | 2225712 | 2225711 |
| Empirical formula | C11H13NO2 | C12H13NO2 | C8H7NO3 | C8H3Cl4NO2 |
| Crystal system | monoclinic | monoclinic | orthorhombic | monoclinic |
| Space group | P21/c (No. 14) | P21/c (No. 14) | P212121 (No. 19) | P21/c (No. 14) |
| Temperature (K) | 125 | 173 | 173 | 93 |
| Crystal form | colourless plate | colourless prism | colourless prism | colourless plate |
| Size (mm) | 0.12 × 0.04 × 0.01 | 0.12 × 0.10 × 0.03 | 0.15 × 0.15 × 0.08 | 0.10 × 0.10 × 0.02 |
| Unit cell | a = 11.3039 (4) | a = 10.978 (3) | a = 7.0068 (7) | a = 6.4439 (4) |
| dimensions (Å, °) | b = 7.28194 (18) | b = 11.441 (3) | b = 8.1073 (8) | b = 5.8690 (3) |
| c = 12.3790 (4) | c = 8.122 (2) | c = 12.7199 (13) | c = 26.8276 (19) | |
| β = 113.542 (2) | β = 92.417 (7) | — | β = 95.696 (6) | |
| Volume (Å3) | 934.16 (6) | 1019.2 (5) | 722.57 (13) | 1009.59 (11) |
| Z | 4 | 4 | 4 | 4 |
| Dc (g cm−3) | 1.360 | 1.324 | 1.518 | 1.888 |
| Absorption coefficient | 0.763 mm−1 | 0.090 mm−1 | 0.118 mm−1 | 1.143 mm−1 |
| Radiation type, wavelength | Cu Kα, 1.54184 Å | Mo Kα, 0.71075 Å | Mo Kα, 0.71075 Å | Mo Kα, 0.71075 Å |
| F(000) | 408.00 | 432.00 | 344.00 | 568.00 |
| θ range | 4.266–75.347° | 1.857–25.361° | 2.980–25.375° | 3.052–28.139° |
| Limiting indices | −13 ≤ h ≤ 14, −8 ≤ k ≤ 9, −15 ≤ l ≤ 15 | −13 ≤ h ≤ 13, −13 ≤ k ≤ 13, –9 ≤ l ≤ 9 | −8 ≤ h ≤ 7, −9 ≤ k ≤ 9, −15 ≤ l≤ 14 | −8 ≤ h ≤ 8, −7 ≤ k ≤ 6, −32 ≤ l ≤ 32 |
| Reflns collected/unique | 10,206/1886 | 11,923/1856 | 8213/1307 | 9882/2148 |
| Rint | 0.0172 | 0.0286 | 0.0295 | 0.0365 |
| Data/restraints/parameters | 1886/1/131 | 1856/0/142 | 1307/1/113 | 2148/1/140 |
| Data with I > 2σ (I) | 1845 | 1743 | 1296 | 1972 |
| Goodness of fit on F2 | 1.041 | 1.090 | 1.022 | 1.006 |
| R1, wR2 (data I > 2σ (I)) | 0.0360, 0.0967 | 0.0370, 0.0969 | 0.0267, 0.0815 | 0.0303, 0.0848 |
| R1, wR2 (all data) | 0.0364, 0.0970 | 0.0385, 0.0981 | 0.0269, 0.0818 | 0.0324, 0.0862 |
| Largest diff. peak/hole (e Å2) | 0.25 and −0.19 | 0.21 and −0.20 | 0.15 and −0.18 | 0.58 and −0.28 |
| Compound | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|
| CCDC deposit no. | 2225705 | 2225704 | 2225708 | 2225710 | 2225706 |
| Empirical formula | C6H9NO2 | C6H9NO2 | C7H11NO2 | C4H5NO3 | C6H9NO2 |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic | monoclinic |
| Space group | P21/c (No. 14) | P21/c (No. 14) | P21/n (No. 14) | P–1 (No. 2) | P21/n (No. 14) |
| Temperature (K) | 173 | 93 | 93 | 173 | 173 |
| Crystal form | colourless platelet | colourless prism | colourless prism | colourless prism | colourless prism |
| Size (mm) | 0.10 × 0.10 × 0.01 | 0.10 × 0.10 × 0.03 | 0.20 × 0.03 × 0.03 | 0.15 × 0.05 × 0.05 | 0.15 × 0.12 × 0.03 |
| Unit cell | a = 11.795 (8) | a = 10.231 (4) | a = 15.276 (3) | a = 3.92406 (12) | a = 10.9883 (2) |
| dimensions | b = 7.040 (4) | b = 5.949 (2) | b = 6.3447 (11) | b = 6.67474 (18) | b = 7.18070 (11) |
| (Å, °) | c = 7.721 (5) | c = 10.412 (4) | c = 15.358 (3) | c = 9.7328 (3) | c = 8.21778 (18) |
| α = 76.716 (2) | |||||
| β = 103.355 (8) | β = 95.784 (8) | β = 90.108 (5) | β = 82.516 (2) | β = 109.066 (2) | |
| γ = 77.665 (2) | |||||
| Volume (Å3) | 623.8 (7) | 630.5 (4) | 1488.5 (5) | 241.489 (13) | 612.84 (2) |
| Z | 4 | 4 | 8 | 2 | 4 |
| Dc (g cm−3) | 1.354 | 1.339 | 1.260 | 1.583 | 1.378 |
| Absorp’n coefficient | 0.853 mm−1 | 0.101 mm−1 | 0.092 mm−1 | 0.137 mm−1 | 0.868 mm−1 |
| Radiation type, wavelength | Cu Kα, 1.54187 Å | Mo Kα, 0.71075 Å | Mo Kα, 0.71075 Å | Mo Kα, 0.71075 Å | Cu Kα, 1.54184 Å |
| F(000) | 272.00 | 272.00 | 608.00 | 120.00 | 272.00 |
| θ range | 3.852–67.810° | 2.001–25.339° | 2.653–25.335° | 2.158–28.247° | 4.257–68.071° |
| Limiting indices | −13 ≤ h ≤ 14, −7 ≤ k ≤ 8, −8 ≤ l ≤ 9 | −10 ≤ h ≤ 12, −5 ≤ k ≤ 7, −11 ≤ l ≤ 12 | −18 ≤ h ≤ 18, −7 ≤ k≤ 7, −18 ≤ l ≤ 18 | −5 ≤ h ≤ 4, −8 ≤ k ≤ 8, −12 ≤ l ≤ 12 | −13 ≤ h ≤ 12, −5 ≤ k ≤ 8, −9 ≤ l ≤ 9 |
| Refln total/unique | 6167/1122 | 3953/1145 | 15821/2699 | 7485/1052 | 5849/1111 |
| Rint | 0.0670 | 0.0300 | 0.0399 | 0.0254 | 0.0174 |
| Data/restraints /parameters | 1122/1/88 | 1145/1/87 | 2699/2/194 | 1052/1/77 | 1111/187 |
| Data I > 2σ (I) | 1061 | 1067 | 2490 | 965 | 1100 |
| Goodness of fit F2 | 1.081 | 1.090 | 1.196 | 1.076 | 1.273 |
| R1, wR2 (I > 2σ (I)) | 0.0643, 0.1706 | 0.0461, 0.1488 | 0.0452, 0.1592 | 0.0292, 0.0838 | 0.0664, 0.1431 |
| R1, wR2 (all data) | 0.0657, 0.1726 | 0.0615, 0.2137 | 0.0486, 0.1618 | 0.0318, 0.0853 | 0.0666, 0.1433 |
| Largest diff. peak/hole (e Å2) | 0.62 and −0.29 | 0.49 and −0.62 | 0.27 and −0.17 | 0.32 and −0.21 | 0.40 and −0.65 |
| Compound | D–H…A | D–A | D–H | H…A | Angle DHA |
|---|---|---|---|---|---|
| 1 | N(6)–H(6)…O(5) | 2.906(2) | 0.97(2) | 1.94(2) | 170(2) |
| 2 | N(1)–H(1)…O(2) | 2.8438(15) | 0.977(15) | 1.902(15) | 160.9(14) |
| 3 | N(1)–H(1)…O(12) | 2.9057(16) | 0.975(13) | 1.946(12) | 167.6(14) |
| N(11)–H(11)…O(9) | 2.9036(16) | 0.972(13) | 1.963(12) | 162.1(13) | |
| 4 | N(1)–H(1)…O(9) | 2.8432(12) | 0.975(14) | 1.878(13) | 169.8(13) |
| 5 | N(1)–H(1)…O(9) | 2.7991(11) | 0.973(11) | 1.832(11) | 172.4(17) |
| 6 | N(1)–H(1)…O(2) | 2.8616(15) | 0.880(17) | 2.020(17) | 159.8(15) |
| 7 | N(1)–H(1)…O(4) | 2.8735(19) | 0.975(8) | 1.909(8) | 170.0(19) |
| 8 | N(1)–H(1)…O(9) | 2.8576(18) | 0.975(16) | 1.897(18) | 168(2) |
| 9 | N(1)–H(1)…O(6) | 2.908(2) | 0.978(17) | 1.935(18) | 173(2) |
| 10 | N(1)–H(1)…O(2) | 2.9300(17) | 0.973(13) | 1.957(13) | 177.0(16) |
| 11 | N(1)–H(1)…O(6) | 2.849(2) | 0.977(17) | 1.871(17) | 179.1(18) |
| N(11)–H(11)…O(16) | 2.883(2) | 0.977(19) | 1.907(19) | 178(2) | |
| 12 | N(1)–H(1)…O(2) | 2.8872(11) | 0.971(11) | 1.920(11) | 173.3(13) |
| 13 | N(1)–H(1)…O(7) | 2.9278(17) | 0.975(17) | 1.957(17) | 173.1(14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Nelson, A.J.B.; Slawin, A.M.Z.; Sonecha, D.K. Solid State Structure and Hydrogen Bonding of Some Cyclic NH Carboximides. Crystals 2023, 13, 150. https://doi.org/10.3390/cryst13010150
Aitken RA, Nelson AJB, Slawin AMZ, Sonecha DK. Solid State Structure and Hydrogen Bonding of Some Cyclic NH Carboximides. Crystals. 2023; 13(1):150. https://doi.org/10.3390/cryst13010150
Chicago/Turabian StyleAitken, R. Alan, Alexander J. B. Nelson, Alexandra M. Z. Slawin, and Dheirya K. Sonecha. 2023. "Solid State Structure and Hydrogen Bonding of Some Cyclic NH Carboximides" Crystals 13, no. 1: 150. https://doi.org/10.3390/cryst13010150
APA StyleAitken, R. A., Nelson, A. J. B., Slawin, A. M. Z., & Sonecha, D. K. (2023). Solid State Structure and Hydrogen Bonding of Some Cyclic NH Carboximides. Crystals, 13(1), 150. https://doi.org/10.3390/cryst13010150






