The Role of Boron Addition on Solidification Behavior and Microstructural Evolution of a High Niobium-Containing TiAl Alloy
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
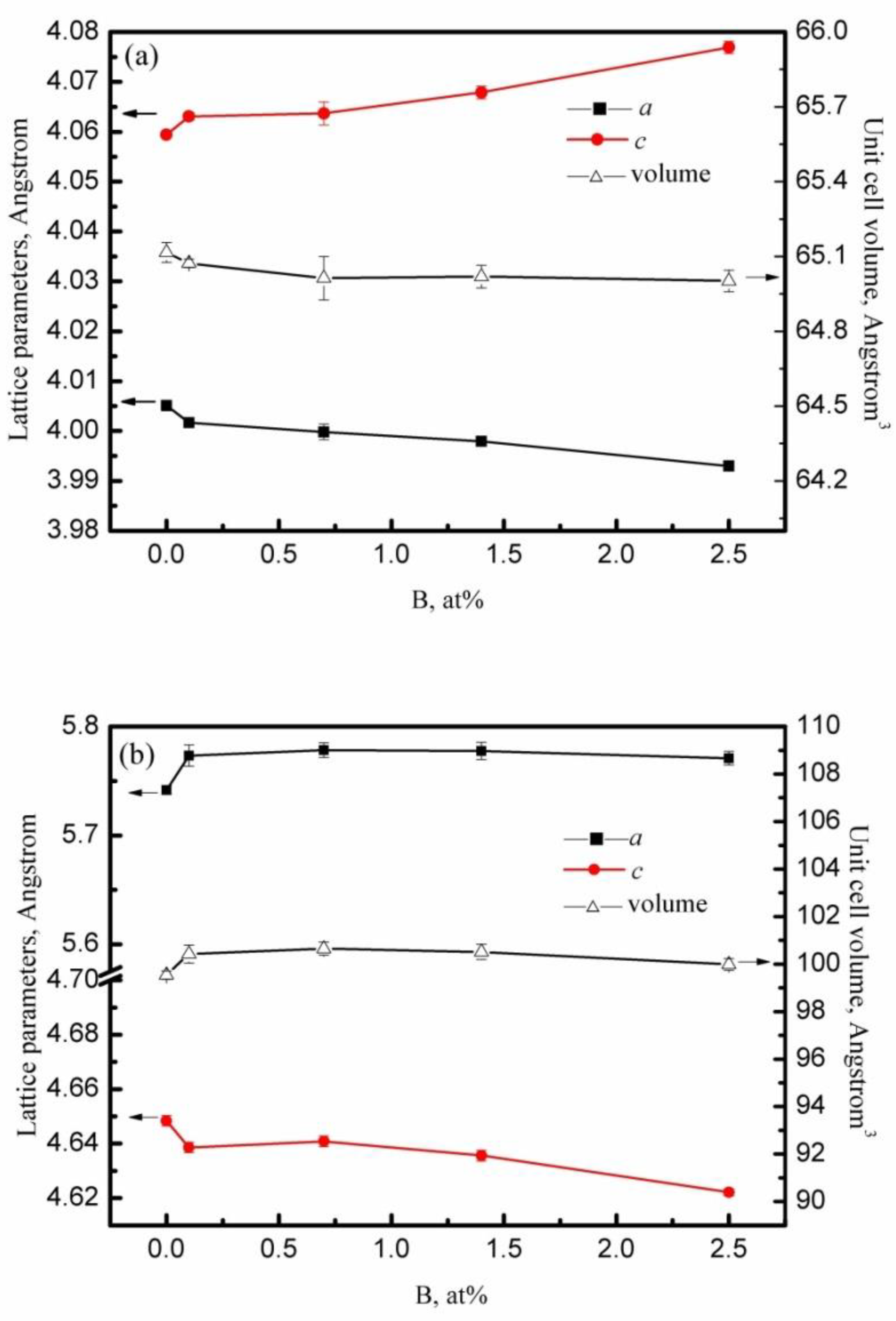
3.1. Phase Composition
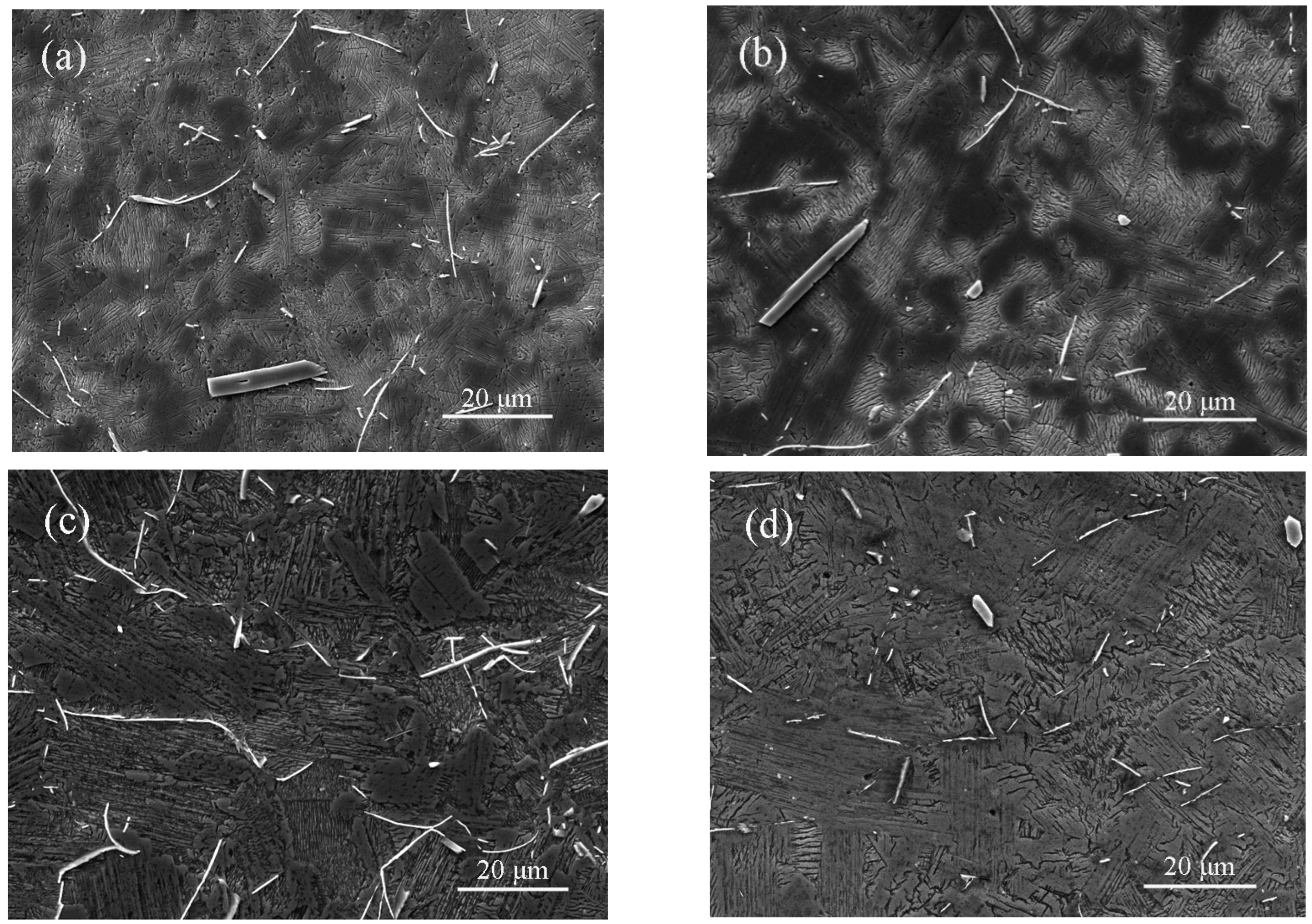
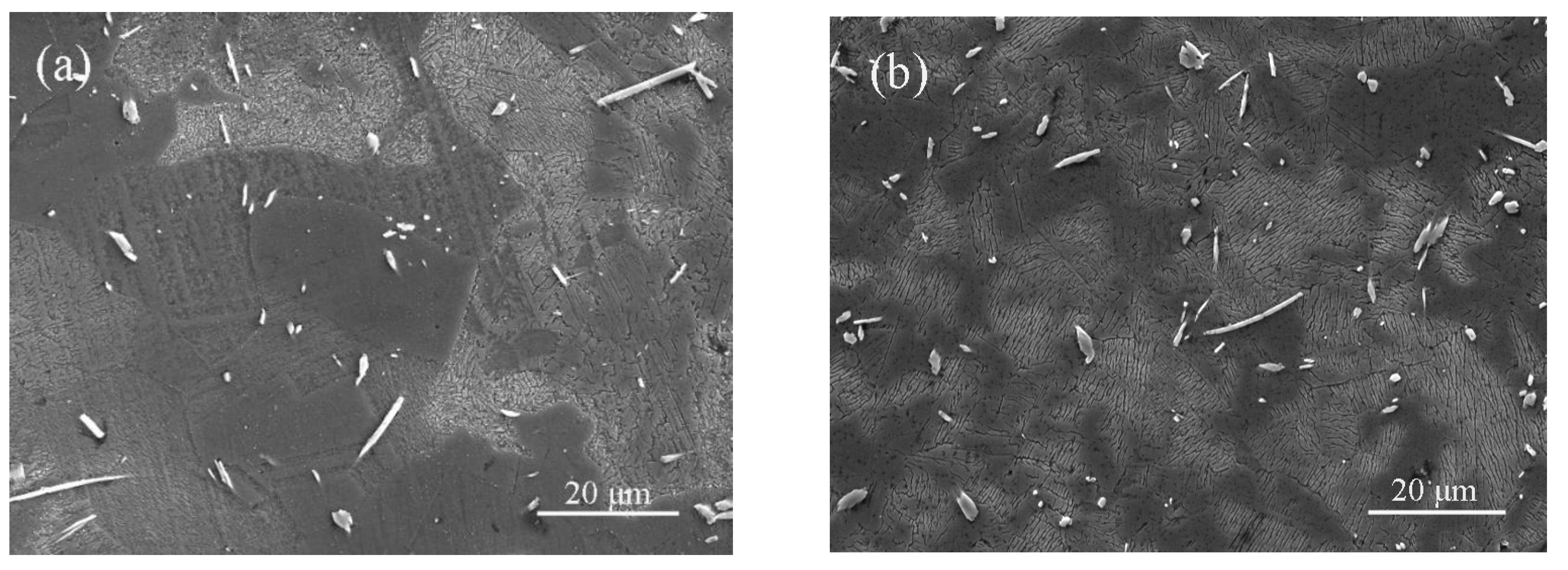
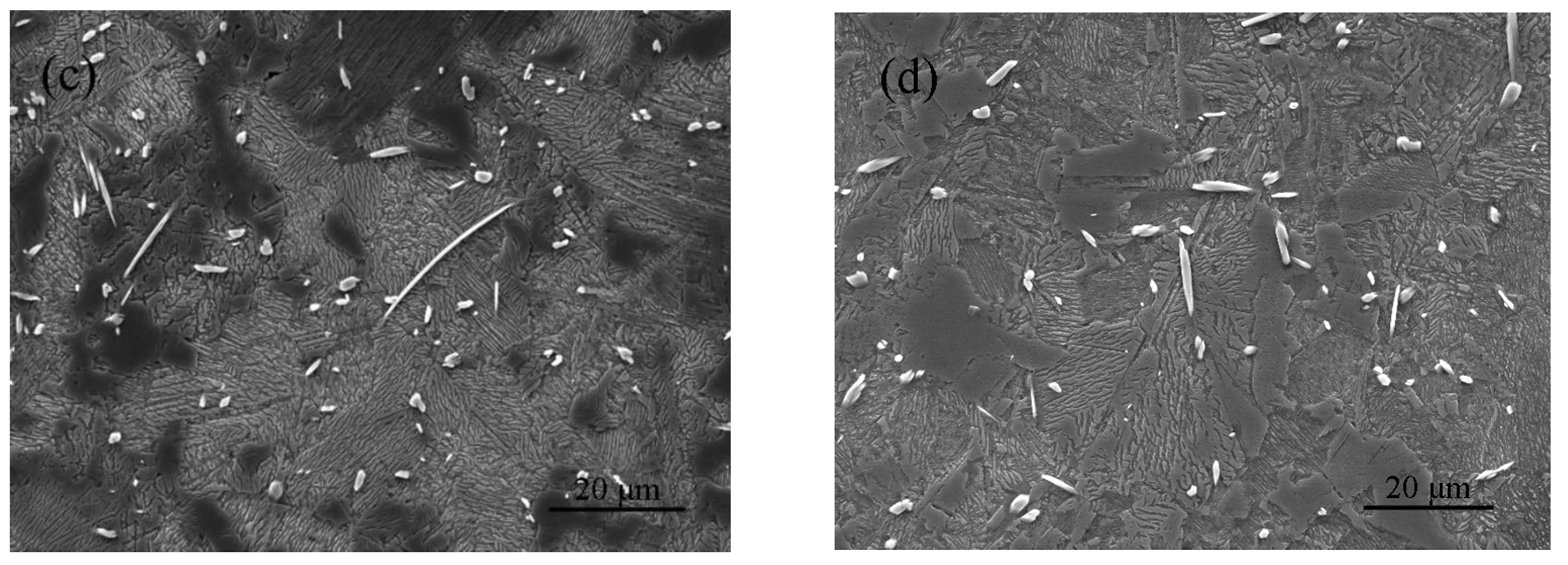
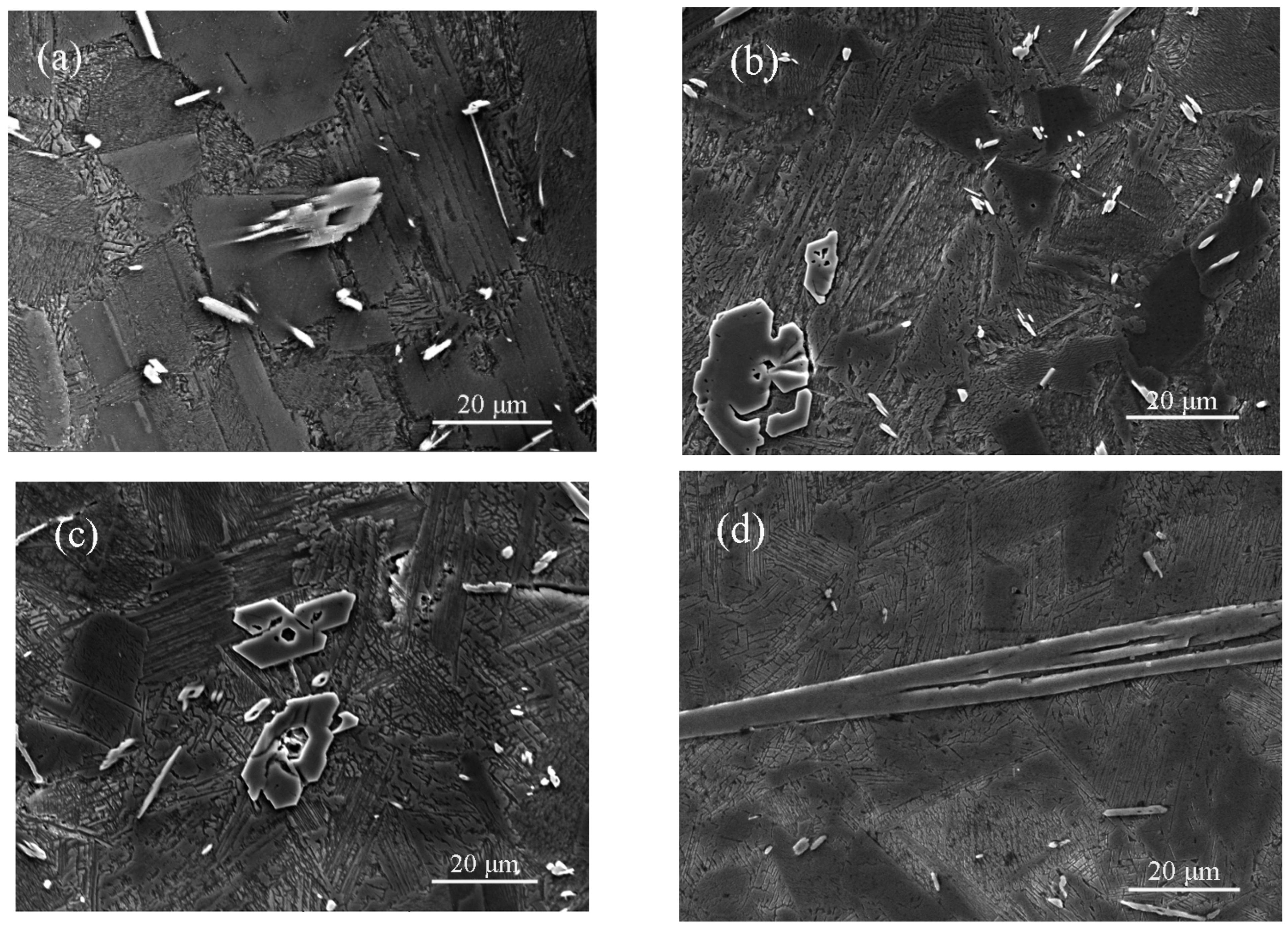
3.2. Microstructural Characterization
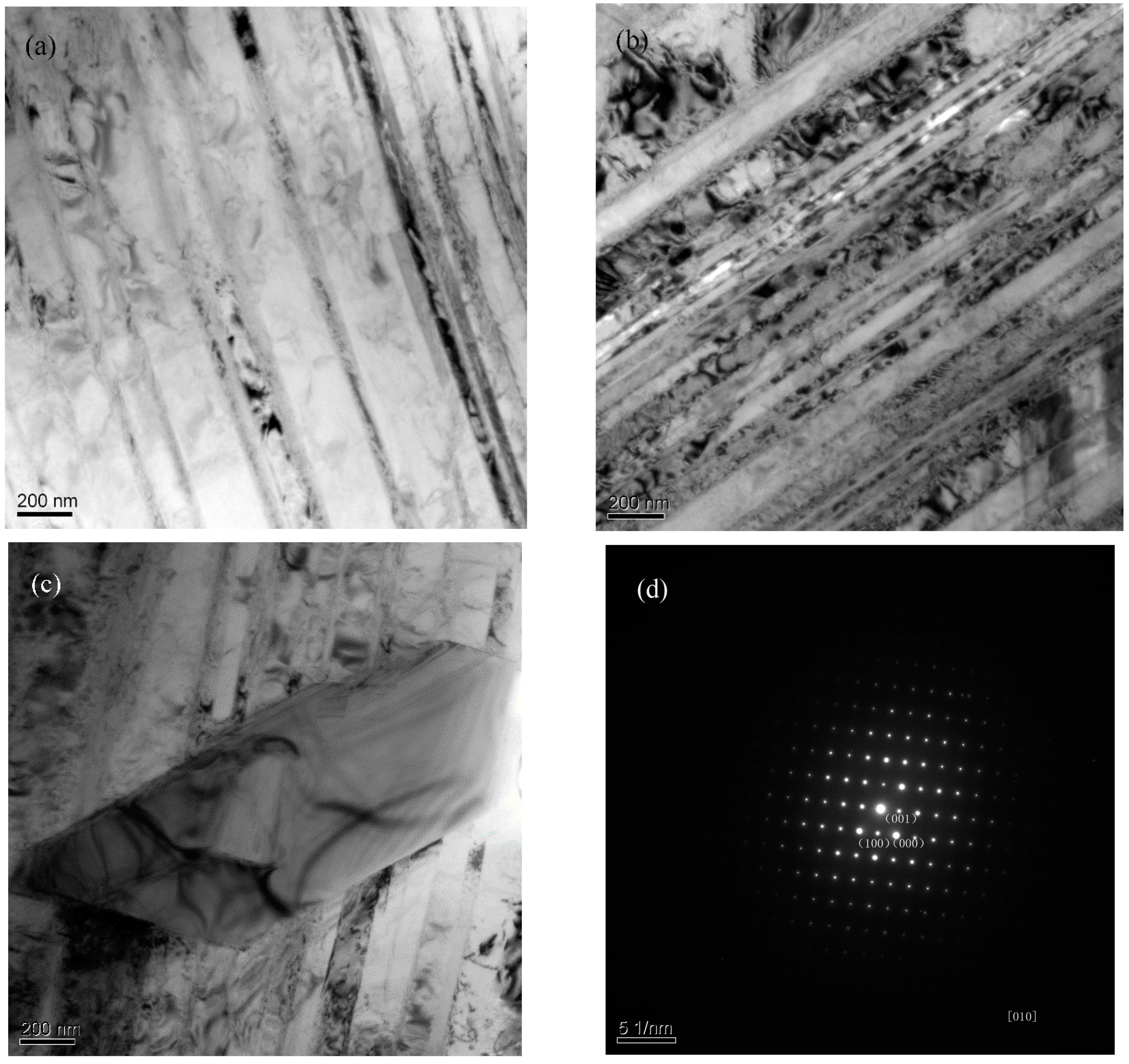
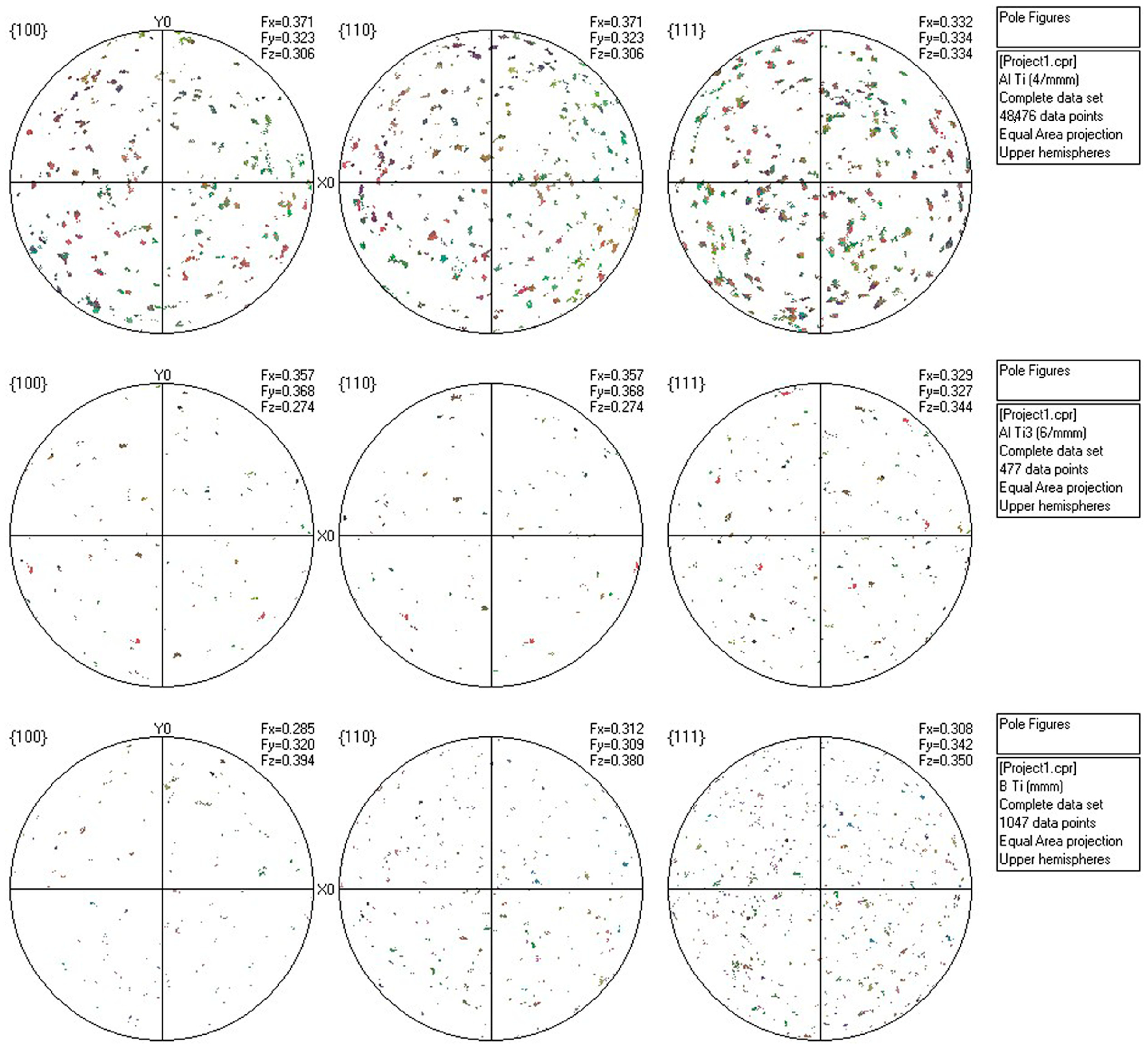
3.3. Microstructural Evolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, J.; Fan, S.; Liu, X.; Ding, X.; Huang, H.; Wu, W.; Wang, Q.; Wang, L.; Su, Y. Pollution-free grain refinement of cast TiAl alloys by vacuum mechanical vibration solidification. Mater. Sci. Eng. A 2023, 865, 144630. [Google Scholar] [CrossRef]
- Park, J.; Yang, G.; Kim, S. A high tensile strength above 900 °C in β-solidified TiAl alloy through alloy design and microstructure optimization. J. Alloys Compd. 2023, 947, 169676. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Z.; Cao, J.; Liang, Y.; Lin, J. Isothermal oxidation behavior of high-Nb-containing TiAl alloys doped with W, B, Y, and C/Si. Corros. Sci. 2023, 213, 110980. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, J.; Niu, H.; Cao, S.; Du, Z.; Jia, Y.; Yao, H.; Zhang, Z.; Han, J. Mechanical and physical characterizations of a three-Phase TiAl alloy during near isothermal forging. Crystals 2022, 12, 1391. [Google Scholar] [CrossRef]
- Abdo, H.; Abdus, S.; Mohammed, J.; Ragab, S.; Seikh, A. Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals 2021, 11, 889. [Google Scholar] [CrossRef]
- Liu, S.; Ding, H.; Chen, R.; Guo, J.; Fu, H. Evolution of rapidly grown cellular microstructure during heat treatment of TiAl-based intermetallic and its effect on micromechanical properties. Intermetallics 2021, 132, 107166. [Google Scholar] [CrossRef]
- Adachi, K.; Waki, H. Elastic constants and internal friction of forged β-solidifying TiAl alloys at room temperature and high temperature. Intermetallics 2022, 142, 107456. [Google Scholar] [CrossRef]
- Huang, L.; Geng, L.; Peng, H. Strengthening and Toughening Mechanisms of the Second Phase in Titanium Alloys and Titanium Matrix Composites. Mater. China 2019, 3, 214–222. [Google Scholar]
- Xu, R.R.; Li, H.; Li, M.Q. Flow softening mechanism in isothermal compression of β-solidifying γ-TiAl alloy. Mater. Des. 2020, 186, 108328. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, Y.; Yao, X.; Tan, Q.; Chang, H.; Wang, D.; Lu, S.; Liu, S.; Xu, J.; Jin, S.; et al. From crack-prone to crack-free: Unravelling the roles of LaB6 in a β-solidifying TiAl alloy fabricated with laser additive manufacturing. Mater. Sci. Eng. A 2022, 861, 144358. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Zhang, Y.; Li, F.; Huang, Y.; Ding, Y.; Xin, J.; Zhou, W.; Hua, X. Effect of Al content on the microstructure and mechanical properties of γ-TiAl alloy fabricated by twin-wire plasma arc additive manufacturing system. Mater. Sci. Eng. A 2021, 826, 142008. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Z.; Zhang, T.; Hu, R.; Wang, X. Microstructure Sensitivity on Environmental Embrittlement of a High Nb Containing TiAl Alloy under Different Atmospheres. Materials 2022, 15, 8508. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, X.; Chen, Y.; Zhao, S.; Liu, G.; Zhang, D. Microstructural stability, phase transformation and mechanical properties of a fully-lamellar microstructure of a Mo-modified high-Nb γ-TiAl alloy. Mater. Sci. Eng. A 2020, 784, 139313. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.; Lee, T.; Ryu, S.; Kim, S.; Kim, S. Origin of enhanced room temperature ductility in TiAl alloys: Reducing activation difference of deformation mechanism of γ phase. J. Alloys Compd. 2022, 899, 163307. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, R.; Yang, Y.; Guo, J.; Su, Y.; Ding, H.; Fu, H. Effects of V and B, Y additions on the microstructure and creep behaviour of high-Nb TiAl alloys. J. Alloys Compd. 2018, 747, 640–647. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Hu, R.; Zhang, F.; Kou, H.; Li, J. Influence of nitrogen on the microstructure and solidification behavior of high Nb containing TiAl alloys. Mater. Des. 2016, 103, 100–105. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, L.; Wang, S.; Chen, R.; Xu, Q.; Yan, Y.; Guo, J. Formation and kinds of boride on microstructure evolution and mechanical properties in Ti47.5Al2.0Cr2.0NbxB alloys. Intermetallics 2022, 141, 107427. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, R.; Fang, H.; Liu, Y.; Cui, H.; Su, Y.; Guo, J.; Fu, H. Enhanced strength and ductility in Ti46Al4Nb1Mo alloys via boron addition. J. Mater. Sci. Technol. 2022, 102, 16–23. [Google Scholar] [CrossRef]
- Yang, C.; Hu, D.; Huang, A.; Dixon, M. Solidification and grain refinement in Ti45Al2Mn2Nb1B subjected to fast cooling. Intermetallics 2013, 32, 64–71. [Google Scholar] [CrossRef]
- Oehring, M.; Stark, A.; Paul, J.D.H.; Lippmann, T.; Pyczak, F. Microstructural refinement of boron-containing β-solidifying γ-titanium aluminide alloys through heat treatments in the β phase field. Intermetallics 2013, 32, 12–20. [Google Scholar] [CrossRef]
- Witusiewicz, T.; Bondar, A.; Hecht, U. Velikanova Ya: The Al-B-Nb-Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al-Nb and ternary Al-Nb-Ti systems. J. Alloys Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic β-stabilized γ-TiAl based alloy. Intermetallics 2014, 46, 173–184. [Google Scholar] [CrossRef]
- Zhu, H.; Wei, T.; Blackford, M.; Short, K.; Carr, D.; Harrison, R.; Edwards, L.; Seo, D.; Maruyama, K. Irradiation behaviour of α2 and γ phases in He ion implanted titanium aluminide alloy. Intermetallics 2014, 50, 28–33. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, R.; Zhang, T.; Zhang, F.; Kou, H.; Li, J. Understanding the role of carbon atoms on microstructure and phase transformation of high Nb containing TiAl alloys. Mater. Charact. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Ouadah, O.; Merad, G.; Abdelkader, H.S. Energetic segregation of B, C, N, O at the γ-TiAl/α2-Ti3Al interface via DFT approach. Vacuum 2021, 186, 110045. [Google Scholar] [CrossRef]
- Kartavykh, A.; Gorshenkov, M.; Tcherdyntsev, V.; Podgorny, D. On the state of boride precipitates in grain refined TiAl-based alloys with high Nb content. J. Alloys Compd. 2014, 586, S153–S158. [Google Scholar] [CrossRef]
- Hu, D. Effect of composition on grain refinement in TiAl-based alloys. Intermetallics 2001, 9, 1037–1043. [Google Scholar] [CrossRef]
- Hecht, U.; Witusiewicz, V.; Drevermann, A.; Zollinger, J. Grain refinement by low boron additions in niobium-rich TiAl-based alloys. Intermetallics 2008, 16, 969–978. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, H.; Hu, D.; Huang, A.; Dixon, M. Effect of boron concentration on phase transformation texture in as-solidified Ti44Al8NbxB. Scr. Mater. 2012, 67, 85–88. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Xing, W.; Liu, R.; Yang, R.; Withey, P.; Zhu, J.; Yu, R. Structural stability and the alloying effect of TiB polymorphs in TiAl alloys. Intermetallics 2017, 90, 97–102. [Google Scholar] [CrossRef]
- Hu, D. Role of boron in TiAl alloy development: A review. Rare Met. 2016, 35, 1–14. [Google Scholar] [CrossRef]


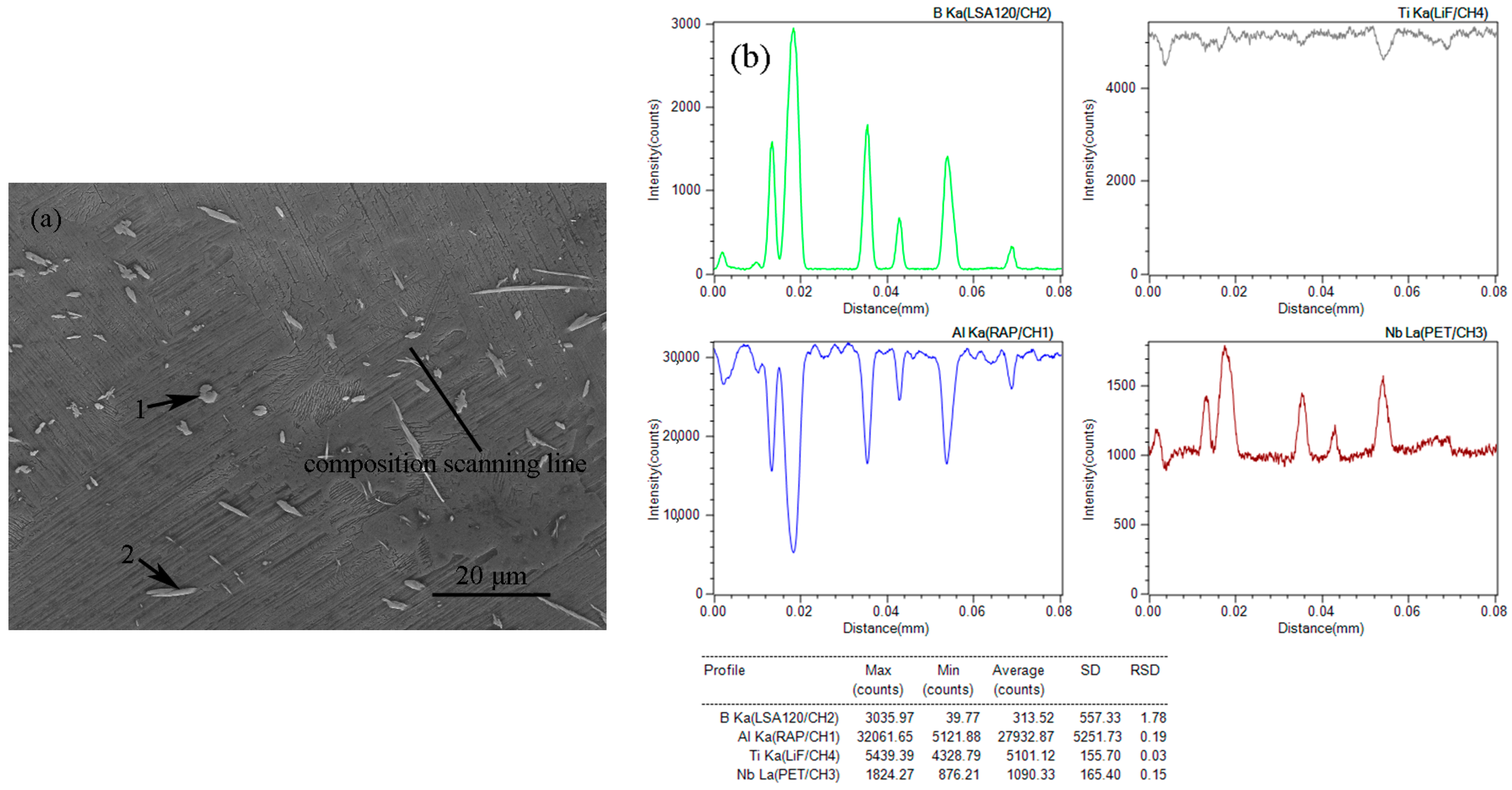
| Data | Al Ka | B Ka | Nb La | Ti Ka | Total |
|---|---|---|---|---|---|
| At% | At% | At% | At% | At% | |
| 1 | 10.13 | 45.398 | 9.263 | 35.209 | 100 |
| 2 | 8.029 | 46.468 | 10.497 | 35.007 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wu, Z.; Wang, X.; Zhang, T.; Zhang, Y.; Li, Q. The Role of Boron Addition on Solidification Behavior and Microstructural Evolution of a High Niobium-Containing TiAl Alloy. Crystals 2023, 13, 1494. https://doi.org/10.3390/cryst13101494
Zhang F, Wu Z, Wang X, Zhang T, Zhang Y, Li Q. The Role of Boron Addition on Solidification Behavior and Microstructural Evolution of a High Niobium-Containing TiAl Alloy. Crystals. 2023; 13(10):1494. https://doi.org/10.3390/cryst13101494
Chicago/Turabian StyleZhang, Fan, Zeen Wu, Xiaoye Wang, Tiebang Zhang, Yongchun Zhang, and Qiao Li. 2023. "The Role of Boron Addition on Solidification Behavior and Microstructural Evolution of a High Niobium-Containing TiAl Alloy" Crystals 13, no. 10: 1494. https://doi.org/10.3390/cryst13101494
APA StyleZhang, F., Wu, Z., Wang, X., Zhang, T., Zhang, Y., & Li, Q. (2023). The Role of Boron Addition on Solidification Behavior and Microstructural Evolution of a High Niobium-Containing TiAl Alloy. Crystals, 13(10), 1494. https://doi.org/10.3390/cryst13101494





