A Luminescent MOF Based on Pyrimidine-4,6-dicarboxylate Ligand and Lead(II) with Unprecedented Topology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solvent-Free Synthetic Approach
2.2. Structural Description of {[Pb5(μ3-OH)(μ3-NO3)3(μ6-pmdc)3]·H2O}n (1)
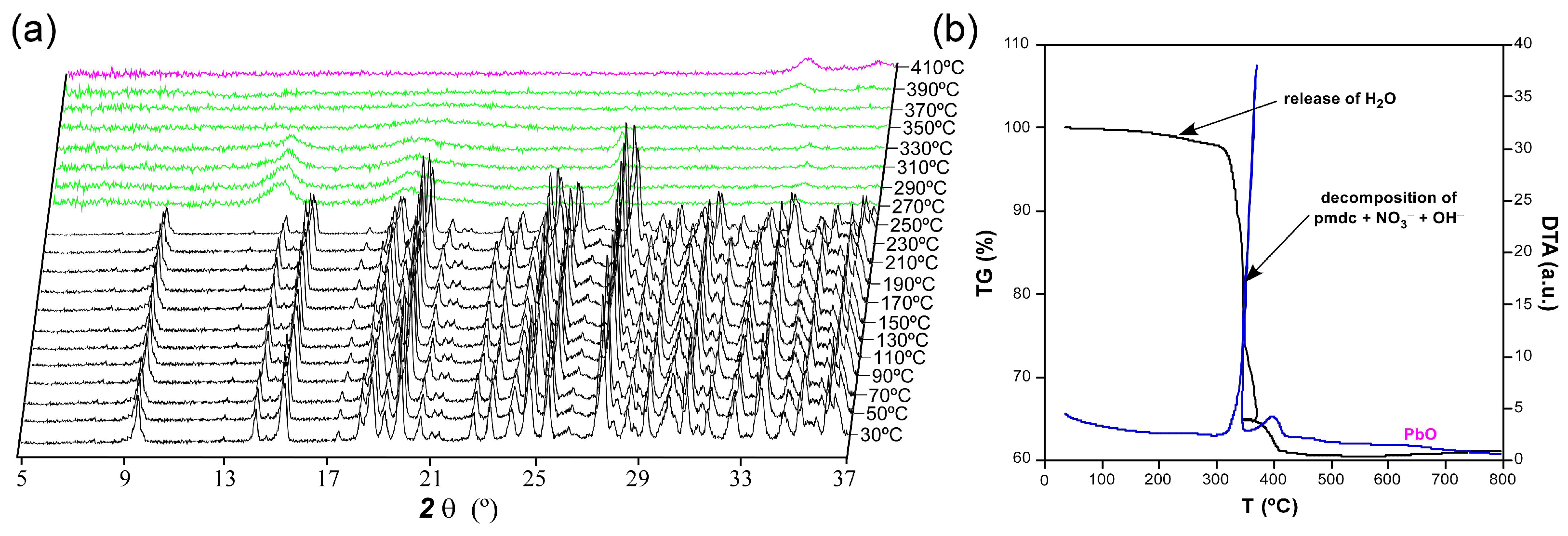
2.3. Thermal Behaviour
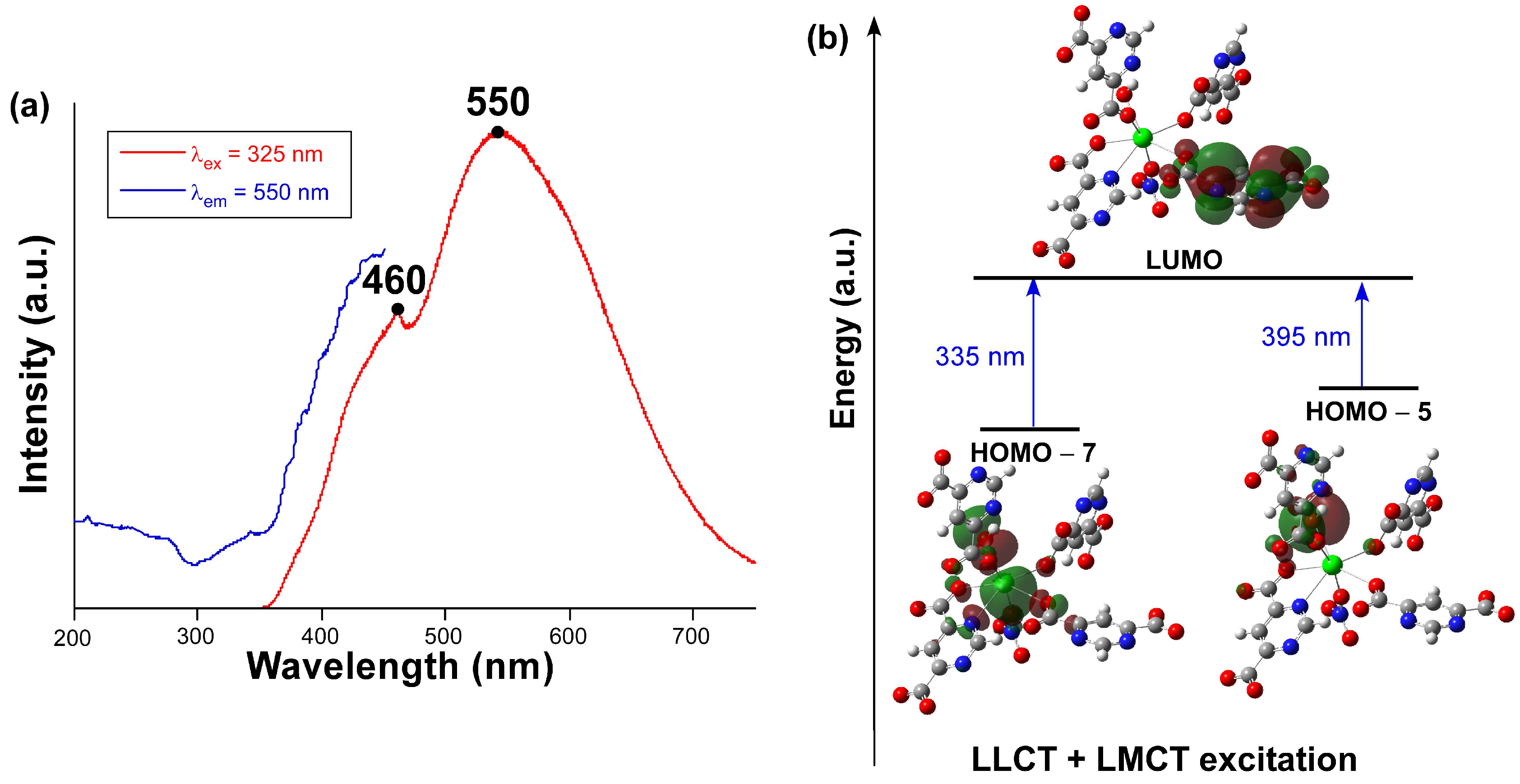
2.4. Photoluminescence Properties
3. Materials and Methods
3.1. Synthesis of {[Pb5(μ3-OH)(μ3-NO3)3(μ6-pmdc)3]·H2O}n (1)
3.2. Physical Measurements
3.3. X-ray Diffraction Data Collection and Structure Determination
3.4. Photoluminescence Measurements
3.5. Computational Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of Metal-Organic Frameworks and Coordination Polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.Ö.; Hupp, J.T. Metal-Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wang, J. Design Strategies for MOF-Derived Porous Functional Materials: Preserving Surfaces and Nurturing Pores. J. Mater. 2021, 7, 440–459. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal-Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically Porous Metal–Organic Frameworks: Synthetic Strategies and Applications. Natl. Sci. Rev. 2019, 7, 1743–1758. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the Structure and Function of Metal-Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Stavila, V. Crystal Engineering, Structure-Function Relationships, and the Future of Metal-Organic Frameworks. CrystEngComm 2015, 17, 229–246. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Morsali, A.; Hashemi, L. Main Group Metal Coordination Chemistry. In Main Group Metal Coordination Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 183–204. ISBN 9781119370772. [Google Scholar]
- Yaghi, O.M. Reticular Chemistry in All Dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef]
- Taddei, M.; Howarth, A.J.; Uemura, T. Introduction to Molecular Engineering in MOFs: Beyond Reticular Chemistry. Mol. Syst. Des. Eng. 2023, 8, 700. [Google Scholar] [CrossRef]
- Fernandez, B.; Beobide, G.; Sanchez, I.; Carrasco-Marin, F.; Seco, J.M.; Calahorro, A.J.; Cepeda, J.; Rodriguez-Dieguez, A. Controlling Interpenetration for Tuning Porosity and Luminescence Properties of Flexible MOFs Based on Biphenyl-4,4′-Dicarboxylic Acid. CrystEngComm 2016, 18, 1282–1294. [Google Scholar] [CrossRef]
- García-Couceiro, U.; Castillo, O.; Cepeda, J.; Lanchas, M.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Vallejo-Sánchez, D.; Garcia-Couceiro, U.; Castillo, O.; et al. Influence of the Synthetic Conditions on the Structural Diversity of Extended Manganese-Oxalato-1,2-Bis(4-Pyridyl)Ethylene Systems. Inorg. Chem. 2010, 49, 11346–11361. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition Metal-Based Bimetallic MOFs and MOF-Derived Catalysts for Electrochemical Oxygen Evolution Reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-Based MOFs: Synthesis Approaches and Applications in Cancer Diagnosis and Therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zhao, Z.-S.; Wang, Z.; Zhang, R.; Liu, L.; Han, Z.-B. Recent Progress in Lanthanide Metal–Organic Frameworks and Their Derivatives in Catalytic Applications. Inorg. Chem. Front. 2021, 8, 590–619. [Google Scholar] [CrossRef]
- San Sebastian, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. Coordination Polymers with Intriguing Photoluminescence Behavior: The Promising Avenue for Greatest Long-Lasting Phosphors. Eur. J. Inorg. Chem. 2018, 2018, 2155–2174. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, C.; Souza, B.E.; Van der Auweraer, M.; Hofkens, J.; Tan, J.-C. Highly Luminescent Silver-Based MOFs: Scalable Eco-Friendly Synthesis Paving the Way for Photonics Sensors and Electroluminescent Devices. Appl. Mater. Today 2020, 21, 100817. [Google Scholar] [CrossRef]
- Karmakar, A.; Li, J. Luminescent MOFs (LMOFs): Recent Advancement towards a Greener WLED Technology. Chem. Commun. 2022, 58, 10768–10788. [Google Scholar] [CrossRef]
- Parr, J. Some Recent Coordination Chemistry of Lead(Ll). Polyhedron 1997, 16, 551–566. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, D.; Han, L.; Ma, E.; Wu, M.; Lin, Z.; Hong, M. Mono- and Bilayered Lead(II)–Bpno Polymers with Unusual Low Energy Emission Properties (Bpno = 4,4′-Bipyridine N,N’-Dioxide). Eur. J. Inorg. Chem. 2005, 2005, 2054–2059. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-Metal Organic Framework (Magnetic-MOF): A Novel Platform for Enzyme Immobilization and Nanozyme Applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Coronado, E. Magnetic Functionalities in MOFs: From the Framework to the Pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting Single-Ion Anisotropy in the Design of f-Element Single-Molecule Magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Mancuso, J.L.; Mroz, A.M.; Le, K.N.; Hendon, C.H. Electronic Structure Modeling of Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8641–8715. [Google Scholar] [CrossRef] [PubMed]
- Karnieli, A.; Tsesses, S.; Bartal, G.; Arie, A. Emulating Spin Transport with Nonlinear Optics, from High-Order Skyrmions to the Topological Hall Effect. Nat. Commun. 2021, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent Developments in Luminescent Coordination Polymers: Designing Strategies, Sensing Application and Theoretical Evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D.; Li, W.; Wen, R.; Liu, X.; Liu, L.; Li, T.; Fan, L. Chemorobust Dye-Encapsulated Framework as Dual-Emission Self-Calibrating Ratiometric Sensor for Intelligent Detection of Toluene Exposure Biomarker in Urine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122637. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Li, W.; Zhang, H.; Li, B.; Hu, T.; Fan, L. Rod-Shaped Units Based Cobalt(II) Organic Framework as an Efficient Electrochemical Sensor for Uric Acid Detection in Serum. Microchem. J. 2023, 185, 108154. [Google Scholar] [CrossRef]
- Yin, J.; Li, W.; Li, W.; Liu, L.; Zhao, D.; Liu, X.; Hu, T.; Fan, L. Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics. Molecules 2023, 28, 6274. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as Multifunctional Luminescent Sensors. Inorg. Chem. Front. 2022, 9, 3259–3266. [Google Scholar] [CrossRef]
- Seco, J.M.; San Sebastián, E.; Cepeda, J.; Biel, B.; Salinas-Castillo, A.; Fernández, B.; Morales, D.P.; Bobinger, M.; Gómez-Ruiz, S.; Loghin, F.C.; et al. A Potassium Metal-Organic Framework Based on Perylene-3,4,9,10-Tetracarboxylate as Sensing Layer for Humidity Actuators. Sci. Rep. 2018, 8, 14414. [Google Scholar] [CrossRef]
- Cepeda, J.; Rodríguez-Diéguez, A. Tuning the Luminescence Performance of Metal-Organic Frameworks Based on D10metal Ions: From an Inherent Versatile Behaviour to Their Response to External Stimuli. CrystEngComm 2016, 18, 8556–8573. [Google Scholar] [CrossRef]
- Briones, D.; Leo, P.; Cepeda, J.; Orcajo, G.; Calleja, G.; Sanz, R.; Rodríguez-Diéguez, A.; Martínez, F. Alkaline-Earth Metal Based MOFs with Second Scale Long-Lasting Phosphor Behavior. CrystEngComm 2018, 20, 4793–4803. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Mendicute-Fierro, C.; Rodríguez-Diéguez, A.; Cepeda, J. A Lamellar Zn-Based Coordination Polymer Showing Increasing Photoluminescence upon Dehydration. Molecules 2023, 28, 5643. [Google Scholar] [CrossRef]
- Cepeda, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Structural Diversity of Coordination Compounds Derived from Double-Chelating and Planar Diazinedicarboxylate Ligands. Coord. Chem. Rev. 2017, 352, 83–107. [Google Scholar] [CrossRef]
- Zangade, S.; Patil, P. A Review on Solvent-Free Methods in Organic Synthesis. Curr. Org. Chem. 2019, 23, 2295–2318. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Mei, J.; Duan, A.; Wang, X. A Brief Review on Solvent-Free Synthesis of Zeolites. Materials 2021, 14, 788. [Google Scholar] [CrossRef]
- López-Cabrelles, J.; Romero, J.; Abellán, G.; Giménez-Marqués, M.; Palomino, M.; Valencia, S.; Rey, F.; Mínguez Espallargas, G. Solvent-Free Synthesis of ZIFs: A Route toward the Elusive Fe(II) Analogue of ZIF-8. J. Am. Chem. Soc. 2019, 141, 7173–7180. [Google Scholar] [CrossRef] [PubMed]
- Baig RB, N.; Varma, R.S. Solvent-Free Synthesis; Future Science Ltd.: London, UK, 2013; pp. 18–38. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener Synthesis of Chemical Compounds and Materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Lin, R.-B.; Cheng, X.-N.; Zhang, J.-P.; Chen, X.-M. Solvent/Additive-Free Synthesis of Porous/Zeolitic Metal Azolate Frameworks from Metal Oxide/Hydroxide. Chem. Commun. 2011, 47, 9185–9187. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-Free Synthesis of a Microporous Metal-Organic Framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Pei, X.; Zhou, J.; Feng, X.; Zhang, S.; Cheng, Y.; Li, H.; Han, R.; Wang, B. A Solvent-Free Hot-Pressing Method for Preparing Metal–Organic-Framework Coatings. Angew. Chem. Int. Ed. 2016, 55, 3419–3423. [Google Scholar] [CrossRef]
- Lanchas, M.; Arcediano, S.; Aguayo, A.T.; Beobide, G.; Castillo, O.; Cepeda, J.; Vallejo-Sánchez, D.; Luque, A. Two Appealing Alternatives for MOFs Synthesis: Solvent-Free Oven Heating vs. Microwave Heating. RSC Adv. 2014, 4, 60409–60412. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous Symmetry Maps and Shape Classification. The Case of Six-Coordinated Metal Compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Cramer, R.E.; Mitchell, K.A.; Hirazumi, A.Y.; Smith, S.L. Crystal Structures of [Pb(NO3)6]4− and [Ba(NO3)6]4− Salts of 24-Pyrimidinium Crown 6 {5,12,19,26,33,40-Hexaamino-3,10,17,24,31,38-Hexamethyl [1.6](1,5)Pyrimidiniophane}. J. Chem. Soc. Dalt. Trans. 1994, 563–569. [Google Scholar] [CrossRef]
- Litecká, M.; Gyepes, R.; Vargová, Z.; Vilková, M.; Almáši, M.; Walko, M.; Imrich, J. Toxic Metal Complexes of Macrocyclic Cyclen Molecule—Synthesis, Structure and Complexing Properties. J. Coord. Chem. 2017, 70, 1698–1712. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Ohto, K.; Schramm, M.P. P-Tert-Butylcalix[6]Arene Hexacarboxylic Acid Conformational Switching and Octahedral Coordination with Pb(Ii) and Sr(Ii). Chem. Commun. 2014, 50, 1903–1905. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package Topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Mechanism of Four-Level Laser Action in Solution Excimer and Excited-State Proton-Transfer Cases. Proc. Natl. Acad. Sci. USA 1983, 80, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.C.; Catalán, J. Kasha’s Rule: A Reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef]
- Agilent CrysAlisPro. Data Collection and Processing Software for Agilent X-ray Diffractometers; Aglient Technologies: Yarnton, UK, 2014; Volume 44, pp. 1–53. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.K.; Puschmann, H. {\it OLEX2}: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Phys. Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16 {R}evision {C}.01. 2016. [Google Scholar]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 720–723. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView {V}ersion {6}. 2019. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Snijders, J.G.; Baerends, E.J. The Zero-order Regular Approximation for Relativistic Effects: The Effect of Spin–Orbit Coupling in Closed Shell Molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, G.L.; Auer, A.A.; Neese, F. Automatic Generation of Auxiliary Basis Sets. J. Chem. Theory Comput. 2017, 13, 554–562. [Google Scholar] [CrossRef] [PubMed]
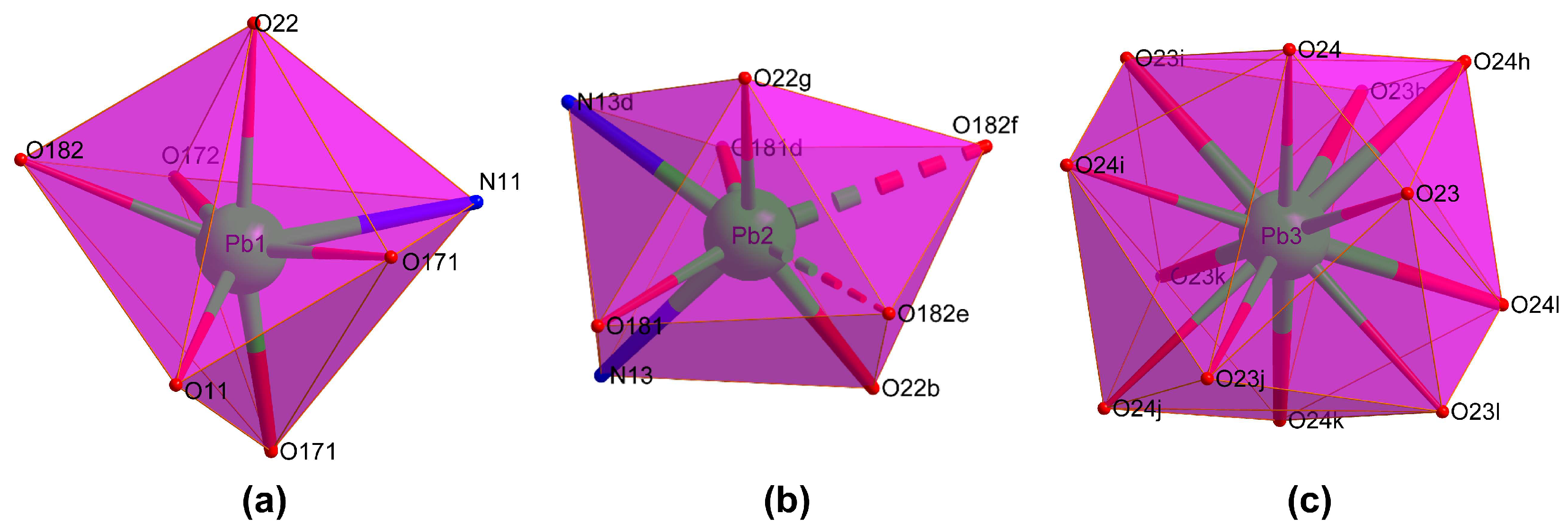

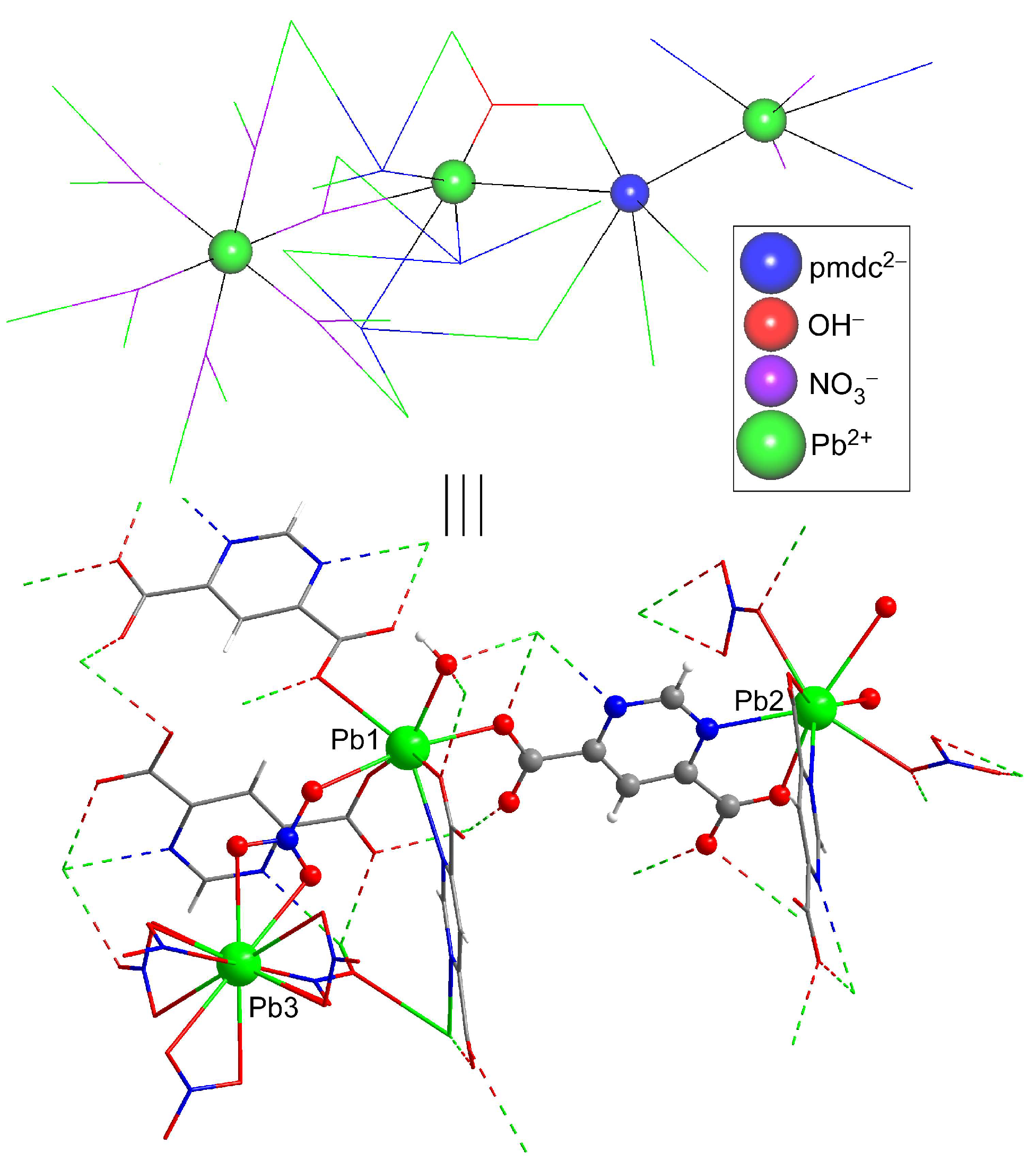

| Pb1–N11 | 2.767(12) | Pb2–N13 | 2.548(12) | Pb3–O23 | 2.744(12) |
| Pb1–O171 | 2.510(12) | Pb2–N13d | 2.548(12) | Pb3–O23h | 2.744(12) |
| Pb1–O171a | 2.432(11) | Pb2–O181 | 2.506(11) | Pb3–O23i | 2.744(12) |
| Pb1–O172b | 2.753(12) | Pb2–O181d | 2.506(11) | Pb3–O23j | 2.744(12) |
| Pb1–O182c | 2.707(10) | Pb2–O182e | 2.914(11) | Pb3–O23k | 2.744(12) |
| Pb1–O11 | 2.478(11) | Pb2–O182f | 2.914(11) | Pb3–O23l | 2.744(12) |
| Pb1–O22 | 2.679(13) | Pb2–O22b | 2.686(12) | Pb3–O24 | 2.865(16) |
| Pb2–O22g | 2.686(12) | Pb3–O24h | 2.865(16) | ||
| Pb3–O24i | 2.865(16) | ||||
| Pb3–O24j | 2.865(16) | ||||
| Pb3–O24k | 2.865(16) | ||||
| Pb3–O24l | 2.865(16) |
| Compound | 1 |
|---|---|
| Empirical formula | C18H9N9O23Pb5 |
| Formula weight (g mol−1) | 1755.31 |
| Crystal system | Trigonal |
| Space group | Rc |
| a (Å) | 14.3004(2) |
| c (Å) | 53.369(1) |
| V (Å3) | 9451.8(3) |
| Z | 6 |
| Reflections collected | 1944 |
| Unique data/parameters | 1926/164 |
| Rint | 0.0549 |
| GoF (S) a | 1.260 |
| R1 b/wR2 [I > 2σ(I)] c | 0.0521/0.1169 |
| R1 b/wR2 [all] c | 0.0524/0.1170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razquin-Bobillo, L.; Pajuelo-Corral, O.; Zabala-Lekuona, A.; Vitorica-Yrezabal, I.; García, J.A.; Moreno, J.M.; Rodríguez-Diéguez, A.; Cepeda, J. A Luminescent MOF Based on Pyrimidine-4,6-dicarboxylate Ligand and Lead(II) with Unprecedented Topology. Crystals 2023, 13, 1490. https://doi.org/10.3390/cryst13101490
Razquin-Bobillo L, Pajuelo-Corral O, Zabala-Lekuona A, Vitorica-Yrezabal I, García JA, Moreno JM, Rodríguez-Diéguez A, Cepeda J. A Luminescent MOF Based on Pyrimidine-4,6-dicarboxylate Ligand and Lead(II) with Unprecedented Topology. Crystals. 2023; 13(10):1490. https://doi.org/10.3390/cryst13101490
Chicago/Turabian StyleRazquin-Bobillo, Laura, Oier Pajuelo-Corral, Andoni Zabala-Lekuona, Iñigo Vitorica-Yrezabal, Jose Angel García, Jose M. Moreno, Antonio Rodríguez-Diéguez, and Javier Cepeda. 2023. "A Luminescent MOF Based on Pyrimidine-4,6-dicarboxylate Ligand and Lead(II) with Unprecedented Topology" Crystals 13, no. 10: 1490. https://doi.org/10.3390/cryst13101490
APA StyleRazquin-Bobillo, L., Pajuelo-Corral, O., Zabala-Lekuona, A., Vitorica-Yrezabal, I., García, J. A., Moreno, J. M., Rodríguez-Diéguez, A., & Cepeda, J. (2023). A Luminescent MOF Based on Pyrimidine-4,6-dicarboxylate Ligand and Lead(II) with Unprecedented Topology. Crystals, 13(10), 1490. https://doi.org/10.3390/cryst13101490











