Scanning Tunneling Microscopy of Intermediate Transformation Structures in Electric Arc Surfacing Modified with Titanium Carbonitrides on Pipe Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Fracture Toughness
3.2. Optical Microscopy
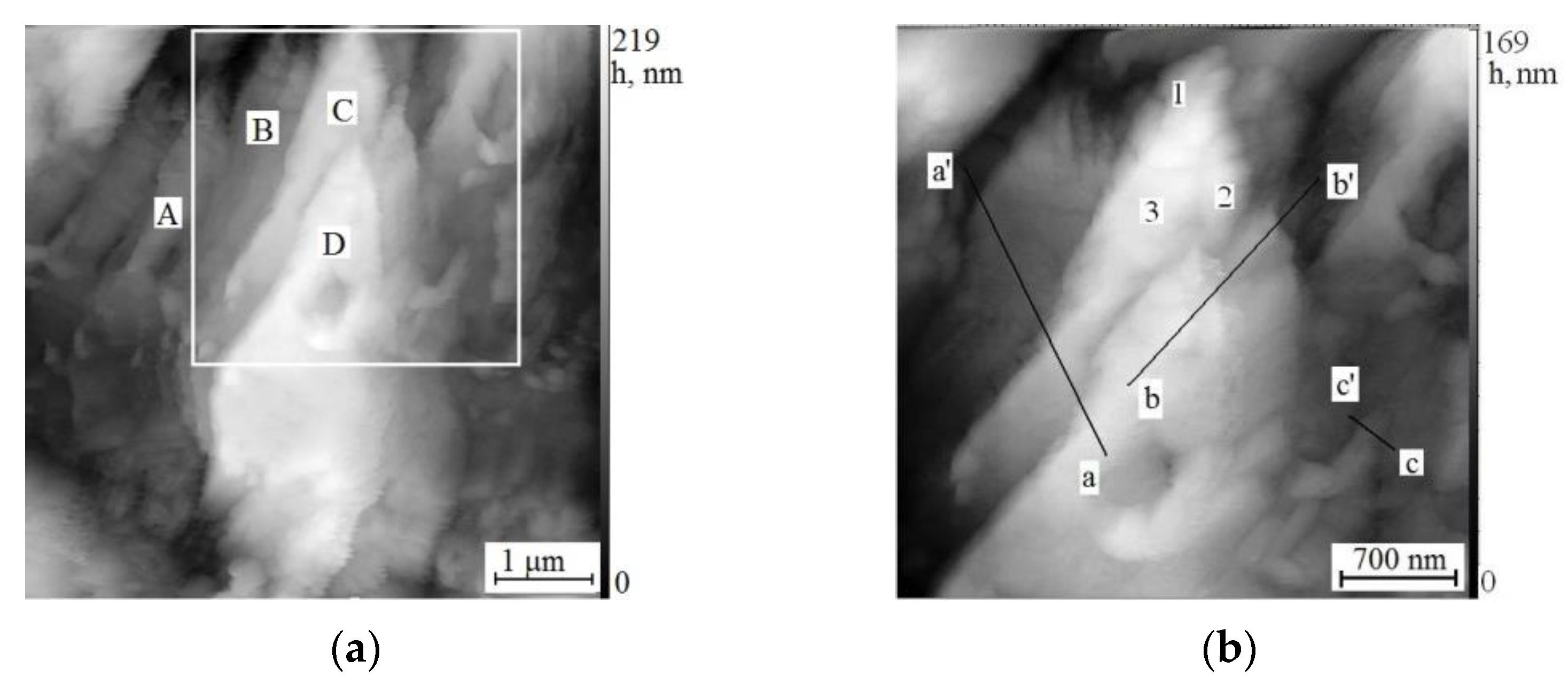
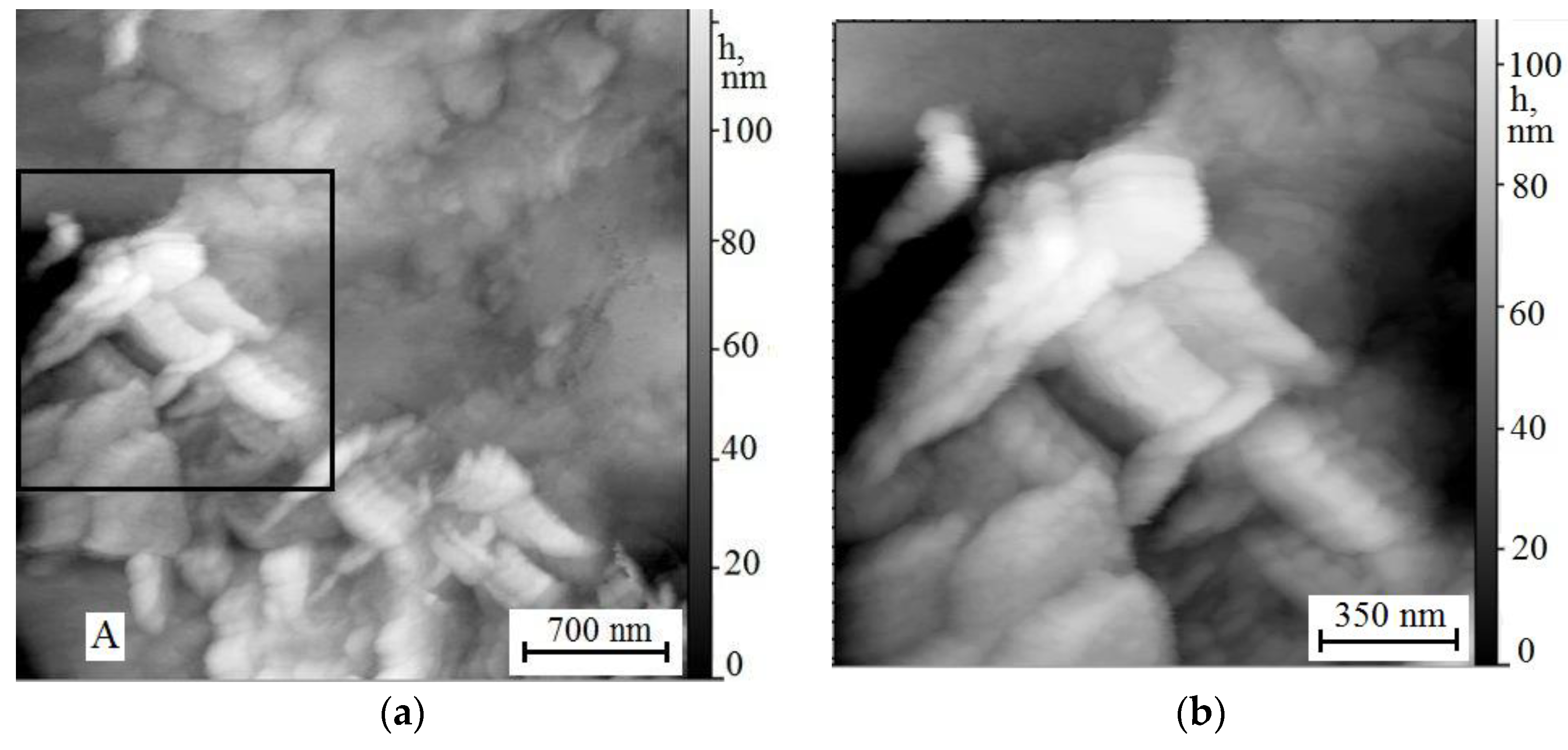
3.3. Scanning Tunneling Microscopy
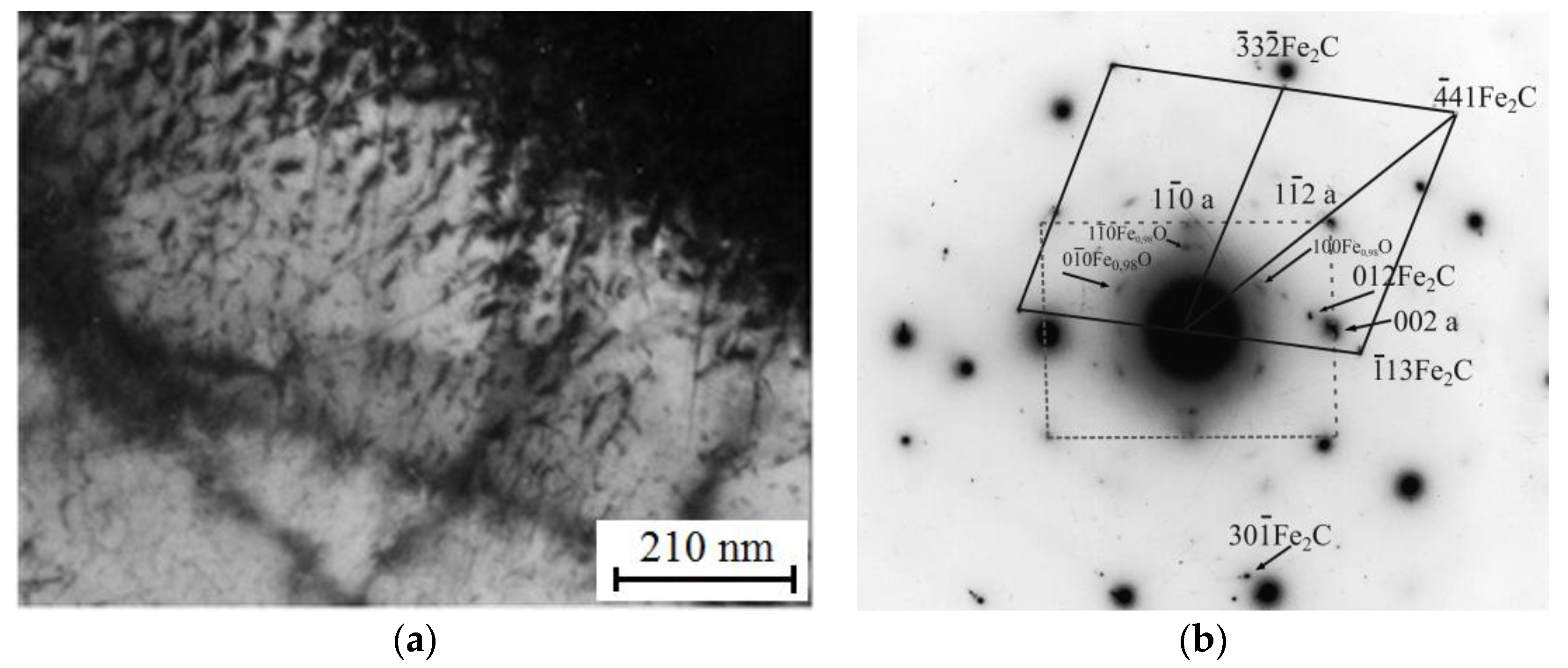
3.4. Transmission Electron Microscope
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, M. (Ed.) Surface Engineering for Enhanced Performance against Wear; Springer: Berlin/Heidelberg, Germany, 2013; 319p. [Google Scholar]
- Ellis, T.; Garrett, G.G. Influence of Process Variables in Flux Cored Arc Welding of Hardfacing Deposits. Surf. Eng. 1986, 2, 55–66. [Google Scholar] [CrossRef]
- Singh, M.; Bhagi, L.K.; Arora, H. Effects of Shielded Metal Arc Welding Process Parameters on Dilution in Hardfacing of Mild Steel Using Factorial Design. In Advances in Materials Processing. Lecture Notes in Mechanical Engineering; Singh, S., Prakash, C., Ramakrishna, S., Krolczyk, G., Eds.; Springer: Singapore, 2020; pp. 207–220. [Google Scholar] [CrossRef]
- Dorval, D.C.A.; Tavares, J.R. Photo-Initiated Chemical Vapour Deposition as a Scalable Particle Functionalization Technology (A Practical Review). Powder Technol. 2013, 239, 484–491. [Google Scholar] [CrossRef]
- Selvakumar, N.; Barshilia, H.C. Review of physical vapor deposited (PVD) spectrally selective coatings for mid- and high-temperature solar thermal applications. Sol. Energy Mater. Sol. Cells 2012, 98, 1–23. [Google Scholar] [CrossRef]
- Acciari, H.A.; Palma, D.P.S.; Codaro, E.N.; Zhou, Q.; Wang, J.; Ling, Y.; Zhang, J.; Zhang, Z. Surface modifications by both anodic oxidation and ion beam implantation on electropolished titanium substrates. Appl. Surf. Sci. 2019, 487, 1111–1120. [Google Scholar] [CrossRef]
- Diankun, L.; Bo, G.; Guanglin, Z.; Jike, L.; Liang, H. High-Current Pulsed Electron Treatment of Hypoeutectic Al-10Si Alloy. High Temp. Mater. Process. 2017, 36, 97–100. [Google Scholar] [CrossRef]
- Katipelli, L.R.; Dahotre, N.B. Mechanism of high temperature oxidation of laser surface engineered TiC/Al alloy composite coating on 6061 aluminium alloy. Mater. Sci. Technol. 2001, 17, 1061–1068. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wei, S.; Lu, S. The Effect of Nickel Contents on the Microstructure Evolution and Toughness of 800 MPa Grade Low Carbon Bainite Deposited Metal. Crystals 2021, 11, 6–709. [Google Scholar] [CrossRef]
- Atamert, S.; Bhadeshia, H.K.D.H. Comparison of the microstructures and abrasive wear properties of stellite hardfacing alloys deposited by arc welding and laser cladding. Metall. Mater. Trans. A 1989, 20, 1037–1054. [Google Scholar] [CrossRef]
- Hedenqvist, P.; Olsson, M.; Hogmark, S. Characterisation of Ti(C,N) and TiN Coatings on Powder Metallurgy High Speed Steels. Surf. Eng. 1992, 8, 39–47. [Google Scholar] [CrossRef]
- Artem’ev, A.A. Influence of microparticles of titanium diboride and nanoparticles of titanium carbonitride on the structure and properties of the deposited metal. MiTOM 2011, 12, 32–37. [Google Scholar]
- Golovko, G.G.; Kostin, V.A.; Grigorenko, G.M. Peculiarities of the influence of complex alloying on structure formation and mechanical properties of welds on low-alloyed high- strength steels. Paton Weld. J. 2011, 7, 11–17. [Google Scholar]
- Dong, J.; Liu, C.; Liu, Y.; Li, C.; Guo, Q.; Li, H. Effects of two different types of MX carbonitrides on austenite growth behavior of Nb-V-Ti microalloyed ultra-high strength steel. Fusion Eng. Des. 2017, 125, 415–422. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels: Transformations, Microstructure and Properties, 2nd ed.; The University Cambridge Press: Cambridge, UK, 2001; 454p. [Google Scholar]
- Krauss, G.; Thomson, S.W. Ferritic Microstructures in Continuously Cooled Low- and Ultralow- carbon Steels. ISIJ Int. 1995, 35, 937–945. [Google Scholar] [CrossRef]
- Kang, J.S.; Seol, J.-B.; Parc, C.G. Three-dimensional characterization of bainitic microstructures in low-carbon high-strength low-alloy steel studied by electron backscatter diffraction. Mater. Charact. 2013, 79, 110–121. [Google Scholar] [CrossRef]
- Araki, T.; Enomoto, M.; Shibata, K. Microstructural Aspects of Bainitic-like Ferritic Structures of Continuously Cooled Low Carbon (<0.1%) HSLA Steels. Mater. Trans. JIM 1991, 32, 729–736. [Google Scholar] [CrossRef][Green Version]
- Bamfitt, B.L.; Speer, J.G. Perspective on the morphology of bainite. Metall Mater. Trans. A 1990, 21, 817–829. [Google Scholar] [CrossRef]
- Wilson, E.A. The γ→α-transformation in low-carbon irons. ISIJ Int. 1994, 34, 615–630. [Google Scholar] [CrossRef]
- Spanos, G.; Fang, H.S.; Aaronson, H.I. A Mechanism for the Formation of Lower Bainite. Metall Mater. Trans. A 1990, 21, 1381–1390. [Google Scholar] [CrossRef]
- Bo, X.Z.; Fang, H.S. Surface relief effects associated with the formation grain boundary allotriomorph in an Fe–C alloy. Acta Mater. 1998, 46, 2929–2936. [Google Scholar] [CrossRef]
- Yang, H.G.; Wang, J.J.; Li, C.M.; Fang, H.S.; Zheng, Y.K. Ledges in Widmanstatten ferrite observed by scanning tunneling microscopy. J. Mater. Sci. Lett. 1998, 17, 331–333. [Google Scholar] [CrossRef]
- Yan, J.; Yu, H.; Ge, Z.; Huang, Y.; Fang, H.; Wang, J.; Yang, Z.; Deng, X. Scanning tunneling microscopy investigation of bainite in steel. J. Vac. Sci. Technol. B 1994, 12, 1793–1796. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, C.; Bai, B.Z.; Fang, H.S. Observation of bainite surface reliefs in Fe–C alloy by atomic force microscopy. Mater. Lett. 2001, 48, 292–298. [Google Scholar] [CrossRef]
- Fang, H.S.; Yang, J.B.; Yang, Z.G.; Bai, B.Z. The mechanism of bainite transformation in steels. Scr. Mater. 2002, 47, 157–162. [Google Scholar] [CrossRef]
- Swallow, E.; Bhadeshia, H.K.D.H. High resolution observations of displacements caused by bainitic transformation. Mater. Sci. Tech. 1996, 12, 121–125. [Google Scholar] [CrossRef]
- Wang, J.J.; Fang, H.S.; Yang, Z.G.; Zheng, Y.K. Fine Structure and Formation Mechanism of Bainite in Steels. ISIJ Int. 1995, 35, 992–1000. [Google Scholar] [CrossRef]
- Wang, J.J.; Fang, H.S.; Zheng, Y.K.; Yang, Z.G. Use of scanning tunneling microscopy in metallography. Mater. Charact. 1994, 33, 169–174. [Google Scholar] [CrossRef]
- Bergeon, N.; Kajiwara, S.; Kikuchi, T. Atomic force microscopy study of stress induced martensite formation and its reverse transformation in a thermomechanically treated Fe-Mn-Si-Cr-Ni alloy. Acta Mater. 2000, 48, 4053–4064. [Google Scholar] [CrossRef]
- Girault, E.; Jacques, P.; Harlet, P.; Mols, K.; Van Humbeeck, J.; Aernoudt, E.; Delannay, F. Metallographic Methods for Revealing the Multiphase Microstructure of TRIP-Assisted Steels. Mater. Charact. 1998, 40, 111–118. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Kuznetsov, P.V.; Galchenko, N.K.; Rakhmatulina, T.V.; Samartsev, V.P.; Kolesnikova, K.A.; Laptev, R.S.; Babikhina, M.N. Scanning tunnel microscopy of coatings with titan carbonitride nanoparticles and their properties. AIP Conf. Proc. 2017, 1909, 020114. [Google Scholar] [CrossRef]
- Kuznetsov, P.V.; Galchenko, N.K.; Belaeva, I.V.; Kozlova, T.V. Structural Features of the Two-Layer Electric Arc Coating with of Additives of Titanium Carbonitride Nanoparticles on Low-Carbon Steel. Russ. Phys. J. 2021, 64, 605–611. [Google Scholar] [CrossRef]
- Fang, H.S.; Wang, J.J.; Yang, Z.G.; Li, C.M.; Zheng, Y.K.; Li, C.X. Formation of Bainite through Sympathetic Nucleation and Ledgewise Growth Mechanism in Ferrous and Nonferrous Alloys. Metall. Mater. Trans. A 1996, 27, 1535–1545. [Google Scholar] [CrossRef]
- Sun, J.; Lu, H.; Kang, M. The Nature of Lower Bainite Midrib. Metall. Trans. A 1992, 23A, 2483–2490. [Google Scholar] [CrossRef]
- Ohmori, Y.; Ohtsubo, H.; Jung, Y.C.; Okaguchi, S.; Ohtani, H. Morphology of Bainite and Widmanstatten Ferrite. Metall. Mater. Trans. A 1994, 25, 1981–1989. [Google Scholar] [CrossRef]
- Ohmori, Y. Crystallographic analysis of lower bainite transformation in Fe-0.7% C alloy. Mater. Trans. JIM 1989, 487–497. [Google Scholar] [CrossRef]
- Spanos, G. The Fine Structure and Formation Mechanism of Lower Bainite. Metal. Mater. Trans. A 1994, 25, 1967–1980. [Google Scholar] [CrossRef]
- Li, C.M.; Fang, H.S.; Yang, Z.G.; Bo, X.Z.; Wang, J.J.; Zheng, Y.K. Scanning tunnelling microscopy and transmission electron microscopy study of ultrafine structure and surface relief of bainite in a Cu–Zn–Al alloy. J. Mater. Sci. 1998, 33, 487–496. [Google Scholar] [CrossRef]
- Fang, H.S.; Wang, J.J.; Zheng, Y.K. Formation Mechanism of Bainitic Ferrite and Carbide. Metall. Mater. Trans. A 1994, 25, 2001–2007. [Google Scholar] [CrossRef]
- Yu, D.; Chen, D.; Zheng, J.; He, Y.; Shen, F. Phase Transformation Unit of Bainitic Ferrite And Its Surface Relief In Low And Medium Carbon Alloy Steels. Acta Metall. Sin. (Engl. Ed.) Ser. A 1989, 2, 161–167. [Google Scholar]
- Loder, D.; Michelic, S.K.; Bernhard, C. Acicular Ferrite Formation and Its Influencing Factors. A Review. J. Mater. Sci. Res. 2017, 6, 24–43. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Boyd, J.D. Bainite Transformation in Deformed Austenite. Metall. Mater. Trans. A 2010, 41, 1449–1459. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in Steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Dirand, M.; Afqir, L. Identification structural precise des carbures precipites dans les aciers faiblement allies aux divers stades du revenu. Mecanismes de precipitation. Acta. Metall. 1983, 31, 1089–1107. [Google Scholar] [CrossRef]
- Huang, D.H.; Thomas, G. Metallography of bainitic Transformation in Silicon Containing Steels. Metall. Trans. A 1977, 8, 1661–1673. [Google Scholar] [CrossRef]
- Sandvik, B.P.J. The Bainite Reaction in Fe-Si-C Alloys: The Primary Stage. Metall. Trans. A 1982, 13, 777–787. [Google Scholar] [CrossRef]
- Lai, G.Y. On the Precipitation of Epsilon-Carbide in Lower Bainite. Metall. Trans. A 1975, 6, 1469–1471. [Google Scholar] [CrossRef]






| C | Si | Mn | Ni | S | P | Cr | N | Cu | As | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5–0.8 | 1.3–1.7 | up to 0.3 | 0.04 | 0.035 | 0.3 | 0.008 | 0.3 | 0.08 | 96–97 |
| C | Mn | Si | S | P |
|---|---|---|---|---|
| 0.10 | 0.58 | 0.17 | 0.030 | 0.035 |
| Content of TiCN nanoparticles, wt % | 0.15 | 0.2 | 0.25 | 1 | Commercial MP-3 electrode |
| Fracture toughness of a specimen, J/cm2 | 104 | - * | 89 | 86 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, K.; Nina, G.; Yury, P. Scanning Tunneling Microscopy of Intermediate Transformation Structures in Electric Arc Surfacing Modified with Titanium Carbonitrides on Pipe Steel. Crystals 2023, 13, 146. https://doi.org/10.3390/cryst13010146
Pavel K, Nina G, Yury P. Scanning Tunneling Microscopy of Intermediate Transformation Structures in Electric Arc Surfacing Modified with Titanium Carbonitrides on Pipe Steel. Crystals. 2023; 13(1):146. https://doi.org/10.3390/cryst13010146
Chicago/Turabian StylePavel, Kuznetsov, Galchenko Nina, and Pochivalov Yury. 2023. "Scanning Tunneling Microscopy of Intermediate Transformation Structures in Electric Arc Surfacing Modified with Titanium Carbonitrides on Pipe Steel" Crystals 13, no. 1: 146. https://doi.org/10.3390/cryst13010146
APA StylePavel, K., Nina, G., & Yury, P. (2023). Scanning Tunneling Microscopy of Intermediate Transformation Structures in Electric Arc Surfacing Modified with Titanium Carbonitrides on Pipe Steel. Crystals, 13(1), 146. https://doi.org/10.3390/cryst13010146







