Insight into the Structural, Mechanical and Optoelectronic Properties of Ternary Cubic Barium-Based BaMCl3 (M = Ag, Cu) Chloroperovskites Compounds
Abstract
1. Introduction
2. Computational Methodologies
3. Structural Properties
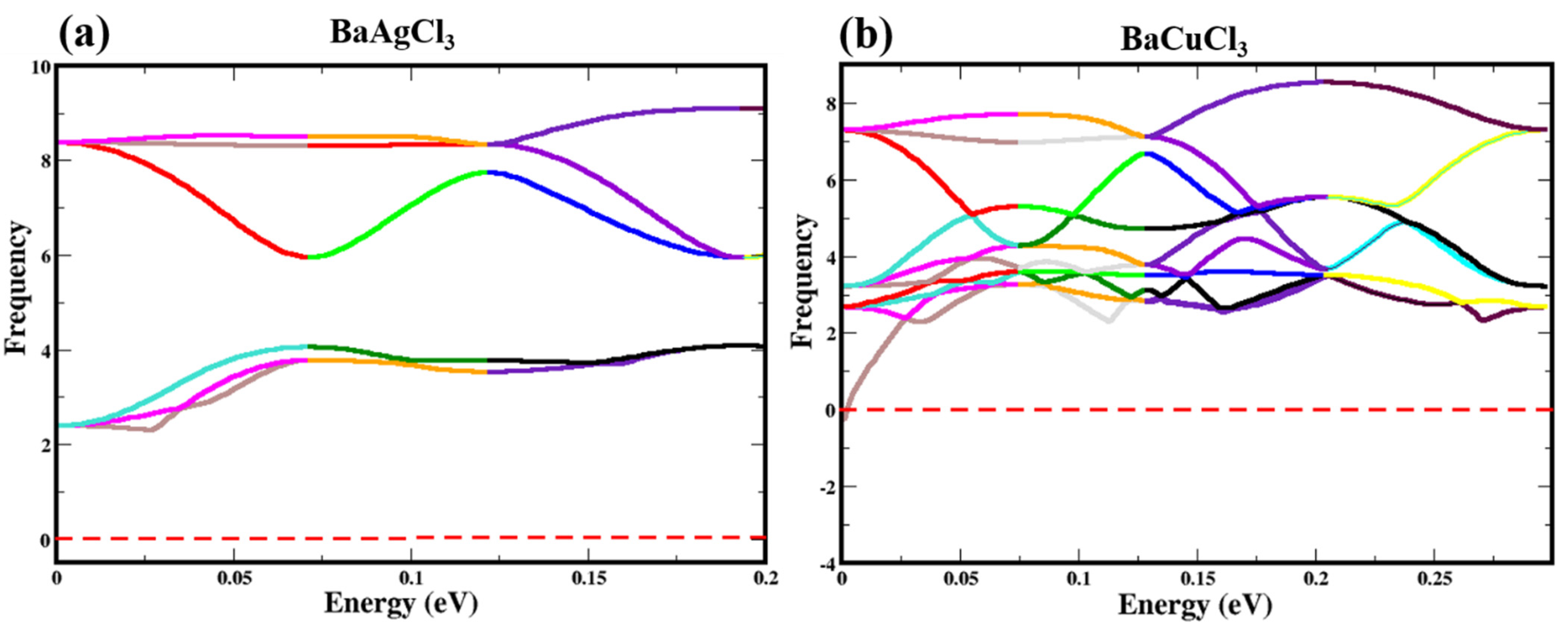
Phonon Properties
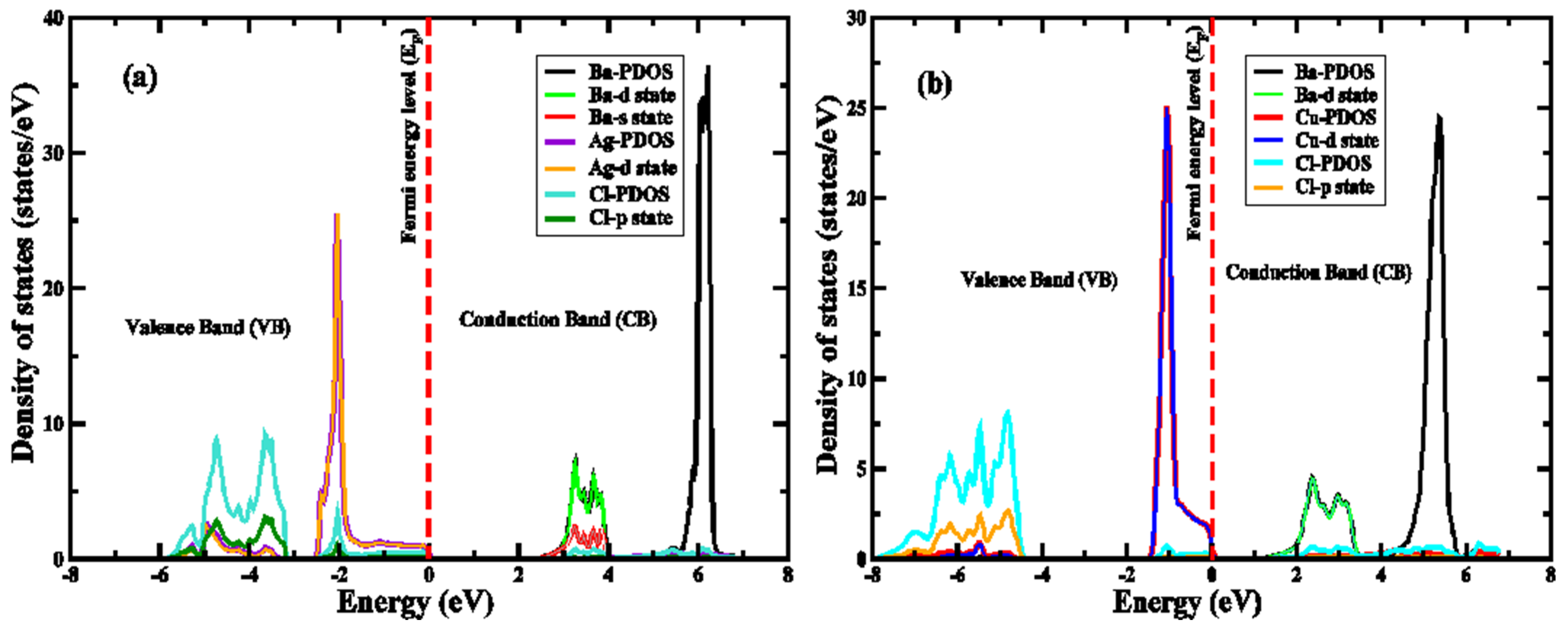
4. Electronic Properties
5. Elastic Properties
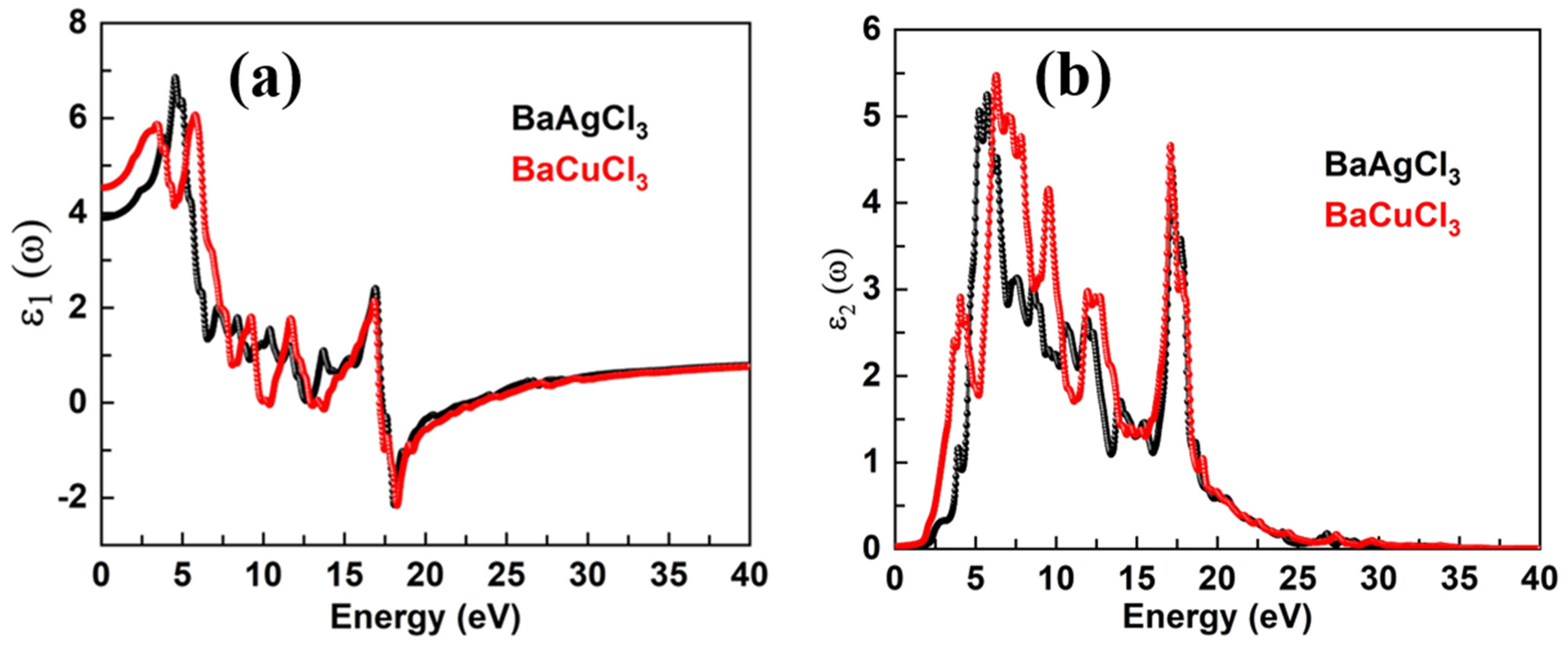
6. Optical Properties
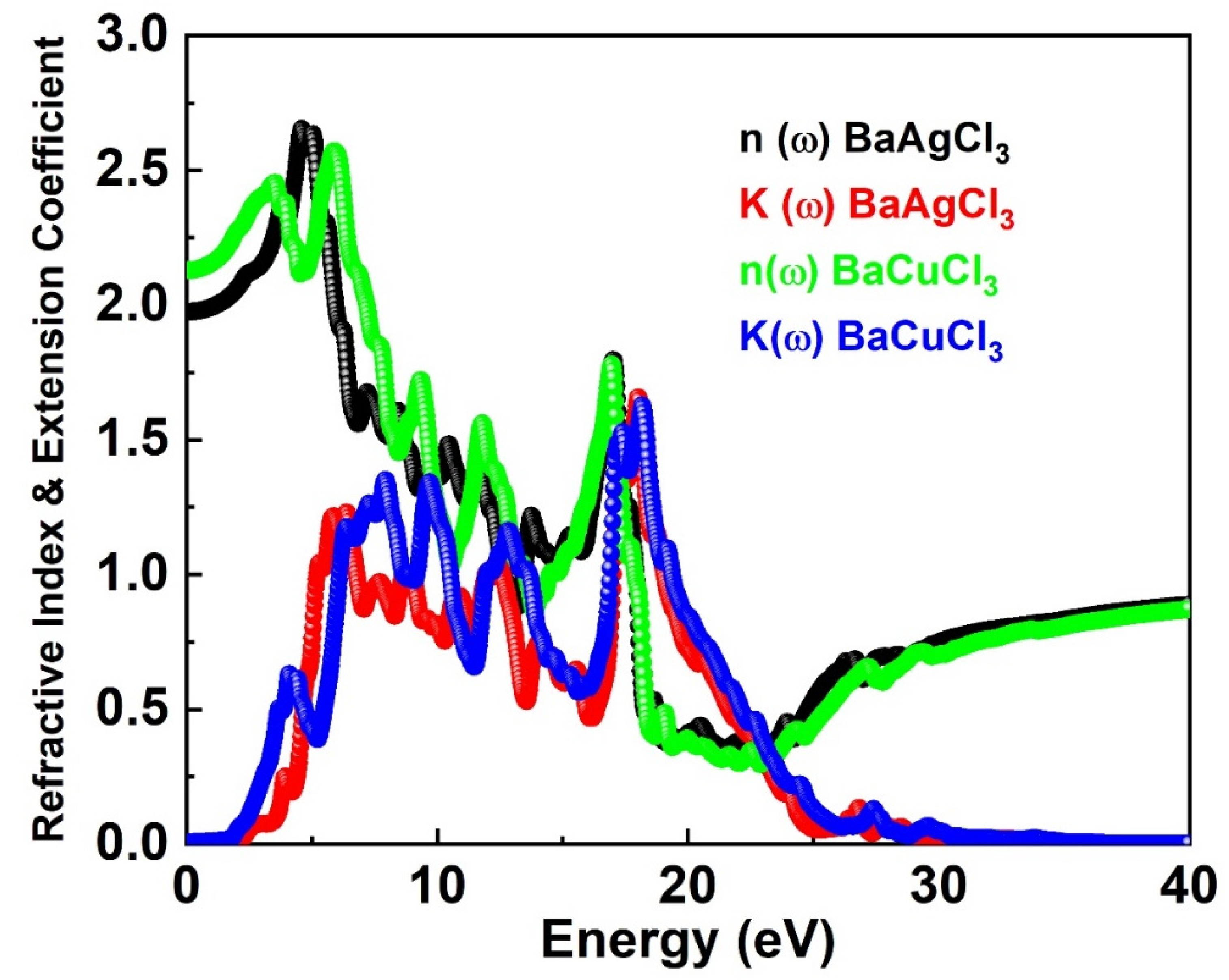
6.1. Extension Coefficient and Refractive Index
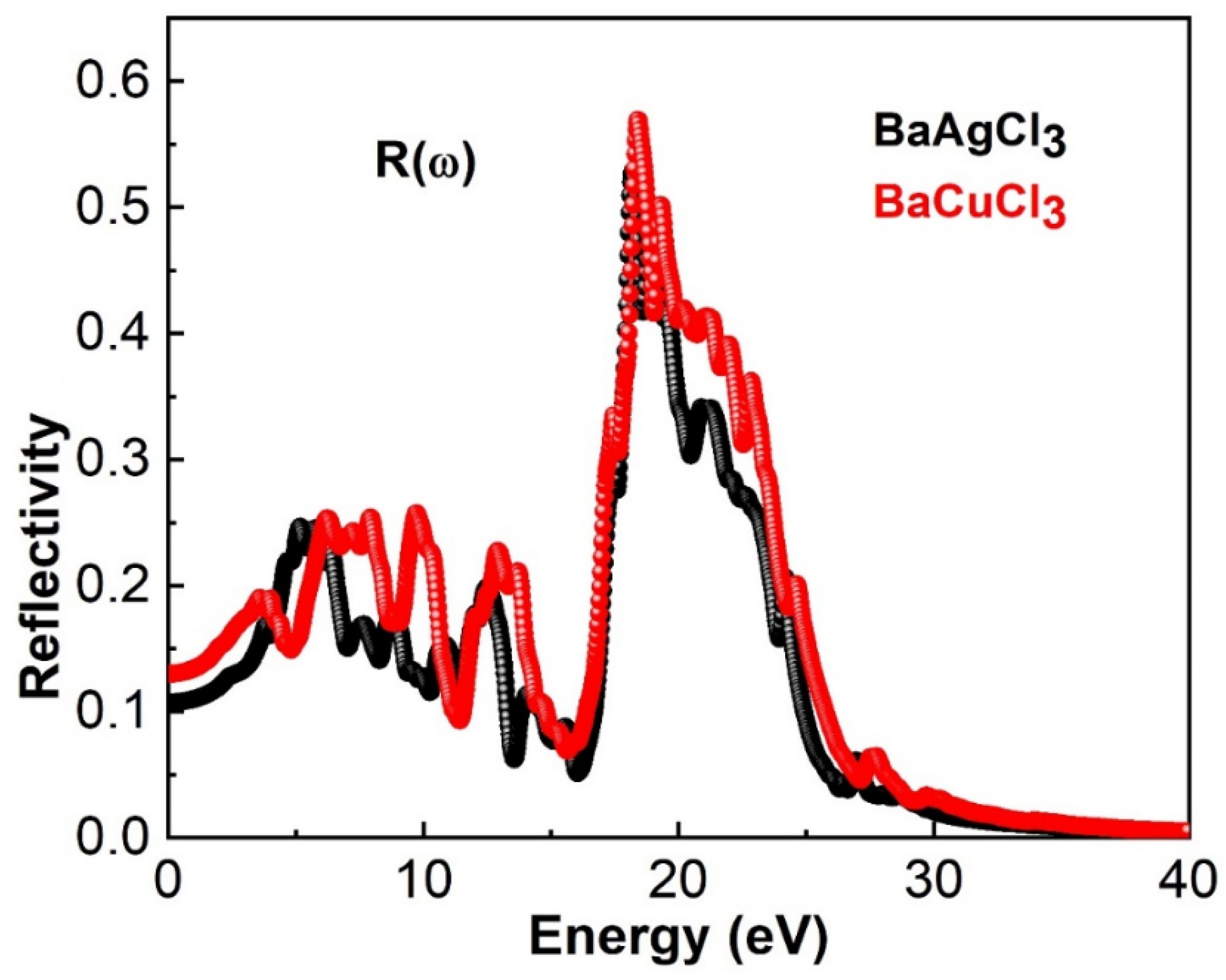
6.2. Reflectivity
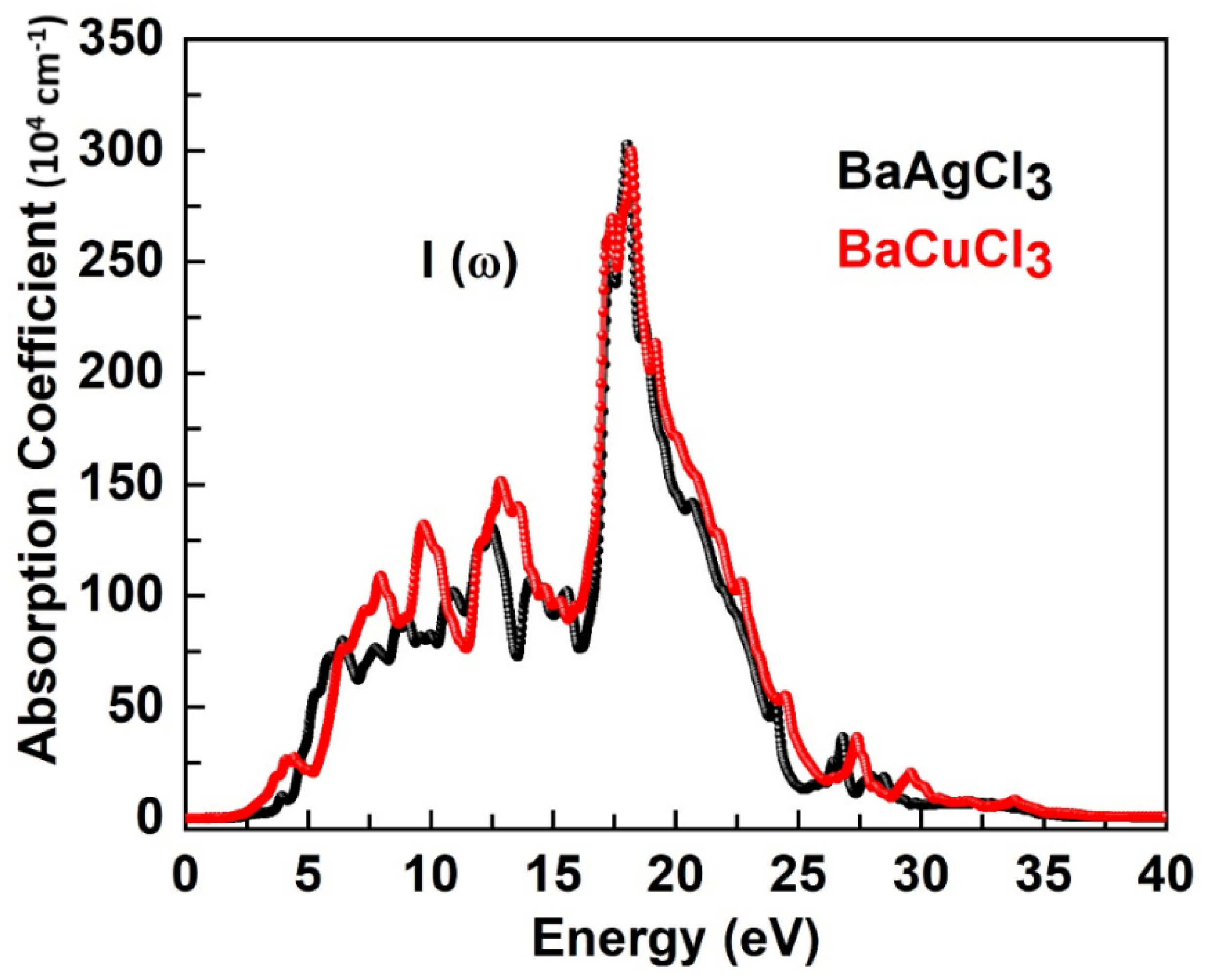
6.3. Absorption Coefficient
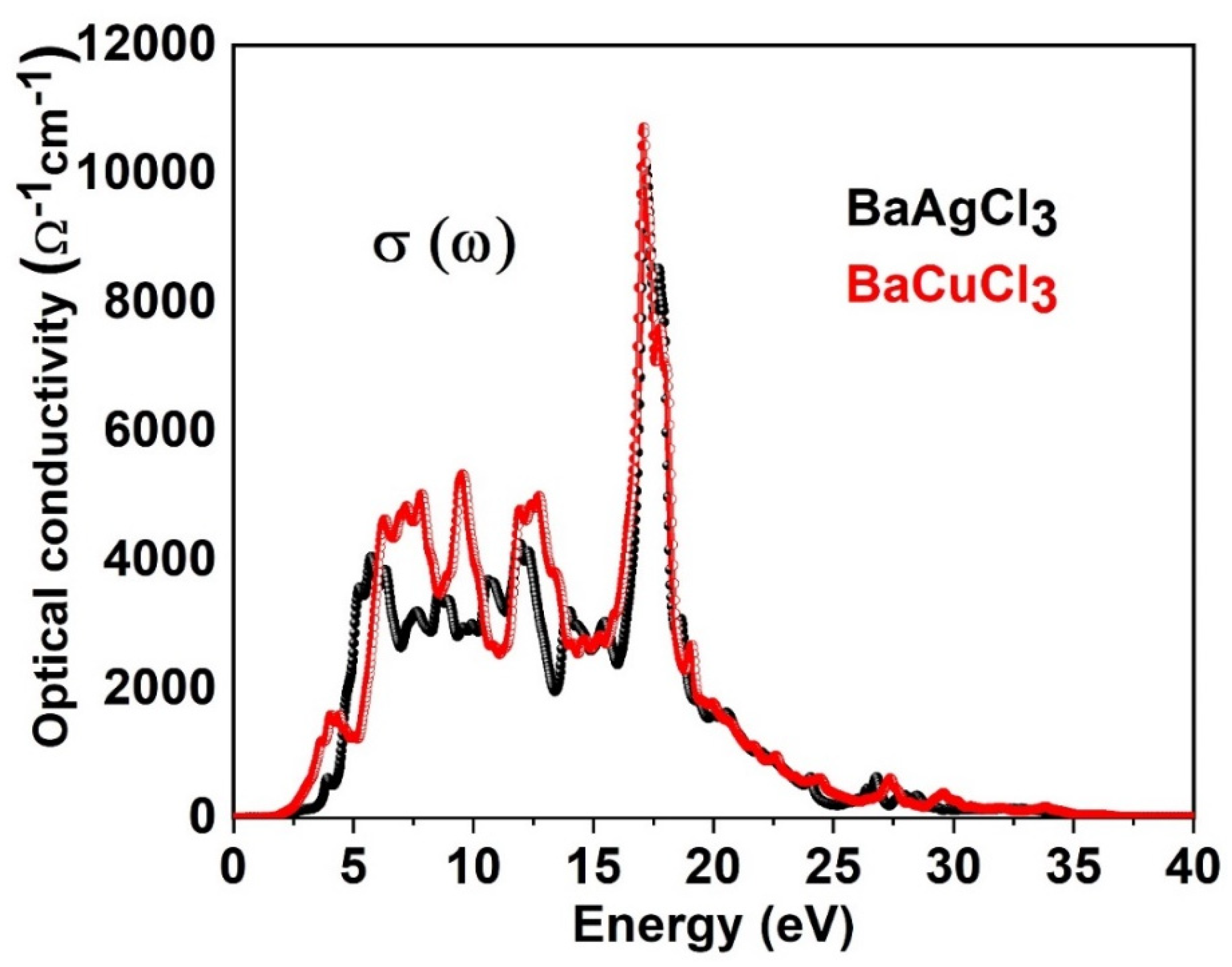
6.4. Optical Conductivity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukhatme, S.P.; Nayak, J.K. Solar Energy; McGraw-Hill Education: New York, NY, USA, 2017; ISBN 9352607120. [Google Scholar]
- Asafu-Adjaye, J. The relationship between energy consumption, energy prices and economic growth: Time series evidence from Asian developing countries. Energy Econ. 2000, 22, 615–625. [Google Scholar] [CrossRef]
- Rodríguez Cervantes, F.J. Optimización de un Secador Solar Basado en el Método de Análisis Exergético. Ph.D. Thesis, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Mexico, 2016. [Google Scholar]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar cells: In research and applications—A review. Mater. Sci. Appl. 2015, 6, 1145. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Rose, G. De Novis Quibusdam Fossilibus Quae in Montibus Uraliis Inveniuntur; Typis AG Schadii: Berlin, Germany, 1839. [Google Scholar]
- Husain, M.; Rahman, N.; Albalawi, H.; Ezzine, S.; Amami, M.; Zaman, T.; Rehman, A.U.; Sohail, M.; Khan, R.; Khan, A.A. Examining computationally the structural, elastic, optical, and electronic properties of CaQCl 3 (Q = Li and K) chloroperovskites using DFT framework. RSC Adv. 2022, 12, 32338–32349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Poddar, S.; Shu, L.; Fu, Y.; Fan, Z. Recent progress on interface engineering for high-performance, stable perovskites solar cells. Adv. Mater. Interfaces 2020, 7, 2000118. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Ayaz, U.; Shazia; Abdullah; Husain, M.; Rahman, N.; Bonyah, E. Ab initio investigation of structural, electronic, magnetic, elastic, and optical properties of Cs-based chloro-perovskites CsXCl3 (X = Be and Rh). AIP Adv. 2021, 11, 105215. [Google Scholar] [CrossRef]
- Cherrad, D.; Maouche, M.; Maamache, M.; Krache, L. Influence of valence electron concentration on elastic, electronic and optical properties of the alkaline-earth tin oxides A3SnO (A = Ca, Sr and Ba): A comparative study with ASnO3 compounds. Phys. B Condens. Matter 2011, 406, 2714–2722. [Google Scholar] [CrossRef]
- Murad, K.; Murtaza, G.; Noman, M.; Khan, S. To study the structural, electronic and optical properties of predicted stable halide perovskites ABX3. arXiv 2021, arXiv:2102.11577. [Google Scholar]
- Gómez–Peralta, J.I.; Bokhimi, X. Discovering new perovskites with artificial intelligence. J. Solid State Chem. 2020, 285, 121253. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D.; Luitz, J. wien2k. An Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties; Vienna University of Technology: Vienna, Austria, 2001; Volume 60. [Google Scholar]
- Vujović, M.; Ragavendran, V.; Arsić, B.; Kostić, E.; Mladenović, M. DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones. Open Chem. 2020, 18, 185–195. [Google Scholar] [CrossRef]
- Laurent, A.D.; Jacquemin, D. TD-DFT benchmarks: A review. Int. J. Quantum Chem. 2013, 113, 2019–2039. [Google Scholar] [CrossRef]
- Singh, D.J.; Nordstrom, L. Planewaves, Pseudopotentials, and the LAPW Method; Springer Science & Business Media: Berlin, Germany, 2006; ISBN 0387296840. [Google Scholar]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.K.H.; Marks, L.D. WIEN2k: An APW+ lo program for calculating the properties of solids. J. Chem. Phys. 2020, 152, 74101. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Band gaps from the Tran-Blaha modified Becke-Johnson approach: A systematic investigation. J. Chem. Phys. 2013, 138, 134115. [Google Scholar] [CrossRef] [PubMed]
- Murnaghan, F.D. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef]
- Koller, D.; Tran, F.; Blaha, P. Improving the modified Becke-Johnson exchange potential. Phys. Rev. B 2012, 85, 155109. [Google Scholar] [CrossRef]
- Khan, S.; Zaman, S.U.; Ahmad, R.; Mehmood, N.; Arif, M.; Kim, H.J. Ab initio investigations of structural, elastic, electronic and optical properties of the fluoroperovskite TIXF3 (X = Ca, Cd, Hg, and Mg) compounds. Mater. Res. Express 2020, 6, 125923. [Google Scholar] [CrossRef]
- Ambrosch-Draxl, C.; Sofo, J.O. Linear optical properties of solids within the full-potential linearized augmented planewave method. Comput. Phys. Commun. 2006, 175, 1–14. [Google Scholar] [CrossRef]
- Berri, S.; Ibrir, M.; Maouche, D.; Attallah, M. Robust half-metallic ferromagnet of quaternary Heusler compounds ZrCoTiZ (Z = Si, Ge, Ga and Al). Comput. Condens. Matter 2014, 1, 26–31. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An extended tolerance factor approach for organic–inorganic perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef]
- Rahman, N.; Husain, M.; Yang, J.; Murtaza, G.; Sajjad, M.; Habib, A.; Karim, A.; Haq, M.U.; Rauf, A.; Nisar, M. First Principle Study of Structural, Electronic, Elastic, and Magnetic Properties of Half-Heusler Compounds ScTiX (X = Si, Ge, Pb, In, Sb, and Tl). J. Supercond. Nov. Magn. 2020, 33, 3915–3922. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, X.F.; De Jonghe, L.C.; Ritchie, R.O. Elastic constants and tensile properties of Al 2 OC by density functional calculations. Phys. Rev. B 2007, 75, 104114. [Google Scholar] [CrossRef]
- Łepkowski, S.P.; Gorczyca, I. Ab initio study of elastic constants in In x Ga 1−x N and In x Al 1−x N wurtzite alloys. Phys. Rev. B 2011, 83, 203201. [Google Scholar] [CrossRef]
- Jamal, M.; Bilal, M.; Ahmad, I.; Jalali-Asadabadi, S. IRelast package. J. Alloys Compd. 2018, 735, 569–579. [Google Scholar] [CrossRef]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 0080542867. [Google Scholar]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Mott, P.H.; Dorgan, J.R.; Roland, C.M. The bulk modulus and Poisson’s ratio of “incompressible” materials. J. Sound Vib. 2008, 312, 572–575. [Google Scholar] [CrossRef]
- Wachter, P.; Filzmoser, M.; Rebizant, J. Electronic and elastic properties of the light actinide tellurides. Phys. B Condens. Matter 2001, 293, 199–223. [Google Scholar] [CrossRef]
- Pettifor, D.G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Technol. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Pathak, A.K.; Vazhappilly, T. Ab initio study on structure, elastic, and mechanical properties of lanthanide sesquioxides. Phys. Status Solidi 2018, 255, 1700668. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-shells in the transition metals. II. Ferromagnetic compounds of manganese with perovskite structure. Phys. Rev. 1951, 82, 403. [Google Scholar] [CrossRef]
- Mayer, B.; Anton, H.; Bott, E.; Methfessel, M.; Sticht, J.; Harris, J.; Schmidt, P.C. Ab-initio calculation of the elastic constants and thermal expansion coefficients of Laves phases. Intermetallics 2003, 11, 23–32. [Google Scholar] [CrossRef]
- Adler, S.L. Quantum theory of the dielectric constant in real solids. Phys. Rev. 1962, 126, 413. [Google Scholar] [CrossRef]
- Smith, N. V Photoelectron energy spectra and the band structures of the noble metals. Phys. Rev. B 1971, 3, 1862. [Google Scholar] [CrossRef]
- Kramers, H.A. La diffusion de la lumiere par les atomes. Atti Cong. Intern. Fisica Como 1927, 2, 545–557. [Google Scholar]
- Kronig, R.d.L. On the theory of dispersion of X-rays. Josa 1926, 12, 547–557. [Google Scholar] [CrossRef]



| Structural Parameters | BaAgCl3 | BaCuCl3 |
|---|---|---|
| Lattice constant (Bohr) | 9.90 | 9.38 |
| Bulk modulus (B) | 34.19 | 43.03 |
| Ground state volume (V0) | 968.2 | 820.78 |
| Bulk modulus derivative (B/) | 5.52 | 7.66 |
| Ground state energy (E0) | −29,682.92 | −22,358.39 |
| Tolerance factor (τ) | 0.93 | 0.97 |
| Compounds | C11 | C12 | C44 | B | A | G | E | υ | B/G | ζ |
|---|---|---|---|---|---|---|---|---|---|---|
| BaAgCl3 | 89.42 | 25.31 | 3.35 | 46.68 | 0.10 | 4.80 | 13.9 | 0.66 | 9.73 | 0.793 |
| BaCuCl3 | 105.16 | 40.6 | 19.93 | 62.12 | 0.61 | 15 | 2.00 | 0.74 | 4.14 | 0.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, M.; Ullah, A.; Algahtani, A.; Tirth, V.; Al-Mughanam, T.; Alghtani, A.H.; Sfina, N.; Briki, K.; Albalawi, H.; Amin, M.A.; et al. Insight into the Structural, Mechanical and Optoelectronic Properties of Ternary Cubic Barium-Based BaMCl3 (M = Ag, Cu) Chloroperovskites Compounds. Crystals 2023, 13, 140. https://doi.org/10.3390/cryst13010140
Husain M, Ullah A, Algahtani A, Tirth V, Al-Mughanam T, Alghtani AH, Sfina N, Briki K, Albalawi H, Amin MA, et al. Insight into the Structural, Mechanical and Optoelectronic Properties of Ternary Cubic Barium-Based BaMCl3 (M = Ag, Cu) Chloroperovskites Compounds. Crystals. 2023; 13(1):140. https://doi.org/10.3390/cryst13010140
Chicago/Turabian StyleHusain, Mudasser, Abd Ullah, Ali Algahtani, Vineet Tirth, Tawfiq Al-Mughanam, Abdulaziz H. Alghtani, Nourreddine Sfina, Khaoula Briki, Hind Albalawi, Mohammed A. Amin, and et al. 2023. "Insight into the Structural, Mechanical and Optoelectronic Properties of Ternary Cubic Barium-Based BaMCl3 (M = Ag, Cu) Chloroperovskites Compounds" Crystals 13, no. 1: 140. https://doi.org/10.3390/cryst13010140
APA StyleHusain, M., Ullah, A., Algahtani, A., Tirth, V., Al-Mughanam, T., Alghtani, A. H., Sfina, N., Briki, K., Albalawi, H., Amin, M. A., Azzouz-Rached, A., & Rahman, N. (2023). Insight into the Structural, Mechanical and Optoelectronic Properties of Ternary Cubic Barium-Based BaMCl3 (M = Ag, Cu) Chloroperovskites Compounds. Crystals, 13(1), 140. https://doi.org/10.3390/cryst13010140






