Corrosion Mechanism and the Effect of Corrosion Time on Mechanical Behavior of 5083/6005A Welded Joints in a NaCl and NaHSO3 Mixed Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
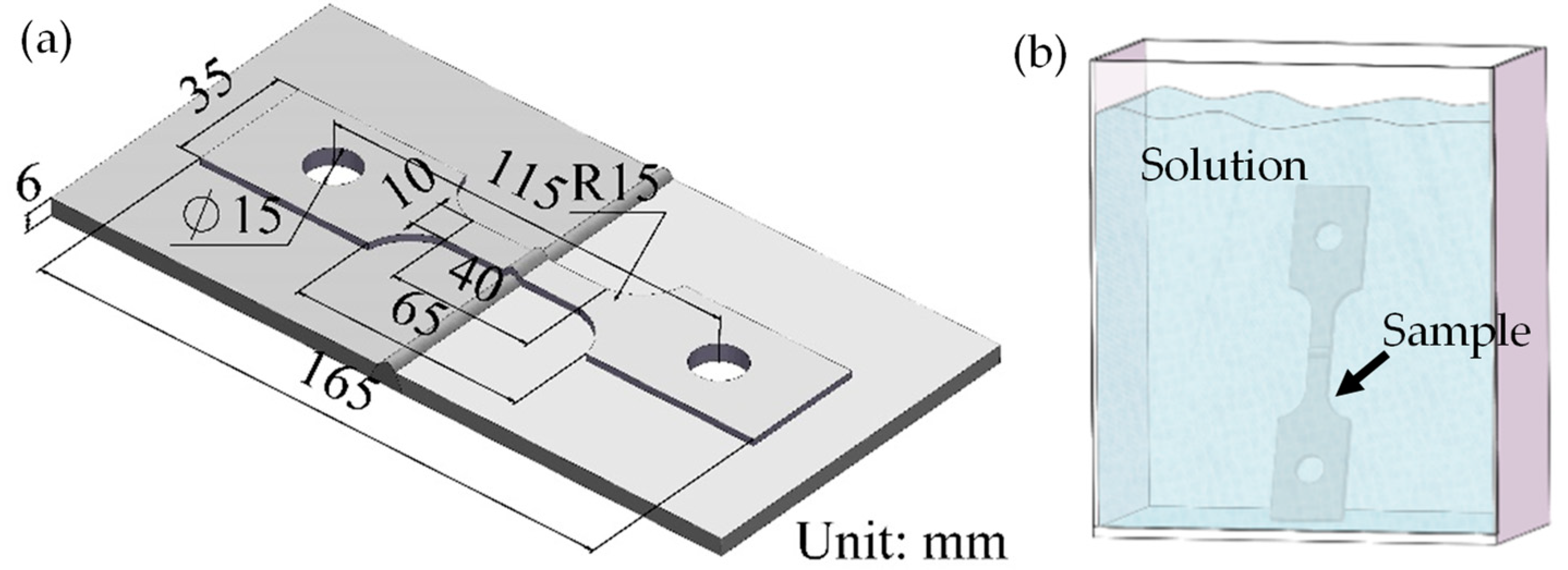
2.2. Corrosion Tests
2.3. Tensile and Fatigue Tests
2.4. Electrochemical Measurement
2.5. Characterization Techniques
3. Results and Discussion
3.1. Effect of Corrosion Time on Mechanical Properties
3.2. Effect of Corrosion Time on Corrosion Resistance
3.3. Analysis of Corrosion Products
3.4. Corrosion Mechanism and Model
3.5. Prediction Model of Fatigue Life after Corrosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, L.; Ma, J.J.; Zou, C.X.; Gao, J.; Wang, H.S.; Sun, J.; Guan, Q.M.; Wang, J.; Tang, B.; Li, J.S.; et al. Revealing foundations of the intergranular corrosion of 5xxx and 6xxx Al alloys. Mater. Lett. 2020, 271, 127767. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Xu, J.B.; Pan, S.P.; Li, N.B.; Ou, C.G.; Liu, W.H.; Song, Y.F.; Tan, X.R.; Liu, Y. Effect of T6I6 treatment on dynamic mechanical behavior of Al-Si-Mg-Cu cast alloy and impact resistance of its cast motor shell. J. Cent. South Univ. 2022, 29, 924–936. [Google Scholar] [CrossRef]
- Arifurrahman, F.; Budiman, B.A.; Aziz, M. On the lightweight structural design for electric road and railway vehicles using fiber reinforced polymer composites-a review. Int. J. Sustain. Transp. 2018, 1, 21–29. [Google Scholar] [CrossRef]
- Lu, W.; Ma, C.P.; Gou, G.Q.; Fu, Z.H.; Sun, W.G.; Che, X.L.; Chen, H.; Gao, W. Corrosion fatigue crack propagation behavior of A7N01P-T4 aluminum alloy welded joints from high-speed train underframe after 1.8 million km operation. Mater. Corros. 2021, 72, 879–887. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G.; Rengsomboon, A. Fatigue characteristics of 6061 aluminum alloy subject to 3.5% NaCl environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Wang, L.W.; Liang, J.M.; Li, H.; Cheng, L.J.; Cui, Z.Y. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl−. Corros. Sci. 2021, 178, 109076. [Google Scholar] [CrossRef]
- Shen, L.; Chen, H.; Che, X.L.; Xu, L.D. Corrosion—Fatigue crack propagation of aluminum alloys for high-speed trains. Int. J. Mod. Phys. B 2017, 31, 1744009. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.L.; Sun, J.; Zhu, M.T.; Yao, Q.; Zhang, D.J.; Zhang, B.W.; Xiao, K.; Wu, J.S. Corrosion behaviors of AA5083 and AA6061 in artificial seawater, effects of Cl−, HSO3− and temperature. Int. J. Electrochem. Sci. 2020, 15, 1218–1229. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Guo, C.; Niu, K.K.; Zhao, J.B.; Huang, Y.H.; Li, X.G. Long-term corrosion behavior of the 7A85 aluminum alloy in an industrial-marine atmospheric environment. J. Mater. Res. Technol. 2021, 12, 1350–1359. [Google Scholar] [CrossRef]
- Mishra, R.K. Study the effect of pre-corrosion on mechanical properties and fatigue life of aluminum alloy 8011. Mater. Today Proc. 2020, 25, 602–609. [Google Scholar] [CrossRef]
- Ghosh, R.; Venugopal, A.; Rao, G.S.; Narayanan, P.R.; Pant, B.; Cherian, R. Effect of temper condition on the corrosion and fatigue performance of AA2219 aluminum alloy. J. Mater. Eng. Perform. 2018, 27, 423–433. [Google Scholar] [CrossRef]
- Ma, Q.N.; Shao, F.; Bai, L.Y.; Xu, Q.; Xie, X.K.; Shen, M. Corrosion fatigue fracture characteristics of FSW 7075 aluminum alloy joints. Materials 2020, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, R.; Ravisankar, V.; Balasubramanian, V. Corrosion performance of friction stir welded AA2024 aluminium alloy under salt fog conditions. Trans. Nonferrous Met. Soc. China 2015, 25, 1427–1438. [Google Scholar] [CrossRef]
- Ge, F.; Zhang, L.; Tian, H.Y.; Yu, M.D.; Liang, J.M.; Wang, X. Stress corrosion cracking behavior of 2024 and 7075 high-strength aluminum alloys in a simulated marine atmosphere contaminated with SO2. J. Mater. Eng. Perform. 2020, 29, 410–422. [Google Scholar] [CrossRef]
- Weber, M.; Eason, P.D.; Özdeş, H.; Tiryakioğlu, M. The effect of surface corrosion damage on the fatigue life of 6061-T6 aluminum alloy extrusions. Mater. Sci. Eng. A 2017, 690, 427–432. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.W.; Su, Y.; Mou, X.L.; Xiao, Y.; Liu, J. Investigation on the corrosion behavior of aluminum alloys 3A21 and 7A09 in chloride aqueous solution. Mater. Des. 2013, 50, 15–21. [Google Scholar] [CrossRef]
- Yang, X.K.; Zhang, L.W.; Zhang, S.Y.; Liu, M.; Zhou, K.; Mu, X.L. Properties degradation and atmospheric corrosion mechanism of 6061 aluminum alloy in industrial and marine atmosphere environments. Mater. Corros. 2017, 68, 529–535. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.G. Microstructure, elevated-temperature mechanical properties and creep resistance of dispersoid-strengthened Al-Mn-Mg 3xxx alloys with varying Mg and Si contents. Mater. Sci. Eng. A 2017, 708, 383–394. [Google Scholar] [CrossRef]
- Sakairi, M.; Otani, K.; Kaneko, A.; Seki, Y.; Nagasawa, D. Analysis of chemical compositions and morphology of surface films formed on 3003 aluminum alloy by immersion in different cation containing model tap waters. Surf. Interface Anal. 2013, 45, 1517–1521. [Google Scholar] [CrossRef]
- Dong, C.; Kui, X.; Lin, X.; Sheng, H. Characterization of 7A04 aluminum alloy corrosion under atmosphere with chloride ions using electrochemical techniques. Rare Met. Mater. Eng. 2011, 40, 275–279. [Google Scholar]
- Hoar, T.P.; Mears, D.C.; Rothwell, G.P. The relationships between anodic passivity, brightening and pitting. Corros. Sci. 1965, 5, 279–289. [Google Scholar] [CrossRef]
- Hoar, T.P. The production and breakdown of the passivity of metals. Corros. Sci. 1967, 7, 341–355. [Google Scholar] [CrossRef]
- Kumar, K.K.; Kumar, A.; Satyanarayana, M. Effect of friction stir welding parameters on the material flow, mechanical properties and corrosion behavior of dissimilar AA5083–AA6061 joints. Proc. Inst. Mech. Eng. Part C 2022, 236, 2901–2917. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, H.; Pan, S.P.; Song, Y.F.; Liu, W.H. Effects of service environment and pre-deformation on the fatigue behaviour of 2524 aluminium alloy. Arch. Civ. Mech. Eng. 2020, 20, 5. [Google Scholar] [CrossRef]
- Nan, Z.Y.; Ishihara, S.; Goshima, T. Corrosion fatigue behavior of extruded magnesium alloy AZ31 in sodium chloride solution. Int. J. Fatigue 2008, 30, 1181–1188. [Google Scholar] [CrossRef]
- Mohabeddine, A.; Correia, J.A.F.O.; Montenegro, P.A.; Castro, J.M. Fatigue crack growth modelling for cracked small-scale structural details repaired with CFRP. Thin Wall Struct. 2021, 161, 107525. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, H.; Song, W.W.; Pan, S.P.; Liu, W.H.; Zhu, B.W.; Song, Y.F.; Zhou, W. Acceleration effect of a graphite dust environment on the fatigue crack propagation rates of Al alloy. Int. J. Fatigue 2019, 126, 20–29. [Google Scholar] [CrossRef]
- Newman, J.C. A crack opening stress equation for fatigue crack growth. Int. J. Fatigue 1984, 24, R131–R135. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Tang, Z.H.; Pan, S.P.; Liu, W.H.; Song, Y.F.; Zhu, B.W.; Liu, Y.; Wen, Z.L. The fatigue crack behavior of 7N01-T6 aluminum alloy in different particle environments. Arch. Civ. Mech. Eng. 2020, 20, 129. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Tang, Z.H.; Pan, S.P.; Liu, W.H.; Song, Y.F.; Liu, Y.; Zhu, B.W. The fatigue crack growth behaviour of 2524-T3 aluminium alloy in an Al2O3 particle environment. Fatigue Fract. Eng. Mater. Struct. 2020, 43, 2376–2389. [Google Scholar] [CrossRef]
- Newman, J.C.; Raju, I.S. Stress-intensity factor equations for cracks in three-dimensional. In Proceedings of the Fourteenth National Symposium on Fracture Mechanics, Los Angeles, CA, USA, 30 June 1981. [Google Scholar]
- Rubio-González, C.; Ocaña, J.L.; Gomez-Rosas, G. Effect of laser shock processing on fatigue crack growth and fracture toughness of 6061-T6 aluminum alloy. Mater. Sci. Eng. A 2004, 386, 291–295. [Google Scholar] [CrossRef]
- Mrowka-Nowotnik, G. Influence of precipitation strengthening process on tensile and fracture behaviour of the 6005 and 6082 alloys. Adv. Manuf. Sci. Technol. 2008, 32, 31–50. [Google Scholar]












| Material | Si | Fe | Cu | Mn | Mg | Zn | Cr | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 5083 | 0.09 | 0.20 | 0.01 | 0.75 | 4.98 | 0.02 | 0.09 | 0.05 | Balance |
| 6005A | 0.50 | 0.19 | 0.01 | 0.26 | 0.71 | 0.02 | 0.16 | 0.06 | Balance |
| ER5356 | 0.12 | 0.12 | 0.08 | 0.15 | 4.90 | 0.12 | 0.11 | 0.12 | Balance |
| Specimen | Uncorroded | 3 Days | 7 Days | 15 Days | 30 Days |
|---|---|---|---|---|---|
| Nf/ cycles | 107 | 1,159,257 | 884,098 | 805,323 | 449,833 |
| 6,804,918 | 907,457 | 672,835 | 353,091 | 267,661 |
| Experimental Conditions | 5083 | 6005A | ||
|---|---|---|---|---|
| Ecorr (vs. SCE)/ (V) | Jcorr/ (μA·cm−2) | Ecorr (vs. SCE)/ (V) | Jcorr/ (μA·cm−2) | |
| Uncorroded | −0.594 | 2.0 | −0.617 | 1.95 |
| After being corroded for 3 days | −0.626 | 4.11 | −0.647 | 2.89 |
| After being corroded for 7 days | −0.650 | 4 | −0.663 | 2.50 |
| After being corroded for 15 days | −0.668 | 5.01 | −0.693 | 2.18 |
| After being corroded for 30 days | −0.685 | 0.812 | −0.731 | 1.38 |
| Sample | ap/μm | bp/μm | t/mm | w/mm | θ | σ0/MPa | Experimental Fatigue Life (Average)/Cycle | Predicted Fatigue Life/Cycles |
|---|---|---|---|---|---|---|---|---|
| 3 days | 198 | 91 | 6 | 5 | 0.125π | 190.3 | 1,033,357 | 1,075,621 |
| 7 days | 673 | 541 | 6 | 5 | 0.125π | 188.6 | 778,467 | 852,645 |
| 15 days | 721 | 547 | 10 | 3 | 0.1875π | 187.0 | 579,207 | 604,837 |
| 30 days | 1076 | 934 | 6 | 5 | 0.125π | 182.8 | 338,747 | 309,234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, H.; Wang, X.; Zeng, X.; Huang, L.; Gu, H.; Li, H. Corrosion Mechanism and the Effect of Corrosion Time on Mechanical Behavior of 5083/6005A Welded Joints in a NaCl and NaHSO3 Mixed Solution. Crystals 2022, 12, 1150. https://doi.org/10.3390/cryst12081150
Chen Y, Wu H, Wang X, Zeng X, Huang L, Gu H, Li H. Corrosion Mechanism and the Effect of Corrosion Time on Mechanical Behavior of 5083/6005A Welded Joints in a NaCl and NaHSO3 Mixed Solution. Crystals. 2022; 12(8):1150. https://doi.org/10.3390/cryst12081150
Chicago/Turabian StyleChen, Yuqiang, Hailiang Wu, Xiangdong Wang, Xianghao Zeng, Liang Huang, Hongyu Gu, and Heng Li. 2022. "Corrosion Mechanism and the Effect of Corrosion Time on Mechanical Behavior of 5083/6005A Welded Joints in a NaCl and NaHSO3 Mixed Solution" Crystals 12, no. 8: 1150. https://doi.org/10.3390/cryst12081150
APA StyleChen, Y., Wu, H., Wang, X., Zeng, X., Huang, L., Gu, H., & Li, H. (2022). Corrosion Mechanism and the Effect of Corrosion Time on Mechanical Behavior of 5083/6005A Welded Joints in a NaCl and NaHSO3 Mixed Solution. Crystals, 12(8), 1150. https://doi.org/10.3390/cryst12081150





