Interactions of Cr3+, Ni2+, and Sr2+ with Crushed Concrete Fines
Abstract
1. Introduction
2. Materials and Methods
2.1. Prepraration and Characterization of Crushed Concrete Fines (CCF)
2.2. Uptake of Aqueous Cr3+, Ni2+, and Sr2+ Ions by CCF
2.3. Leaching of Cr3+, Ni2+, and Sr2+ Ions from Metal-Bearing CCF
2.4. Characterization of Metal-Bearing CCF by SEM and EDX
3. Results
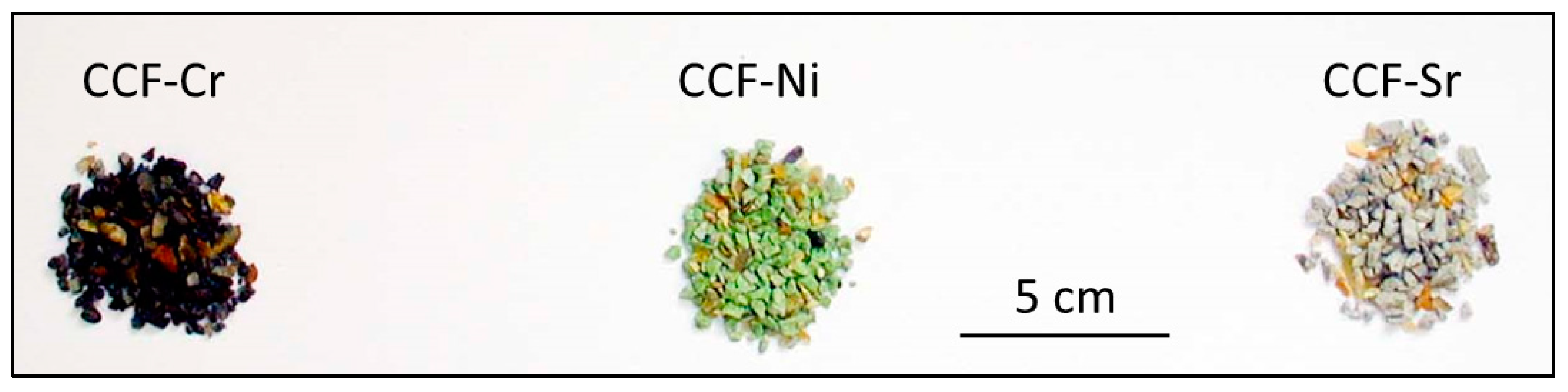
3.1. Removal of Cr3+, Ni2+, and Sr2+ Ions by CCF
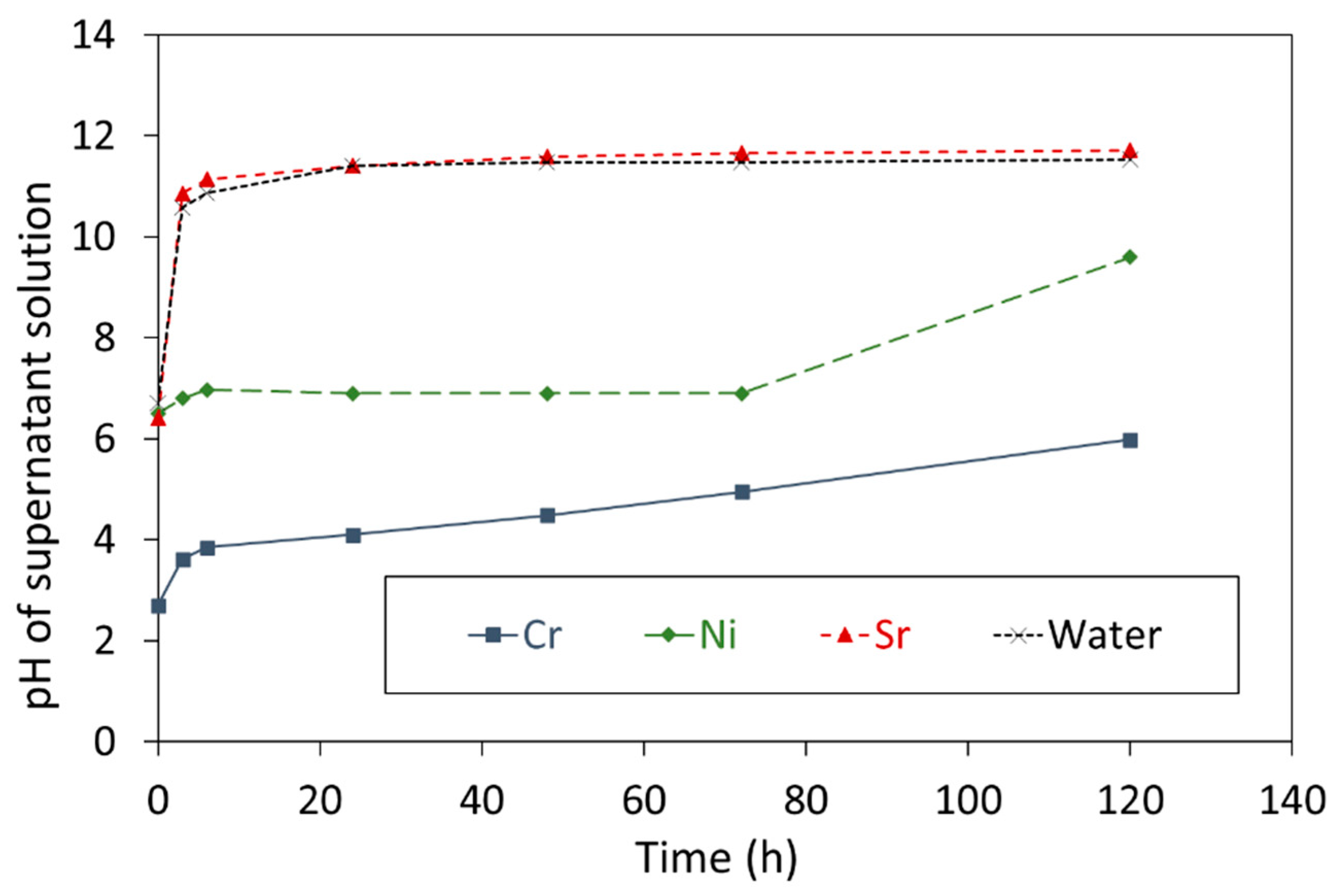
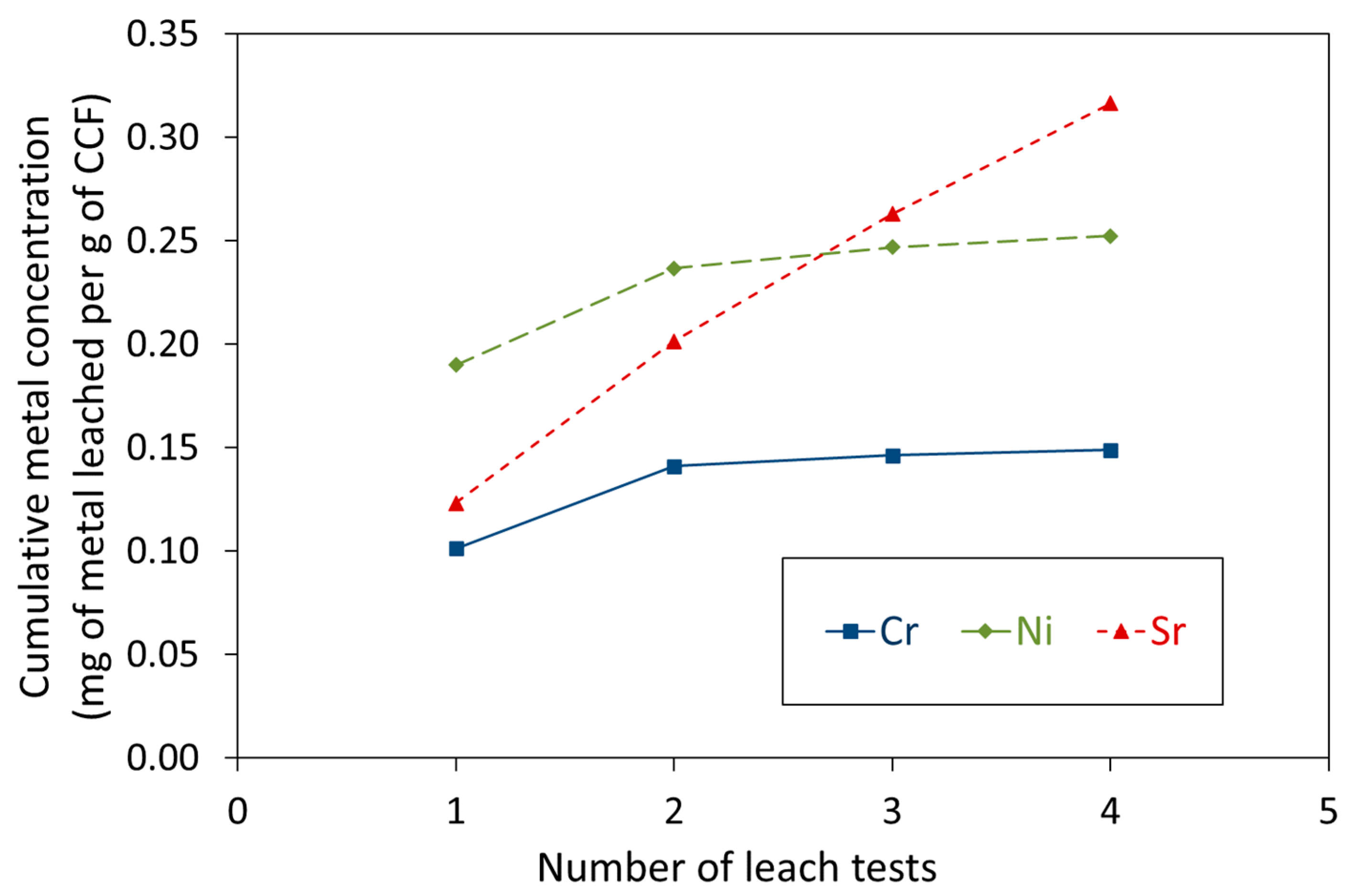
3.2. Leaching of Cr3+, Ni2+, and Sr2+ Species Bound to CCF
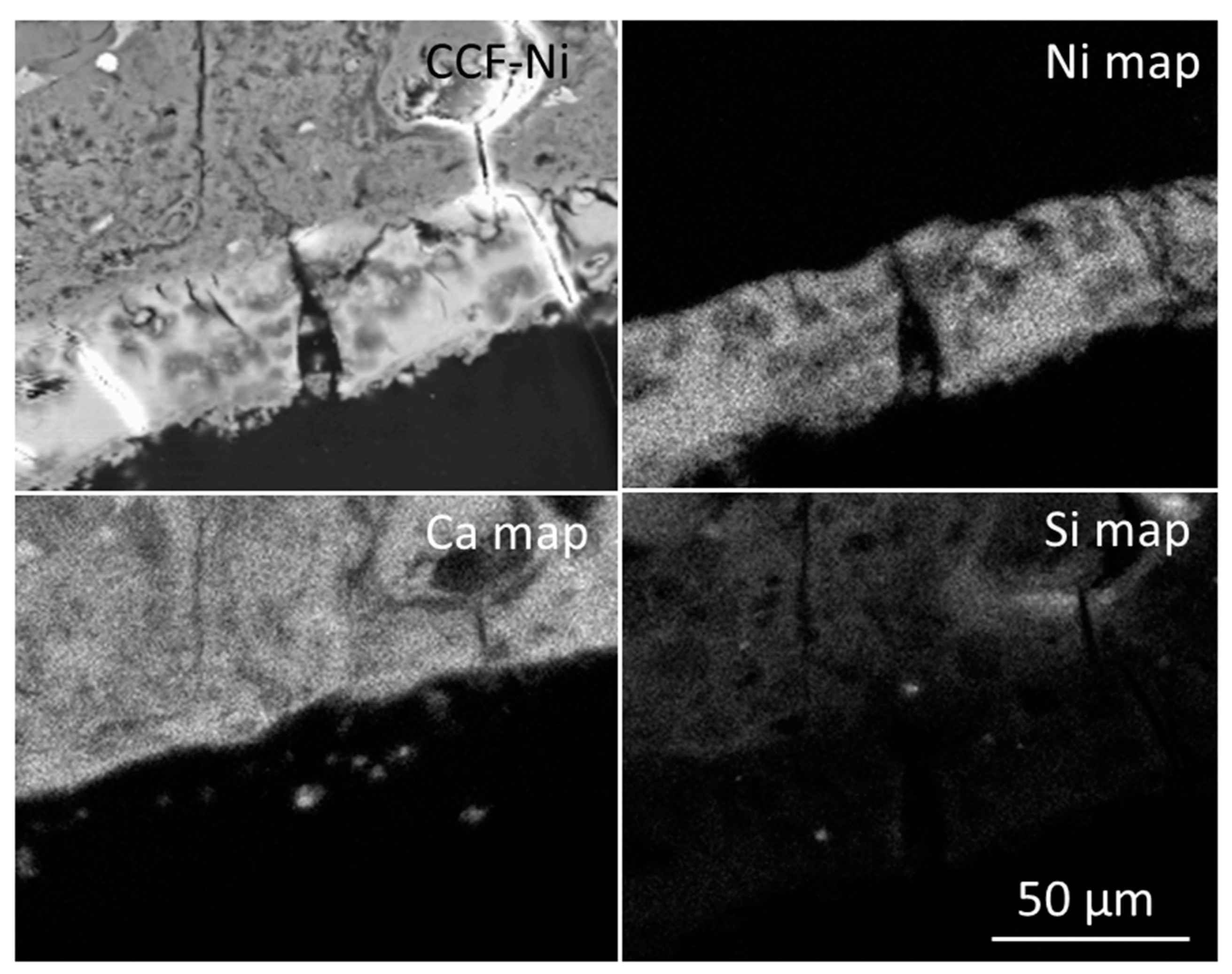
3.3. The Fate of Metal Species Bound to CCF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimbili, O.; Salim, W.; Ndambuki, M. A review on the usage of ceramic wastes in concrete production. Int. J. Civil Archit. Struct. Constr. Eng. 2014, 8, 91–95. [Google Scholar]
- Meng, T.; Hong, Y.; Ying, K.; Wang, Z. Comparison of technical properties of cement pastes with different activated recycled powder from construction and demolition waste. Cem. Concr. Compos. 2021, 120, 104065. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.-L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and demolition waste best management practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Kua, H.; Geng, Y.; Bleischwitz, R.; Ren, J. Construction and demolition waste management in China through the 3R principle. Resour. Conserv. Recycl. 2018, 129, 36–44. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.-S.; Xue, Q.; Zhan, B.; Chen, X.; Wan, Y.; Zhao, Y.; Sun, Y.; Poon, C.S. Deep insight on mechanism and contribution of As(V) removal by thermal modification waste concrete powder. Sci. Total Environ. 2022, 807, 150764. [Google Scholar] [CrossRef]
- Gartner, E.M.; Young, J.F.; Damidot, D.A.; Jawed, I. Hydration of Portland cement. In Structure and Performance of Cements, 2nd ed.; Bensted, J., Barnes, P., Eds.; Spon Press: London, UK, 2002; pp. 57–113. [Google Scholar]
- Conner, J.R.; Hoeffner, S.L. The history of stabilization/solidification technology. Crit. Rev. Environ. Sci. Technol. 1998, 28, 325–396. [Google Scholar] [CrossRef]
- Evans, N.D.M. Binding mechanisms of radionuclides to cement. Cem. Concr. Res. 2008, 38, 543–553. [Google Scholar] [CrossRef]
- Shin, W.-S.; Na, K.-R.; Kim, Y.-K. Adsorption of metal ions from aqueous solution by recycled aggregate: Estimation of pretreatment effect. Desalin. Water Treat. 2016, 57, 9366–9374. [Google Scholar] [CrossRef]
- Damrongsiri, S. Feasibility of using demolition waste as an alternative heavy metal immobilising agent. J. Environ. Manag. 2017, 192, 197–202. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Kawamoto, K.; Saito, T. Evaluation of autoclaved aerated concrete fines for removal of Cd(II) and Pb(II) from wastewater. J. Environ. Eng. 2019, 145, 04019078. [Google Scholar] [CrossRef]
- Coleman, N.J.; Lee, W.E.; Slipper, I.J. Interactions of aqueous Cu2+, Zn2+ and Pb2+ ions with crushed concrete fines. J. Hazard. Mater. 2005, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.F.; Abd Ali, Z.T. Removal of lead ions from wastewater using crushed concrete demolition waste. Assoc. Arab Univ. J. Eng. Sci. 2019, 26, 22–29. [Google Scholar] [CrossRef]
- Ali, A.F.; Abd Ali, Z.T. Interaction of aqueous Cu2+ ions with granules of crushed concrete. Iraqi J. Chem. Pet. Eng. 2019, 20, 31–38. [Google Scholar] [CrossRef]
- Yoo, J.; Shin, H.; Ji, S. Evaluation of the applicability of concrete sludge for the removal of Cu, Pb, and Zn from contaminated aqueous solutions. Metals 2018, 8, 666. [Google Scholar] [CrossRef]
- Deng, Y.; Wheatley, A. Mechanisms of phosphorus removal by recycled crushed concrete. Int. J. Environ. Res. Public Health 2018, 15, 357. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Vujović, Z.; Smičiklas, I. Radionuclide immobilization by sorption onto waste concrete and bricks-experimental design methodology. Water Air Soil Pollut. 2019, 230, 242. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Ivana, J.; Dimović, S.; Antonijević, D.; Jović, M.; Mraković, A.; Smičiklas, I. Exploring innovative solutions for aged concrete utilization: Treatment of liquid radioactive waste. Clean Technol. Environ. Policy 2018, 20, 1343–1354. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Mirković, M.; Smičiklas, I. The applicability of construction and demolition waste components for radionuclide sorption. J. Clean. Prod. 2018, 171, 322–332. [Google Scholar] [CrossRef]
- Shin, W.-S.; Kim, Y.-K. Removal characteristics of heavy metals from aqueous solution by recycled aggregate and recycled aggregate/steel slag composites as industrial byproducts. Appl. Chem. Eng. 2015, 26, 477–482. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Yang, Y.; Wan, N. Kinetic studies of phosphate adsorption onto construction solid waste (CSW). Water Qual. Res. J. Can. 2014, 49, 307–318. [Google Scholar] [CrossRef]
- Egemose, S.; Sønderup, M.J.; Beinthin, M.V.; Reitzel, K.; Hoffmann, C.C.; Flindt, M.R. Crushed concrete as a phosphate binding material: A potential new management tool. J. Environ. Qual. 2012, 41, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tang, J.; Zhang, S.; Wang, J.; Ding, X. Using recycled concrete as an adsorbent to remove phosphate from polluted water. J. Environ. Qual. 2019, 48, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, G.S.; Cazacliu, B.G.; Corread, C.R.; Ovsyannikovad, E.; Krused, A.; Sampaio, C.H.; Lima, E.C.; Dotto, G.L. Adsorption and recovery of phosphate from aqueous solution by the construction and demolition wastes sludge and its potential use as phosphate-based fertilizer. J. Environ. Chem. Eng. 2020, 8, 103605. [Google Scholar] [CrossRef]
- Kittnerová, J.; Drtinová, B.; Štamberg, K.; Vopálka, D.; Evans, N.; Deissmann, G.; Lange, S. Comparative study of radium and strontium behaviour in contact with cementitious materials. Appl. Geochem. 2020, 122, 104713. [Google Scholar] [CrossRef]
- Wieland, E.; Tits, J.; Kunz, D.; Dähn, R. Strontium uptake by cementitious materials. Environ. Sci. Technol. 2008, 42, 403–409. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Vincent, J.B. New Evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Moulin, I.; Rosea, J.; Stone, W.; Bottero, J.-Y.; Mosnier, F.; Haelmel, C. Lead, zinc and chromium (III) and (VI) speciation in hydrated cement phases. In Waste Materials in Construction; Woolley, G.R., Goumans, J.J.J.M., Wainwright, P.J., Eds.; Elsevier: Oxford, UK, 2000; Volume 1, pp. 269–280. [Google Scholar]
- Johnson, D.C.; Coleman, N.J.; Lane, J.; Hills, C.D.; Poole, A.B. A preliminary investigation of the removal of heavy metal species from aqueous media using crushed concrete fines. In Waste Materials in Construction; Woolley, G.R., Goumans, J.J.J.M., Wainwright, P.J., Eds.; Elsevier: Oxford, UK, 2000; Volume 1, pp. 1044–1049. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Colarusso, P.; Guo, B.; Zhang, K.-Q.; Bernath, P.F. High-resolution infrared emission spectrum of strontium monofluoride. J. Mol. Spectrosc. 1996, 175, 158–171. [Google Scholar] [CrossRef]
- Zamburlini, M.; Campbell, J.L.; de Silveira, G.; Butler, R.; Pejović-Milić, A.; Chettle, D.R. Strontium depth distribution in human bone measured by micro-PIXE. X-ray Spectrom. 2009, 38, 271–277. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements, 2nd ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Iwaida, T.; Nagasaki, S.; Tanaka, S. Sorption study of strontium onto hydrated cement phases using a sequential desorption method. Radiochim. Acta 2000, 88, 483–486. [Google Scholar] [CrossRef]
- Elmes, V.K.; Coleman, N.J. Interactions of Cd2+, Co2+ and MoO42− ions with crushed concrete fines. J. Compos. Sci. 2021, 5, 42. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Yarusova, S.B.; Gordienko, P.S.; Yudakov, A.A.; Azarova, Y.A.; Yashchuk, R.D. Kinetics of the sorption of heavy-metal ions by a sorbent obtained from boric acid production waste. Theor. Found Chem. Eng. 2016, 50, 841–845. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Zou, J.; Guo, C.; Zhou, X.; Sun, Y.; Yang, Z. Sorption capacity and mechanism of Cr3+ on tobermorite derived from fly ash acid residue and carbide slag. Colloids Surf. A 2018, 538, 825–833. [Google Scholar] [CrossRef]
- Niuniavaite, D.; Baltakys, K.; Dambrauskas, T.; Eisinas, A. Cu2+, Co2+ and Cr3+ adsorption by synthetic dibasic calcium silicate hydrates and their thermal stability in a 25–1000 °C temperature range. J. Therm. Anal. Calorim. 2019, 138, 2241–2249. [Google Scholar] [CrossRef]
- Ghorbel-Abid, I.; Jrad, A.; Nahdi, K.; Trabelsi-Ayadi, M. Sorption of chromium (III) from aqueous solution using bentonitic clay. Desalination 2009, 246, 595–604. [Google Scholar] [CrossRef]
- Flieger, J.; Kawka, J.; Płaziński, W.; Panek, R.; Madej, J. Sorption of heavy metal ions of chromium, manganese, selenium, nickel, cobalt, iron from aqueous acidic solutions in batch and dynamic conditions on natural and synthetic aluminosilicate sorbents. Materials 2020, 13, 5271. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.F.; Abd Ali, Z.T. Sustainable use of concrete demolition waste as reactive material in permeable barrier for remediation of groundwater: Batch and continuous study. J. Environ. Eng. 2020, 146, 04020048. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Šerović, R.; Smičiklas, I. Utilization of waste ceramics and roof tiles for radionuclide sorption. Process Saf. Environ. Protect. 2017, 105, 348–360. [Google Scholar] [CrossRef]
- Coleman, N.J.; Brassington, D.S.; Raza, A.; Mendham, A.P. Sorption of Co2+ and Sr2+ by waste-derived 11 Å tobermorite. Waste Manag. 2006, 26, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Wu, Y.-C. Highly efficient adsorption of Sr2+ and Co2+ ions by ambient prepared alkali activated metakaolin. Polymers 2022, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Rumble, J. (Ed.) CRC Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 5–189. ISBN 1138561630. [Google Scholar]
- Regmi, G.; Indraratna, B.; Nghiem, L.D.; Golab, A.; Prasad, B.G. Treatment of acid groundwater in acid sulphate soil terrain using recycled concrete: Column experiments. J. Environ. Eng. 2011, 137, 433–443. [Google Scholar] [CrossRef]
- Banasiak, L.; Indraratna, B.; Regmi, G.; Golab, A.; Lugg, G. Characterisation and assessment of recycled concrete aggregates used in a permeable reactive barrier for the treatment of acidic groundwater. Geomech. Geoengin. 2013, 8, 155–166. [Google Scholar] [CrossRef]
- Banasiak, L.B.; Indraratna, B.; Lugg, G.; Pathirage, U.; McIntosh, G.; Rendell, N. Permeable reactive barrier rejuvenation by alkaline wastewater. Environ. Geotech. 2015, 2, 45–55. [Google Scholar] [CrossRef]
- Medawela, S.; Indraratna, B.; Athuraliya, S.; Lugg, G.; Nghiem, L.D. Monitoring the performance of permeable reactive barriers constructed in acid sulfate soils. Eng. Geol. 2022, 296, 106465. [Google Scholar] [CrossRef]





| Constituent | Mass (kg) | Cement Content (%) |
|---|---|---|
| OPC | 28.32 | - |
| Water | 11.37 | - |
| 5 mm aggregate | 13.90 | - |
| 10 mm aggregate | 44.22 | - |
| 20 mm aggregate | 43.87 | - |
| Cement content of block | - | ~28 |
| Cement content of CCF | - | 50.1 ± 0.4 |
| Kinetic Parameter | Cr | Ni | Sr |
|---|---|---|---|
| Pseudo-first-order model | |||
| k1 (min−1) | 2.3 × 10−4 | 5.7 × 10−4 | 4.9 × 10−4 |
| qe calc. (mg g−1) | 32.7 | 37.3 | 4.14 |
| R2 | 0.991 | 0.998 | 0.945 |
| Pseudo-second-order model | |||
| k2 (g mg−1 min−1) | 1.0 × 10−5 | 1.4 × 10−5 | 3.1 × 10−4 |
| qe calc. (mg g−1) | 40.3 | 46.7 | 7.36 |
| R2 | 0.877 | 0.996 | 0.999 |
| Property | Control | Cr | Ni | Sr |
|---|---|---|---|---|
| Metal ion removal (mg g−1) | - | 33.9 ± 1.2 | 35.8 ± 1.1 | 7.16 ± 0.31 |
| Calcium dissolution (mg g−1) | 7.42 ± 0.32 | 42.8 ± 1.7 | 29.2 ± 0.9 | 12.1 ± 0.5 |
| Aluminum dissolution (mg g−1) | 0.13 ± 0.01 | b.d.l. | b.d.l. | 0.13 ± 0.01 |
| Silicon dissolution (mg g−1) | 0.18 ± 0.02 | 0.08 ± 0.03 | 0.03 ± 0.02 | 0.14 ± 0.02 |
| Structure | Relative Elemental Composition (Moles per Mole of Al) 1 | |||
|---|---|---|---|---|
| Al | Si | Ca | Metal Ion | |
| CCF-Cr | ||||
| Platy deposits | 1.00 ± 0.45 | 4.07 ± 2.33 | 33.8 ± 3.2 | 117 ± 10 |
| Rhomboids | 1.00 ± 0.25 | 3.35 ± 0.67 | 201 ± 19 | 24.0 ± 8.5 |
| CCF-Ni | ||||
| Colloform network | 1.00 ± 0.37 | 2.48 ± 0.65 | 1.38 ± 0.37 | 28.7 ± 0.76 |
| Cubes | 1.00 ± 0.47 | 1.40 ± 0.26 | 239 ± 9.8 | 9.45 ± 3.1 |
| CCF-Sr | ||||
| Spear-headed fronds | 1.00 ± 0.31 | 4.79 ± 0.72 | 168 ± 3.9 | 22.2 ± 1.8 |
| Sorbent | 1 Ci Range (ppm) | Solid:Liquid Ratio (mg cm−3) | 2 qm (mg g−1) | 3 teq (min) | Ref |
|---|---|---|---|---|---|
| Chromium, Cr3+ | |||||
| Laboratory CCF | 850 | 25 | 33.9 | >7200 | This study |
| Geopolymer | 50–300 | 0.001–0.02 | 19.9 | 4320 | [41] |
| Waste-derived tobermorite | 520–2600 | 7 | 253 | 360 | [42] |
| Calcium silicate hydrate | 1000 | 10 | 100 | 300 | [43] |
| Bentonitic clay | 10–300 | 0.5–6 | 117.5 | 60 | [44] |
| Fly ash-derived zeolite A | 100 | 25 | 4.0 | 30 | [45] |
| Nickel, Ni2+ | |||||
| Laboratory CCF | 900 | 25 | 35.8 | 7200 | This study |
| Demolition CCF | 5.87–470 | 5 | 31.7 | 1440 | [19] |
| Demolition CCF | 100–750 | 50–3000 | 13.6 | 300 | [46] |
| NaOH-pretreated CCF | 10 | 1 | 0.531 | 360 | [9] |
| Waste-derived calcium silicate | 200 | 2.5 | 79.2 | 180 | [39] |
| Waste ceramic tiles | 5.87–470 | 5 | 7.04 | 3000 | [47] |
| Strontium, Sr2+ | |||||
| Laboratory CCF | 991 | 25 | 7.16 | 4320 | This study |
| Laboratory CCF | 0–30.7 | 0.0017–0.1 | 0.675 | 5760 | [25] |
| Demolition CCF | 8.76–701 | 5 | 21.9 | 1440 | [19] |
| Waste-derived tobermorite | 0–100 | 50 | 1.52 | 7200 | [48] |
| Waste ceramic tiles | 8.76–701 | 5 | 3.07 | 1500 | [47] |
| Alkali-activated metakaolin | 10–3000 | 10 | 180 | 240 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurt, A.P.; Coleman, A.A.; Coleman, N.J. Interactions of Cr3+, Ni2+, and Sr2+ with Crushed Concrete Fines. Crystals 2022, 12, 717. https://doi.org/10.3390/cryst12050717
Hurt AP, Coleman AA, Coleman NJ. Interactions of Cr3+, Ni2+, and Sr2+ with Crushed Concrete Fines. Crystals. 2022; 12(5):717. https://doi.org/10.3390/cryst12050717
Chicago/Turabian StyleHurt, Andrew P., Aimee A. Coleman, and Nichola J. Coleman. 2022. "Interactions of Cr3+, Ni2+, and Sr2+ with Crushed Concrete Fines" Crystals 12, no. 5: 717. https://doi.org/10.3390/cryst12050717
APA StyleHurt, A. P., Coleman, A. A., & Coleman, N. J. (2022). Interactions of Cr3+, Ni2+, and Sr2+ with Crushed Concrete Fines. Crystals, 12(5), 717. https://doi.org/10.3390/cryst12050717







