Abstract
Novel triarylazoimidazoles containing strong electron donors (p-NEt2) or acceptors (p-NO2) by the azoaryl group, and their group 12 metal complexes were synthesized and fully characterized, including X-ray analysis for several complexes. Novel complexes exhibit red photo-luminescence emission (Φ up to 0.21) in a solution. Moreover, the antibacterial activity of complexes was tested against Gram-positive microorganism S. aureus and Gram-negative microorganism E. coli.
Keywords:
azo dyes; nitrogen heterocycles; fluorescence; photoswitchable materials; Zn; Cd; Hg complexes; azoimidazoles 1. Introduction
Imidazole function is an important group in biology, often playing a role as a supporting ligand in metal-containing systems [1,2,3]. This stimulates the synthesis and structural characterization of imidazole metal complexes to better understand the role of these ligands. Azoimidazoles constitute an important class of photoactive materials, which were intensively studied earlier [4,5,6,7,8,9]. In addition, they are interesting chelating ligands, which electronics could be easily modulated via facile structural tuning [6,8,10]. Recently we started exploring triarylazoimidazoles and their coordination compounds [2,11,12]. Surprisingly, triarylazoimidazoles received almost no attention in the literature. In our recent publication, we showed that coordination of some triarylazoimidazoles to ZnII, CdII, and HgII led to the formation of bright red emissive complexes (quantum yields up to 44%), which could be excited with a visible light [2]. Here, we report the synthesis and structural characterization of several novel triarylazoimidazoles and their emissive group 12 metal complexes (Φ up to 0.21). Introduction of the p-bromo substituent to the azoaryl substituent of the Zn-coordinated triarylazoimidazole shifts an emission maximum to 677 nm relatively to the unsubstituted triarylazoimidazole complex. In addition, we report antibacterial properties of novel complexes.
2. Materials and Methods
General remarks. Unless stated otherwise, all the reagents used in this study were obtained from the commercial sources (Aldrich (Schnelldorf, Germany), TCI-Europe (Zwijndrecht, Belgium), Strem (Bischheim, France), and ABCR (Karlsruhe, Germany)). NMR spectra were recorded on a Bruker Avance III (Karlsruhe, Germany) (1H: 400 MHz); chemical shifts (δ) are given in ppm relative to TMS, coupling constants (J) in Hz. The solvent signals were used as references (CDCl3: δC = 77.16 ppm; residual CHCl3 in CDCl3: δH = 7.26 ppm; CD2Cl2: δC = 53.84 ppm; residual CHDCl2 in CD2Cl2: δH = 5.32 ppm); 1H and 13C assignments were established using NOESY, HSQC, and HMBC experiments; numbering schemes as shown in the inserts. C, H, and N elemental analyses were carried out on a Euro EA 3028HT CHNS/O analyzer (Pavia, Italy). Mass-spectra were obtained on a Bruker micrOTOF spectrometer equipped with the electrospray ionization (ESI) source (Bremen, Germany); MeOH, CH2Cl2, or MeOH/CH2Cl2 mixture was used as a solvent. Solvents were purified by distillation over the indicated drying agents and were transferred under Ar: Et2O (Mg/anthracene), CH2Cl2 (CaH2), hexane (Na/K). Flash chromatography: Merck Geduran® Si 60 (40–63 μm). Absorption spectra were measured in a 3.8 mL quartz cuvette using a UV-VIS spectrometer (UV-3600, Shimadzu, Kyoto, Japan) and photoluminescence emission and excitation spectra in the same cuvette, using a spectrofluorometer (RF-5031PC, Shimadzu). The luminescence quantum yield was determined using the slope method relative to the reference fluorophore, which the ethanol solution of Nile Blue dye (Φ = 0.27) excited at 590–625 nm, using a series of ethanol solutions of the sample with varying concentrations.
Synthesis of 9. Methanol solution of NaOMe (30 wt %, 1.9 mmol, 356 µL) and a solution of p-diethylaminophenyldiazonium tetrafluoroborate (1.9 mmol, 500.0 mg) in 2 mL MeCN were sequentially added to a solution of diphenylimidazole 1 (1.9 mmol, 418.7 mg) in EtOH (10 mL). The resulting mixture was stirred for 1 h, evaporated, redissolved in CH2Cl2, filtered, evaporated again, washed with Et2O (3 × 3 mL), and dried under vacuum. Yield: 674.2 mg (90%) Elem. anal. calcd. for C25H25N5: C, 75.92; H, 6.37; N, 17.71. Found: C 76.13; H 6.54; N 17.58. 1H NMR (400 MHz, CDCl3): δ 9.74 (s, br, 1H), 7.90 (d, J = 9.1 Hz, 2H), 7.64 (m, 4 H), 7.38–7.31 (m, 6 H), 6.71 (d, J = 9.1 Hz, 2H), 3.47 (q, J = 7.0 Hz, 4H), 1.24 (t, J = 7.0 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 211.8, 154.6, 150.9, 142.8, 135.5, 128.7, 128.5, 128.0, 126.2, 111.3, 44.9, 12.8. UV/Vis (CH2Cl2): λmax = 493 nm, ε = 3.83 × 104 M−1 cm−1.
Synthesis of 10. Methanol solution (2 mL) of potassium tert-butoxide (189.4 mg, 1.69 mmol) and a solution of p-nitrophenyldiazonium tetrafluoroborate (1.69 mmol, 401.7 mg) in MeCN (10 mL) were sequentially added to a solution of di-p-methoxyphenylimidazole (1.69 mmol, 473.3 mg) in MeOH (20 mL). The resulting mixture was stirred for 1 h, evaporated, redissolved in CH2Cl2, filtered, evaporated again, washed with Et2O (3 × 3 mL), and dried under vacuum. Yield: 374.1 mg (52%) Elem. anal. calcd. for C23H19N5O4: C, 64.33; H, 4.46; N, 16.31. Found: C 64.58; H 4.69; N 16.22. 1H NMR (400 MHz, CDCl3): δ 10.05 (s, br, 1H), 8.35 (d, J = 9.0 Hz, 2H), 8.01 (d, J = 9.0 Hz, 2H), 7.59 (m, 4H), 6.93 (d, J = 8.0 Hz, 4H), 3.85 (s, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 160.1, 159.4, 155.5, 153.9, 148.2, 129.7, 129.3, 124.9, 123.0, 114.3, 55.4. UV/Vis (CH2Cl2): λmax = 512 nm, ε = 1.92 × 104 M−1 cm−1.
Synthesis of 11. Methanol solution (2 mL) of potassium t-butoxide (165.6 mg, 1.49 mmol) and a solution of p-bromophenyldiazonium tetrafluoroborate (1.49 mmol, 403.5 mg) in MeCN (10 mL) were sequentially added to a solution of di-p-methoxyphenylimidazole (1.49 mmol, 413.5 mg) in MeOH (20 mL). The resulting mixture stirred for 1 h, evaporated, redissolved in CH2Cl2, filtered, evaporated again, washed with Et2O (3 × 3 mL), and dried under vacuum. Yield: 374.1 mg (62%). Elem. anal. calcd. for C23H19N5O4: C 49.14; H 3.37; N 10.42. Found: C 49.56; H 3.74; N 10.11. 1H NMR (400 MHz, CDCl3): δ 9.86 (s, br, 1H), 7.82 (d, J = 8.8 Hz, 2H), 7.63 (d, J = 8.8 Hz, 4H), 7.46 (m, 2H), 6.91 (m, 4H), 3.84 (s, 6H). 13C{1H} NMR (151 MHz, CD3CN): δ 159.5, 158.3, 154.0, 143.0, 132.8, 130.2, 130.0, 124.3, 114.5, 55.9. UV/Vis (CH2Cl2): λmax = 483 nm, ε = 1.86 × 104 M−1 cm−1.
General procedure for the synthesis of 7Zn–11Zn, 7Cd–11Cd, and 7Hg–11Hg. Methanol solution of the ligand 9–11 (2 mL) was added to a solution of an appropriate metal halide (1 eq.) in 1 mL MeOH. The resulting mixture was kept without stirring for 24 h, the formed precipitate was filtered, washed with MeOH (3 × 1 mL), Et2O (3 × 3 mL), and dried under vacuum.
9Zn. 9 (1 eq, 0.13 mmol, 50.4 mg), ZnCl2 (1 eq., 0.13 mmol, 17.8 mg) were used. Yield: 32.6 mg (48.2%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C25H25Cl2N5Zn: C, 56.46; H, 4.74; N, 13.17. Found: C 56.75; H 7.86; N 13.05. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture. UV/Vis (CH2Cl2): λmax = 614 nm, ε = 3.19 × 104 M−1 cm−1.
9Cd. 9 (1 eq, 0.13 mmol, 50.2 mg), CdBr2 4H2O (1 eq., 0.13 mmol, 44.0 mg) were used. Yield: 46.4 mg (54.9%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C25H25Br2N5Cd: C, 44.97; H, 3.77; N, 10.49. Found: C 45.14; H 3.98; N 10.38. UV/Vis (CH2Cl2): λmax = 599 nm, ε = 3.03 × 104 M−1 cm−1.
9Hg. 9 (1 eq, 0.13 mmol, 50.0 mg), HgCl2 (1 eq., 0.13 mmol, 35.1 mg) were used. Yield: 46.0 mg (54.5%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C25H25Cl2N5Hg: C, 45.02; H, 3.78; N, 10.50. Found: C 45.31; H 3.87; N 10.46. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture. UV/Vis (CH2Cl2): λmax = 517 nm, ε = 3.13 × 104 M−1 cm−1.
10Zn. 10 (2 eq., 0.17 mmol, 75.0 mg), ZnCl2 (1 eq., 0.09 mmol, 12.2 mg) were used. Yield: 48.4 mg (55.5%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19Cl2N5O4Zn: C, 48.83; H, 3.39; N, 12.38. Found: C 49.11; H 3.56; N 12.29. UV/Vis (CH2Cl2): λmax = 516 nm, ε = 7.70 × 104 M−1 cm−1.
10Cd. 10 (2 eq., 0.17 mmol, 74.9 mg), CdBr2 4H2O (1 eq., 0.09 mmol, 30.6 mg) were used. Yield: 66.6 mg (67.2%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19Br2N5O4Cd: C, 39.37; H, 2.73; N, 9.98. Found: C 39.71; H 2.94; N 10.05. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture. UV/Vis (CH2Cl2): λmax = 516 nm, ε = 6.67 × 104 M−1 cm−1.
10Hg. 7 (2 eq., 0.17 mmol, 74.9 mg), HgCl2 (1 eq., 0.09 mmol, 23.4 mg) were used. Yield: 45.0 mg (45.8%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19Cl2N5O4Hg: C, 39.41; H, 2.73; N, 9.99. Found: C 39.45; H 2.78; N 10.07. UV/Vis (CH2Cl2): λmax = 517 nm, ε = 6.31 × 104 M−1 cm−1.
11Zn. 11 (1 eq., 0.18 mmol, 50.3 mg), ZnCl2 (1 eq., 0.18 mmol, 14.9 mg) were used. Yield: 26.3 mg (40.5%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19BrCl2ZnN4O2: C, 46.07; H, 3.19; N, 9.34. Found: C 45.87; H 3.08; N 9.36. UV/Vis (CH2Cl2): λmax = 527 nm, ε = 2.14 × 104 M−1 cm−1.
11Cd. 11 (1 eq., 0.11 mmol, 50.2 mg), CdBr2⸱4H2O (1 eq., 0.11 mmol, 37.2 mg) were used. Yield: 31.4 mg (39.7%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19Br3CdN4O2: C, 37.56; H, 2.60; N, 7.62. Found: C 37.64; H 2.66; N 7.81. UV/Vis (CH2Cl2): λmax = 483 nm, ε = 1.03 × 104 M−1 cm 1.
11Hg. 11 (1 eq., 0.11 mmol, 50.0. mg), HgCl2 (1 eq., 0.11 mmol, 29.9 mg) were used. Yield: 33.2 mg (41.62%). 1H and 13C NMR spectra were not obtained due to the low solubility of the compound in the common deuterated solvents. Elem. anal. calcd. for C23H19BrCl2HgN4O2: C, 37.59; H, 2.61; N, 7.62. Found: C 37.81; H 2.54; N 7.58. UV/Vis (CH2Cl2): λmax = 503 nm, ε = 2.24 × 104 M−1 cm 1.
2.1. X-ray Diffraction Studies
XRD data for 9Hg’·2CH3OH, 8·solvate and 10Cd·1.75CH3OH·0.75H2O were collected using equipment of the Center for Molecular Studies of the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences. The X-ray diffraction studies for 9Zn·CH3OH, 9Hg·CH3OH and 10 were performed at the Shared Equipment Center of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences. The X-ray measurements were performed using shared experimental facilities supported by INEOS RAS and IGIC RAS state assignment.
Single crystals of 9Zn·CH3OH, 9Hg·CH3OH, 9Hg’·2CH3OH, 8·solvate, 10 and 10Cd·1.75CH3OH·0.75H2O were obtained from reaction mixtures. Intensities of the reflections for these crystals were collected with Bruker D8 Venture (9Zn·CH3OH, 9Hg·CH3OH, 10) and Bruker Quest (9Hg·2CH3OH, 8·solvate and 10Cd·1.75CH3OH·0.75H2O) diffractometers (Bruker AXS, Inc., Madison, WI, USA) at 100.0(2) K (MoKα-radiation, λ = 0.71073 Å). The structures were solved by the SHELXT method [13] and refined by full-matrix least squares against F2. Non-hydrogen atoms were refined anisotropically. H(N) and H(O) atoms were located on difference Fourier maps, and those of H(C) atoms were calculated. The unit cell of 8·solvate contains two solvent molecules (each occupies 186 Å3 and has 50 counts of electrons), which were treated as a diffuse contribution to the overall scattering without specific atom positions by the SQUEEZE/PLATON procedure [14]. A similar procedure was applied to treat some of the highly disordered solvent molecules in 10Cd; two voids with volumes 624 and 199 Å3 and 399 and 106 counts of electrons were found. All hydrogen atoms were included in a refinement by the riding model with Uiso(H) = 1.5Ueq(X) for methyl and hydroxy groups and 1.2Ueq(X) for the other atoms. All calculations were made using the SHELXL2014 [15] and OLEX2 [16] program packages. Crystallographic parameters and refinement details for all complexes are listed in Table S1. CCDC 2166661–2166666 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/structures/ (accessed on 10 April 2022).
2.2. Computational Details
The DFT calculations based on the experimental X-ray geometries of 9Hg, 9Hg’, 9Zn, and 10Cd were carried out with the help of the Gaussian-09 [17] program package. The topological analysis of the electron density distribution was performed by using the Multiwfn program (version 3.7) [18]. The Cartesian atomic coordinates for model supramolecular associates are presented in the attached xyz-files, Supplementary Materials.
3. Results and Discussion
3.1. Synthesis and Structural Characterization
Novel triarylazoimidazoles 9–11 were readily synthesized by the coupling between the corresponding diazonium salts 4–6 and diarylimidazoles 1 or 2 (Scheme 1). The synthesis of similar triarylazoimidazoles 7 and 8 we reported earlier [2,12].
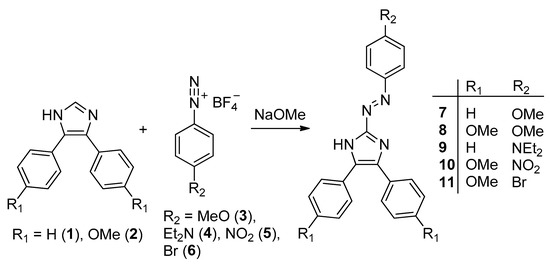
Scheme 1.
Synthesis of triarylazoimidazoles 7–11.
Triarylazoimidazoles 7–11 appear as bright red solids, 7, 8, 10, and 11 [11] could be recrystallized from saturated MeOH solutions to produce crystals, suitable for analysis by single crystal X-ray crystallography (Figure 1). In addition, we obtained single crystals of 8 (Figure 1), which synthesis we reported earlier [2,12].
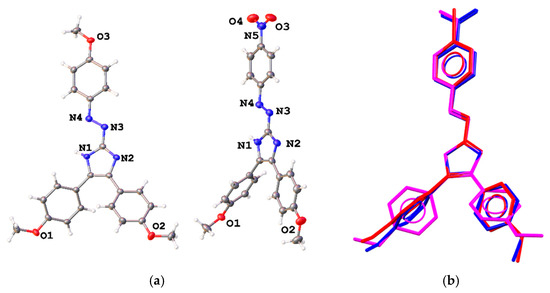
Figure 1.
(a) Molecular structures of 8 and 10 in representation of atoms with thermal ellipsoids (given at p = 50%) and (b) comparison of their conformations with that of 11. Solvent molecules are omitted. Overlaid atoms of 8 (magenta), 10 (blue), and 11 (red) belong to the imidazole ring.
Data about molecular and crystal structures of pure triarylazoimidazoles are limited with those for 2-(p-bromophenyl)diazen-1-yl-containing imidazole 11 [11]. Herein we compare its geometry with that of p-methoxy- and p-nitrosubstituted analogs. The main geometrical parameters of these molecules are listed in Table 1. Bond distances are very close for these molecules (Figure 1, Table 1). All molecules adopt a trans conformation with respect to the double N3=N4 bond. The azo double bond is elongated as compared with that in azobenzene, and the imidazole ring is nearly coplanar with the aryl group attached to the azo group (Figure 1). This fact indicates a prominent electron delocalization within the molecule. The two other substituents at carbon atoms of the five-membered ring can freely rotate along single C–C bonds, visualized in Figure 1.

Table 1.
Selected geometrical parameters (Å, °) for azoimidazoles 7–11 and their group 12 metal complexes.
Subsequent addition of group 12 metal chlorides to 7–11 resulted in the formation of chelates 7Zn–11Zn, 7Cd–11Cd, and 7Hg–11Hg (Scheme 2), which precipitated from the reaction mixtures as dark purple microcrystalline solids. The crystals of complexes 9Zn, 9Hg, and 10Cd were suitable for analysis by single crystal X-ray crystallography. The structural investigations proved the formation of azoimidazole chelate complexes (Figure 2). The Cd complex 10Cd, which contained p-NO2 substituent by the azoaryl group, contained two chelating azoimidazoles in contrast to 9Zn and 9Hg.
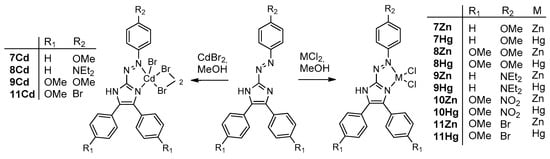
Scheme 2.
Synthesis of triarylazoimidazole complexes 7Zn–11Zn, 7Cd–11Cd, and 7Hg–11Hg.
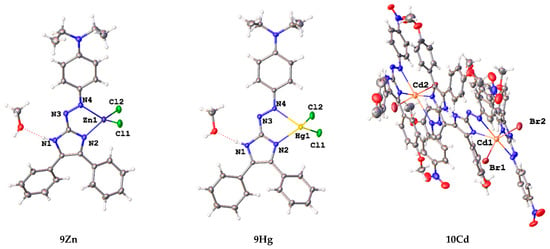
Figure 2.
Asymmetric units of 9Zn, 9Hg, and 10Cd in representation of atoms with thermal ellipsoids (given at p = 50%). Solvent molecules are omitted for 10Cd.
Metal atoms in 9Zn and 9Hg complexes coordinate one azaimidazole ligand in a bidentate-chelate mode, and two terminal chlorine atoms to form a distorted tetrahedral MN2Cl2 coordination polyhedron. Asymmetric unit of 10Cd contains two independent complexes. Their conformations are compared in Figure S2; they differ mainly in disposition of phenylmethoxy and nitrophenyl groups in respect to azoaimidazole fragments. In 10Cd, the large Cd2+ atom binds two chelating aza ligands and two terminal bromine atoms to form an octahedral CdN4Br2 coordination polyhedron with the nitrogen atoms of the azo group being in axial positions. Lengths of M–Cl, M–N1, and M–N4 bonds increase from Zn2+ to Hg2+ and to Cd2+ and N-M–N chelate angles decrease in the same raw (Table 1). Delocalization within the imidazole ring at coordination becomes less pronounced as it follows from elongation of single and shortening of double bonds; however, coordination of the azoaryl moiety results in elongation of the N=N bond and shortening of the N4–Ar and C1–-N3 bonds. For all three complexes, all azoimidazoles retain upon coordination the trans configuration at diazo groups, and nearly planar configuration of the azoimidazole moieties. However, the N4 atom of the azo bond is situated on the same side as the N2 atom of the imidazole ring in respect to the C1–N3 bond, while for neutral ligands, it is always situated on the same side as the N1(H) atom of the ring. Interestingly, the N atom of the diethylamino group is also almost planar with CNC plane being coplanar with the plane of the azoimidazole moiety, which indicates the significant electronic conjugation in the whole system.
Overall, molecular geometry of triarylazoimidazoles in 5, 7–10 is similar to those reported for structurally relevant azo-compounds [5,9,19,20,21,22,23,24,25] and imidazole derivatives [26,27].
Interestingly, the reaction of 9 with HgCl2 resulted in the formation of a very small batch of crystals of 9Hg’ (Figure 3 and Figure S1) along with the main product 9Hg. Although we were not able to reproduce the synthesis of 9Hg’, here we give a description of the crystal structure of 9Hg’ since it is a remarkable one-dimensional coordination polymer. In this complex, chlorine atoms act as terminal ligands, and deprotonated azaimidazole 9 acts as a tridentate bridging and chelating ligand, which coordinates by both N atoms of the imidazole ring and the N4 atom of the azo group. Coordination bonds for the resulting tetrahedral HgN2Cl2 coordination polyhedron are very close to the 9Hg, while delocalization within the five-membered ring becomes even more pronounced than in the neutral uncoordinated ligand (Table 1). Overall complex is a chain coordination polymer parallel with crystallographic axis c (Figure 3).
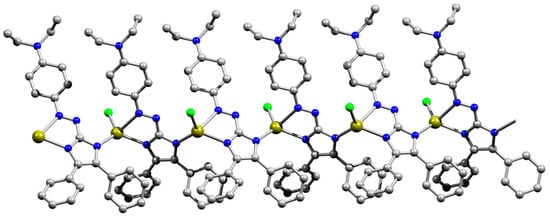
Figure 3.
Ball-and-stick representation of the coordination polymer 9Hg’ in the crystal. Solvent molecules and hydrogen atoms are omitted. Color code: C–grey, Cl–green, H–light grey, Hg–olive green, N–blue, O–red.
Presence of acidic hydrogen atoms in 8, 10, 9Zn, 9Hg, and 10Cd as well as a number of solvent water and MeOH molecules in solid 9Zn, 9Hg, 9Hg’, and 10Cd allows formation of strong H-bonds in these solids. Presence of some of these hydrogen bonds in metal complexes was confirmed using DFT calculations of isolate clusters followed by analysis of electron density distribution within the QTAIM approach (see Section 3.4) [28]. Herein we should note that in pure ligand 10 H-bonded dimers (see Figure S3) connected by two N-H…O bonds with a methoxy group were found (r(N…O) = 2.921(3)Å, NHO = 165.7°), while in 8, one can propose that H(N) atom should be involved in H-bonding with acceptor groups of solvent molecules removed from refinement using the SQUEEZE procedure. In isostructural 9Zn and 9Hg H-bonded dimers (Figure S3) can be found due to N-H…O and O-H…Cl interactions with a hydroxy group of solvent molecule (r(N…O) = 2.747(3)–2.750(3)Å, NHO = 165.6–167.5°; r(O…Cl) = 3.104(3)–3.129(3) Å, OHCl = 163.1–165.0°). In 9Hg’, the amino group is absent, but methanol molecules are connected to the coordination polymer through O-H…O and O-H…Cl interactions (Figure S3; r(O…O) = 2.716(16) Å, OHO = 130.3°; r(O…Cl) = 3.124(10) Å, OHCl = 162.6°). In 10Cd, one of the amino groups interacts with a bromine atom of a neighboring complex, two others with methanol molecules, and the fourth one with a water molecule. Solvent molecules in 10Cd also take part in O-H…O and O-H…Br bonding. Besides a strong hydrogen bond, C-H…Hal and C-H…O interactions can be found in solid 9Zn, 9Hg, 9Hg’, and 10Cd. Planar configuration of the ligand also allows realization of hydrophobic C–H…π and stacking interactions in these compounds. Particularly, in H-bonded dimers in 9Zn and 9Hg the mean plains of ligands are situated parallel at distances 3.298(3) and 3.308(2) Å, respectively. Thus, geometry of these complexes allows realization of both hydrophilic and hydrophobic interactions in solids and ligand–receptor complexes.
3.2. Absorption and Emission Profiles
Ligands 7–10 exhibited a red color in the dichloromethane solution and the main absorption band with the absorption maximum close to 500 nm and additional absorption bands in the UV range (Figure 4, Table 2).
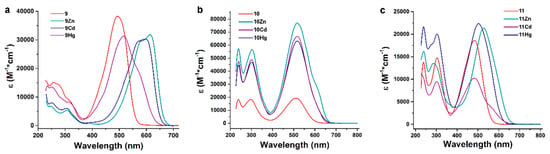
Figure 4.
UV-Vis absorptions spectra of the ligand 9 and its complexes (a), ligand 10 and its complexes (b), ligand 11 and its complexes (c) in dichloromethane.

Table 2.
UV-Vis absorption characteristics of the ligands 9–11 in dichloromethane solution.
Arylazoimidazole compounds have the absorption maximum in the near UV range around 360 nm corresponding to intraligand π–π* charge transfer (ILCT) transitions [29,30], the bathochromic shift of this maximum to 480–510 nm in ligands 9–11 can be partly attributed to electron delocalization effect due to the presence of two additional aromatic rings. Position and intensity of the ILCT absorption band is also affected by electron-donating and electron-withdrawing groups at the para-position of the aryl rings. In ligands 7 and 9, the electron density shifts upon photoexcitation from the electron-donating OMe (Et2N) group to the imidazole ring (electron acceptor). Correspondingly, in agreement with the previous findings [31] a stronger electron donor in the R1 position gives 9 a higher absorption maximum (493 vs. 441 nm) and a larger extinction coefficient (38,300 vs. 28,300 M−1 cm−1) compared to 7 [2]. Planar geometry of the arylazoimidazole fragment facilitates ILCT, thus producing large values of the extinction coefficients of 7 and 9. In 8 and 10–11, electron-donating methoxy groups at the R2 position induce additional charge transfer from the aryl groups attached to the imidazole ring. This effect is further strengthened by the electron-withdrawing Br and NO2 groups at the R1 position, resulting in increase of absorption maximum from 466 (8) to 483 (11) and 512 nm (10). Lack of planarity between imidazole-attached aryl rings and imidazole hinders intraligand charge transfer and results in smaller values of extinction coefficients of the 8, 10, and 11 ligands compared with 7 and 9. Extinction coefficients of 9–11 ligands corresponded to 22–28,000 M−1 cm−1 values reported for similar arylazoimidazole and triarylazoimidazole compounds [2].
The ILCT absorption bands of the ligands 9 and 11 exhibited a bathochromic shift, which was especially strong in case of Zn and Cd complexes of 9 giving them intensive blue colors (Figure 4, Table 2). The same coordination-induced spectral shift was previously discovered for the complexes of 7 and 8 [2]. This shift is attributed to the electron-withdrawing effect of metal ions coordinated to the azo group and imidazole ring and depends on the LUMO at the N1 and N4 nitrogen atoms. Thus, the strongest coordination-induced absorption shift was observed for complexes of 9 (from 493 to ca. 590 nm in 9Zn), where electron density in the excited state was localized at the imidazole ring as a result of charge transfer induced by the Et2N substituent. At the same time, in ligand 10, the nitro group itself exerted a strong electron-withdrawing effect via the aromatic ring and attracted electron density from the azo and imidazole groups; as a result the bathochromic shift of the absorption maximum induced by coordination was small (from 512 to 516 nm). The complexes of 11 with a weakly electron-withdrawing Br atom at the R1 position exhibited an intermediate shift (from 483 to 527 nm). Coordination-induced bathochromic shift of the ILCT band also depended on the metal ion and followed the trend λmaxILCT(Hg) < λmaxILCT(Cd) < λmaxILCT(Zn) [2]. Additional absorption bands appeared in the UV-Vis spectra of complexes in the spectral region between 580 and 620 nm and is attributed to metal-to-ligand charge transfer (MLCT) transition. In case of complexes 9Zn and 9Cd, this coordination-induced MLCT band was even stronger than the ILCT band at 550–560 nm, for others it appeared as a shoulder on the absorption spectrum.
Whereas ligands 9–11 were nonluminescent, all complexes exhibited red photoluminescence emission in dichloromethane solutions with emission maximum between 650 and 680 nm (Figure 4, Table 3), similar orange or red photoluminescence with emission maximum between 600 and 660 nm was previously observed for the metal complexes of 7 and 8 [2].

Table 3.
UV-Vis absorption and photoluminescence characteristics of the complexes 9Zn–11Zn, 9Cd–11Cd, and 9Hg–11Hg in dichloromethane solution.
Excitation and emission maximum wavelengths followed the general trend λex(em) (Hg) < λex(em) (Cd) < λex(em) (Zn), and the luminescence quantum yields–Φ (Hg) < Φ (Cd) < Φ (Zn) in accordance with the trend found for metal complexes of 7 and 8 [2]. Luminescence excitation maxima coincided with the positions of MLCT absorption bands on UV-Vis spectra of complexes, which confirms that emission is excited via coordination-related MLCT transitions. R1 and R2 substituents in 9–11 strongly affected the luminescence quantum yield (Table 3). The maximal quantum yield (21% for 11Zn) is smaller than the yield of 7 and 8 metal complexes (up to 44%) [2] but was still significant.
3.3. Antibacterial Tests
The in vitro antibacterial activity of the 7–11 against Gram-positive microorganism S. aureus and Gram-negative microorganism E. coli was estimated using the agar-well diffusion method. The antibacterial properties of 7–11 (Table 4) were compared with those for the commercially available conventional antibiotics ampicillin and gentamicin. The antibacterial activities of the mentioned organic compounds increase in the row 7 < 8~10 < 11 < 9, where 9 is the undoubted leader (see the corresponding inhibition zones and, especially, MIC values). We assume that the antibacterial pharmacophore is an azaimidazole moiety, which can be highly protonated at physiological pH. The resulting cation interacts with the anionic fragments of bacterial cell surface, which causes an osmotic imbalance, damages of the bacterial cell membrane, and leakage of the cell content into the external environment. The cascade of these unfavorable events for the bacterial cell inevitably leads to the death of the bacterium. The intracellular components of bacteria are characterized by strong absorption at 260 nm. This fact was used in previous studies to monitor the integrity of the bacterial cell membrane by spectrophotometry. We used this approach within the frames of the current work to study the effect of 7–11 on the integrity of the cell membranes of S. aureus and E. coli. We found that treatment of the bacterial suspension with 7–11 caused a dramatic increase in optical density from 0.5 to 0.9 in 6 min (9), 10 min (8, 10, 11), or 20 min (7). Thus, the tested organic compounds effectively damaged the bacterial cell membrane, but the effect of 9 most rapidly developed. The highest antibacterial effect of 9 can be explained by the presence of a larger number of N-atoms that can be protonated at a physiological pH value.

Table 4.
The in-vitro antibacterial activity of the ligands 7–11 and their complexes against Gram-positive microorganism S. aureus and Gram-negative microorganism E. coli.
The antibacterial activity of Zn(II), Cd(II), and Hg(II) complexes derived from 7–11 is substantially higher than that of the staring 7–11 (Table 1). Provided the same metal center, the complexes derived from 9 are characterized by the highest antibacterial effect, while the complexes derived from 7 are the least active. Moreover, provided the same ligand, the antibacterial activity of complexes increases in the rows Zn(II), Cd(II), Hg(II), and this corresponds to the previously reported data about antibacterial activity of Zn(II), Cd(II), and Hg(II) complexes with glibenclamide or 2,6-bis(benzimidazol-2-yl) pyridine ligands [32,33]. Thus, the most effective antibacterial complex proved to be 9Hg. The antibacterial activity of this complex is almost equal to that of the reference antibiotics ampicillin and gentamicin.
3.4. Theoretical Studies of Intermolecular Hydrogen Bonding Interactions N–H···O, O–H···Cl, and N–H···Br in 9Hg, 9Hg’, 9Zn, and 10Cd
In order to confirm or disprove the hypothesis on the existence of various most interesting intermolecular hydrogen bonding interactions N–H···O, O–H···Cl, and N–H···Br in 9Hg, 9Hg’, 9Zn, and 10Cd, and approximately quantify the strength of these supramolecular contacts from a theoretical viewpoint, the DFT calculations followed by the topological analysis of the electron density distribution within the QTAIM approach [28] were carried out at the ωB97XD/SDD level of theory for model supramolecular associates (see computational details and attached xyz-files in Supplementary Materials). Results of QTAIM analysis summarized in Table 5, the contour line diagrams of the Laplacian of electron density distribution ∇2ρ(r), bond paths, and selected zero-flux surfaces, visualization of electron localization function (ELF), and reduced density gradient (RDG) analyses for the selected intermolecular hydrogen bonding interactions N–H···O, O–H···Cl, and N–H···Br in 9Hg and 10Cd are shown in Figure 5 for illustrative purposes.

Table 5.
Characteristics of H-bonds in 9Hg, 9Hg’, 9Zn, and 10Cd *.
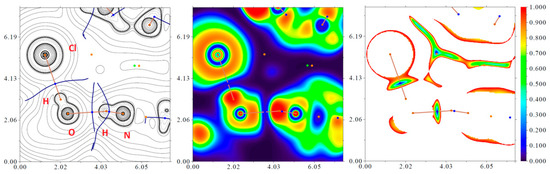
Figure 5.
Contour line diagram of the Laplacian of electron density distribution ∇2ρ(r), bond paths, and selected zero-flux surfaces (left panel), visualization of electron localization function (ELF, center panel), and reduced density gradient (RDG, right panel) analyses for intermolecular hydrogen bonding interactions N–H···O and O–H···Cl in 9Hg. Bond critical points (3, –1) are shown in blue, nuclear critical points (3, –3)—in pale brown, ring critical points (3, +1)—in orange, cage critical points (3, +3)—in light green, bond paths are shown as pale brown lines, length units—Å, and the color scale for the ELF and RDG maps is presented in a.u.
The QTAIM analysis of model supramolecular associates demonstrates the presence of intermolecular hydrogen bonding interactions N–H···O, O–H···Cl, and N–H···Br in 9Hg, 9Hg’, 9Zn, and 10Cd (Table 5). The low magnitude of the electron density (0.020–0.031 a.u.), positive values of the Laplacian of electron density (0.061–0.135 a.u.), and zero or very close to zero positive energy density (0.000–0.002 a.u.) in these bond critical points (3, –1) as well as the estimated strength of appropriate supramolecular contacts (4.7–9.4 kcal/mol) are typical for hydrogen bonds in such chemical systems [23,35,36,37]. The balance between the Lagrangian kinetic energy G(r) and potential energy density V(r) at the bond critical points (3, –1) reveals that a covalent contribution in all discussed intermolecular hydrogen bonding interactions N–H···O, O–H···Cl, and N–H···Br in 9Hg, 9Hg’, 9Zn, and 10Cd is absent, and a sign of λ2 indicates that these supramolecular contacts are attractive.
4. Conclusions
Overall, we reported the synthesis and characterization of novel triarylazoimidazoles and their group 12 metal complexes. Two triarylazoimidazoles (8 and 10) and four of their metal complexes (9Zn, 9Hg, 9Hg, 10Cd) were structurally characterized. Novel complexes exhibit red photoluminescence emission (Φ up to 0.21) in a solution. Moreover, the antibacterial activity of complexes was tested against Gram-positive microorganism S. aureus and Gram-negative microorganism E. coli.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12050680/s1, Figure S1: Asymmetric units of compounds 9Zn·CH3OH, 9Hg·CH3OH, 9Hg’·2CH3OH, 8·solvate, 10 and 10Cd·1.75CH3OH·0.75H2O in representation of atoms with thermal ellipsoids (given at p = 50%). Figure S2. 1H NMR spectrum of 9 (CDCl3). Figure S3. 1H NMR spectrum of 10 (CDCl3). Figure S4. 1H NMR spectrum of 11 (CDCl3). Table S1: Crystallographic data and the refinement parameters for compounds; zip-archive with xyz-files for model supramolecular associates.
Author Contributions
Conceptualization, A.G.T.; methodology, A.S.K. (Andreii S. Kritchenkov); investigation, A.A.A. (Alexey A. Artemjev), A.A.A. (Artyom A. Astafiev), A.V.V., A.S.K. (Alexey S. Kubasov); writing—original draft preparation, A.G.T., G.M.B.; writing—review and editing, A.G.T., A.A.K., A.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed under the support of the Russian Science Foundation (award no. 20-73-00094). Antibacterial tests were performed under the support of the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Otsuki, J.; Suwa, K.; Sarker, K.K.; Sinha, C. Photoisomerization and thermal isomerization of arylazoimidazoles. J. Phys. Chem. A 2007, 111, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Astafiev, A.A.; Repina, O.V.; Tupertsev, B.S.; Nazarov, A.A.; Gonchar, M.R.; Vologzhanina, A.V.; Nenajdenko, V.G.; Kritchenkov, A.S.; Khrustalev, V.N.; Nadtochenko, V.N.; et al. Unprecedented Coordination-Induced Bright Red Emission from Group 12 Metal-Bound Triarylazoimidazoles. Molecules 2021, 26, 1739. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl azo dyes as molecular photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Sarker, K.K.; Chand, B.G.; Suwa, K.; Cheng, J.; Lu, T.H.; Otsuki, J.; Sinha, C. Structural studies and photochromism of mercury(II)-iodo complexes of (arylazo)imidazoles. Inorg. Chem. 2007, 46, 670–680. [Google Scholar] [CrossRef]
- Liu, Y.; Varava, P.; Fabrizio, A.; Eymann, L.Y.M.; Tskhovrebov, A.G.; Planes, O.M.; Solari, E.; Fadaei-Tirani, F.; Scopelliti, R.; Sienkiewicz, A.; et al. Synthesis of aminyl biradicals by base-induced Csp3–Csp3 coupling of cationic azo dyes. Chem. Sci. 2019, 10, 5719–5724. [Google Scholar] [CrossRef] [Green Version]
- Sarker, K.K.; Sardar, D.; Suwa, K.; Otsuki, J.; Sinha, C. Cadmium (II) complexes of (Arylazo)imidazoles: Synthesis, structure, photochromism, and density functional theory calculation. Inorg. Chem. 2007, 46, 8291–8301. [Google Scholar] [CrossRef]
- Das, D.; Nayak, M.K.; Sinha, C. Chemistry of azoimidazoles. Synthesis, spectral characterization and redox studies of N(1)-benzyl-2-(arylazo)imidazolepalladium(II)chloride. Transit. Met. Chem. 1997, 22, 172–175. [Google Scholar] [CrossRef]
- Schütt, C.; Heitmann, G.; Wendler, T.; Krahwinkel, B.; Herges, R. Design and synthesis of photodissociable ligands based on azoimidazoles for light-driven coordination-induced spin state switching in homogeneous solution. J. Org. Chem. 2016, 81, 1206–1215. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Naested, L.C.E.; Solari, E.; Scopelliti, R.; Severin, K. Synthesis of azoimidazolium dyes with nitrous oxide. Angew. Chem. Int. Ed. 2015, 54, 1289–1292. [Google Scholar] [CrossRef]
- Bhunia, P.; Baruri, B.; Ray, U.; Sinha, C.; Das, S.; Cheng, J.; Lu, T.-H. Azoimidazole Complexes of Manganese (II)-thiocyanate. X-ray Structures of 2-(p-tolylazo)imidazole and Bis-[1-methyl-2-(p-tolylazo)imidazole]-Di-thiocyanato-manganese(II). Transit. Met. Chem. 2006, 31, 310–315. [Google Scholar] [CrossRef]
- Temesgen, A.; Tskhovrebov, A.G.; Vologzhanina, A.V.; Le, T.A.; Khrustalev, V.N. Crystal structure of 2-[(E)-2-(4-bromophenyl)diazen-1-yl]-4,5-bis(4-methoxyphenyl)-1H-imidazole: The first example of a structurally characterized triarylazoimidazole. Acta Crystallogr. Sect. E 2021, 77, 305–308. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Tupertsev, B.S.; Nazarov, A.A.; Antonets, A.A.; Astafiev, A.A.; Kritchenkov, A.S.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N. Azoimidazole Gold(III) Complexes: Synthesis, Structural Characterization and Self-Assembly in the Solid State. Inorg. Chim. Acta 2021, 522, 120373. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Solari, E.; Scopelliti, R.; Severin, K. Reactions of grignard reagents with nitrous oxide. Organometallics 2014, 33, 2405–2408. [Google Scholar] [CrossRef] [Green Version]
- Eymann, L.Y.M.; Tskhovrebov, A.G.; Sienkiewicz, A.; Bila, J.L.; Živković, I.; Rønnow, H.M.; Wodrich, M.D.; Vannay, L.; Corminboeuf, C.; Pattison, P.; et al. Neutral Aminyl Radicals Derived from Azoimidazolium Dyes. J. Am. Chem. Soc. 2016, 138, 15126–15129. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Halogenated Diazabutadiene Dyes: Synthesis, Structures, Supramolecular Features, and Theoretical Studies. Molecules 2020, 25, 5013. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Vasileva, A.A.; Goddard, R.; Riedel, T.; Dyson, P.J.; Mikhaylov, V.N.; Serebryanskaya, T.V.; Sorokoumov, V.N.; Haukka, M. Palladium(II)-Stabilized Pyridine-2-Diazotates: Synthesis, Structural Characterization, and Cytotoxicity Studies. Inorg. Chem. 2018, 57, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Repina, O.V.; Novikov, A.S.; Khoroshilova, O.V.; Kritchenkov, A.S.; Vasin, A.A.; Tskhovrebov, A.G. Lasagna-like supramolecular polymers derived from the PdII osazone complexes via C(sp2)–H⋯Hal hydrogen bonding. Inorg. Chim. Acta 2020, 502, 119378. [Google Scholar] [CrossRef]
- Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Tsyrenova, B.D.; Nenajdenko, V.G.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Supramolecular organic frameworks derived from bromoaryl-substituted dichlorodiazabutadienes via Cl·Br halogen bonding. Mendeleev Commun. 2021, 31, 191–193. [Google Scholar] [CrossRef]
- Shikhaliyev, N.G.; Maharramov, A.M.; Suleymanova, G.T.; Babazade, A.A.; Nenajdenko, V.G.; Khrustalev, V.N.; Novikov, A.S.; Tskhovrebov, A.G. Arylhydrazones of α-keto esters via methanolysis of dichlorodiazabutadienes: Synthesis and structural study. Mendeleev Commun. 2021, 31, 677–679. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Lingnau, J.B.; Fürstner, A. Gold Difluorocarbenoid Complexes: Spectroscopic and Chemical Profiling. Angew. Chem. Int. Ed. 2019, 58, 8834–8838. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Goddard, R.; Fürstner, A. Two Amphoteric Silver Carbene Clusters. Angew. Chem. Int. Ed. 2018, 57, 8089–8094. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Ostrowska, K.; Stadnicka, K.M.; Gryl, M.; Klimas, O.; Brela, M.Z.; Goszczycki, P.; Liberka, M.; Grolik, J.; Węgrzyn, A. Influence of Hydrogen Bonds and π–π Interactions on the Fluorescence of Crystalline (N-Alkylpyridyl)enamino-pyrrolo [2,3-b]quinoxalin-2-one Derivatives. Cryst. Growth Des. 2022, 22, 1571–1582. [Google Scholar] [CrossRef]
- Anitha, C.; Sheela, C.D.; Tharmaraj, P.; Shanmugakala, R. Studies on Synthesis and Spectral Characterization of Some Transition Metal Complexes of Azo-Azomethine Derivative of Diaminomaleonitrile. Int. J. Inorg. Chem. 2013, 2013, 436275. [Google Scholar] [CrossRef]
- Pramanik, A.; Basu, A.; Das, G. Coordination assembly of p-substituted aryl azo imidazole complexes: Influences of electron donating substitution and counter ions. Polyhedron 2010, 29, 1980–1989. [Google Scholar] [CrossRef]
- Aghatabay, N.M.; Neshat, A.; Karabiyik, T.; Somer, M.; Haciu, D.; Dülger, B. Synthesis, characterization and antimicrobial activity of Fe(II), Zn(II), Cd(II) and Hg(II) complexes with 2,6-bis(benzimidazol-2-yl) pyridine ligand. Eur. J. Med. Chem. 2007, 42, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, K.; Tariq, M.I.; Munir, C.; Hussain, I.; Siddiqui, H.L. Synthesis, Characterization and Hypoglycemic Activity of Zn(II), Cd(II) and Hg(II) Complexes with Glibenclamide. Chem. Pharm. Bull. 2008, 56, 168–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Novikov, A.S.; Gushchin, P.V.; Haukka, M.; Asfin, R.E.; Tolstoy, P.M.; Kukushkin, V.Y. Identification and H(D)-bond energies of C-H(D) Cl interactions in chloride-haloalkane clusters: A combined X-ray crystallographic, spectroscopic, and theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 14104–14112. [Google Scholar] [CrossRef] [Green Version]
- Tskhovrebov, A.G.; Novikov, A.S.; Odintsova, O.V.; Mikhaylov, V.N.; Sorokoumov, V.N.; Serebryanskaya, T.V.; Starova, G.L. Supramolecular polymers derived from the PtII and PdII schiff base complexes via C(sp2)–H … Hal hydrogen bonding: Combined experimental and theoretical study. J. Organomet. Chem. 2019, 886, 71–75. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Novikov, A.S.; Melnik, M.V.; Tskhovrebov, A.G.; Balova, I.A. Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes. J. Organomet. Chem. 2020, 912, 121174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).