Aurophilic Interactions in Cationic Three-Coordinate Gold(I) Bipyridyl/Isocyanide Complex
Abstract
:1. Introduction
2. Materials and Methods
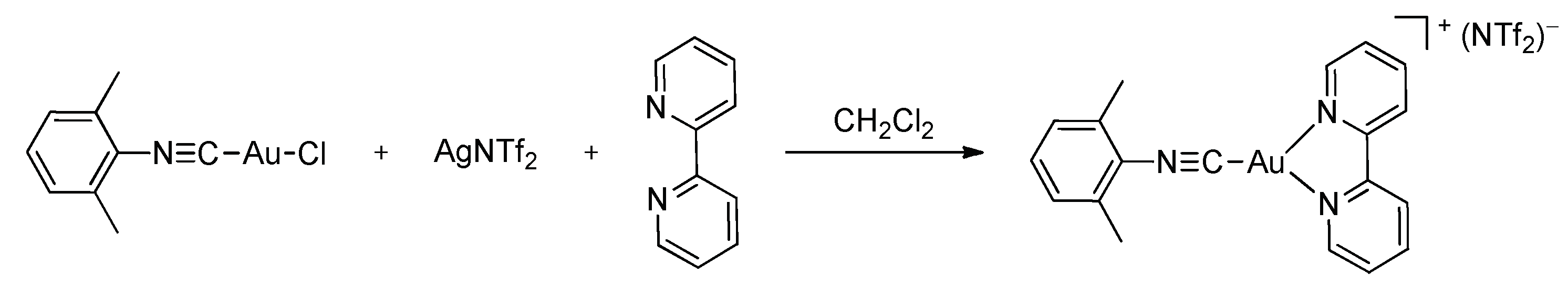
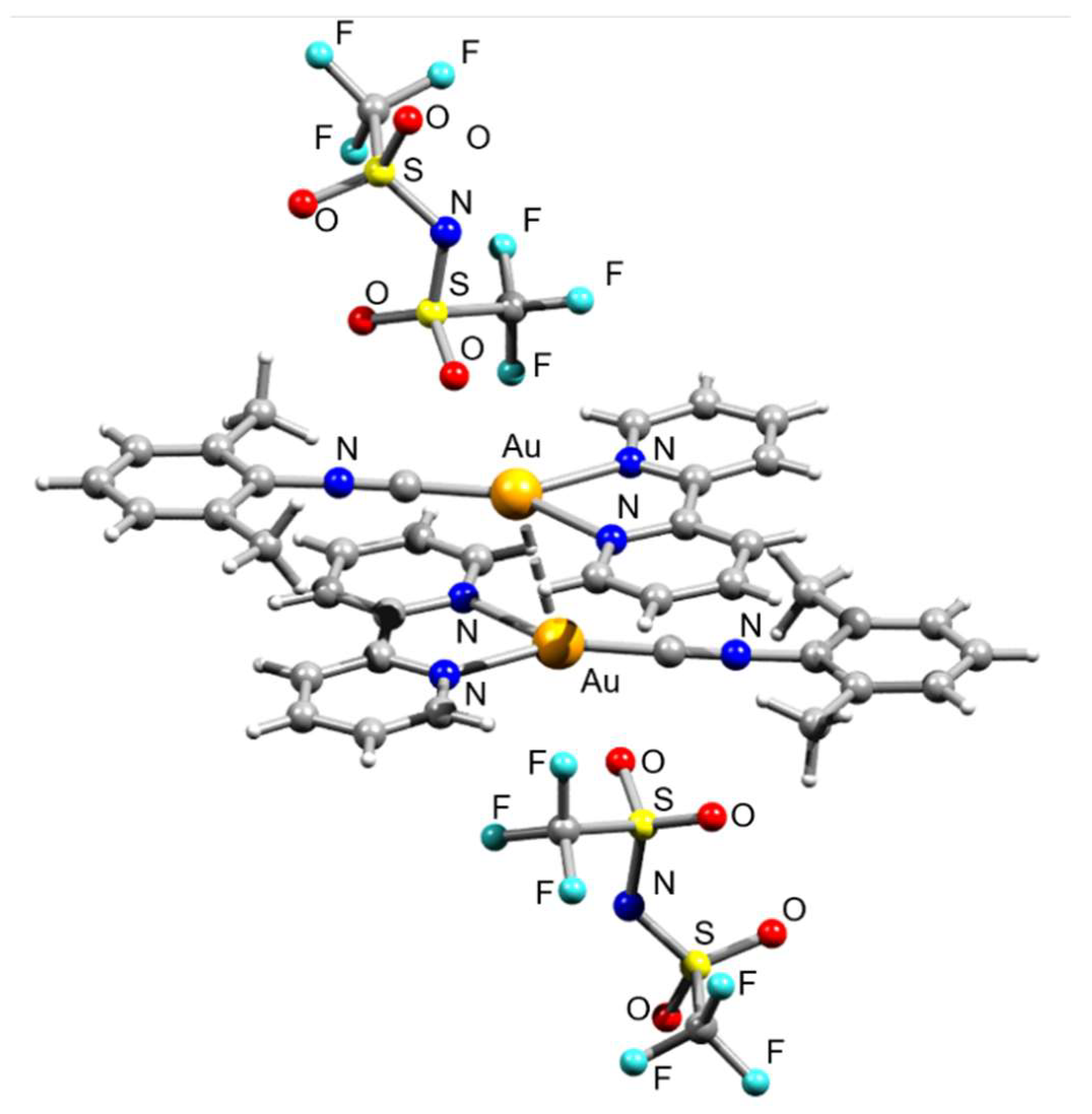
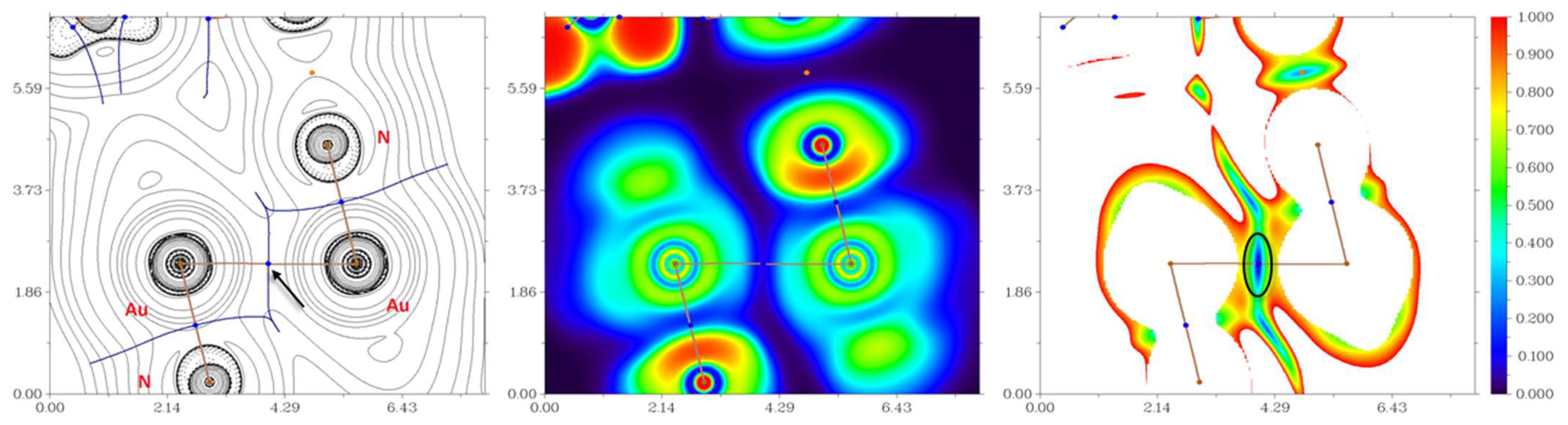
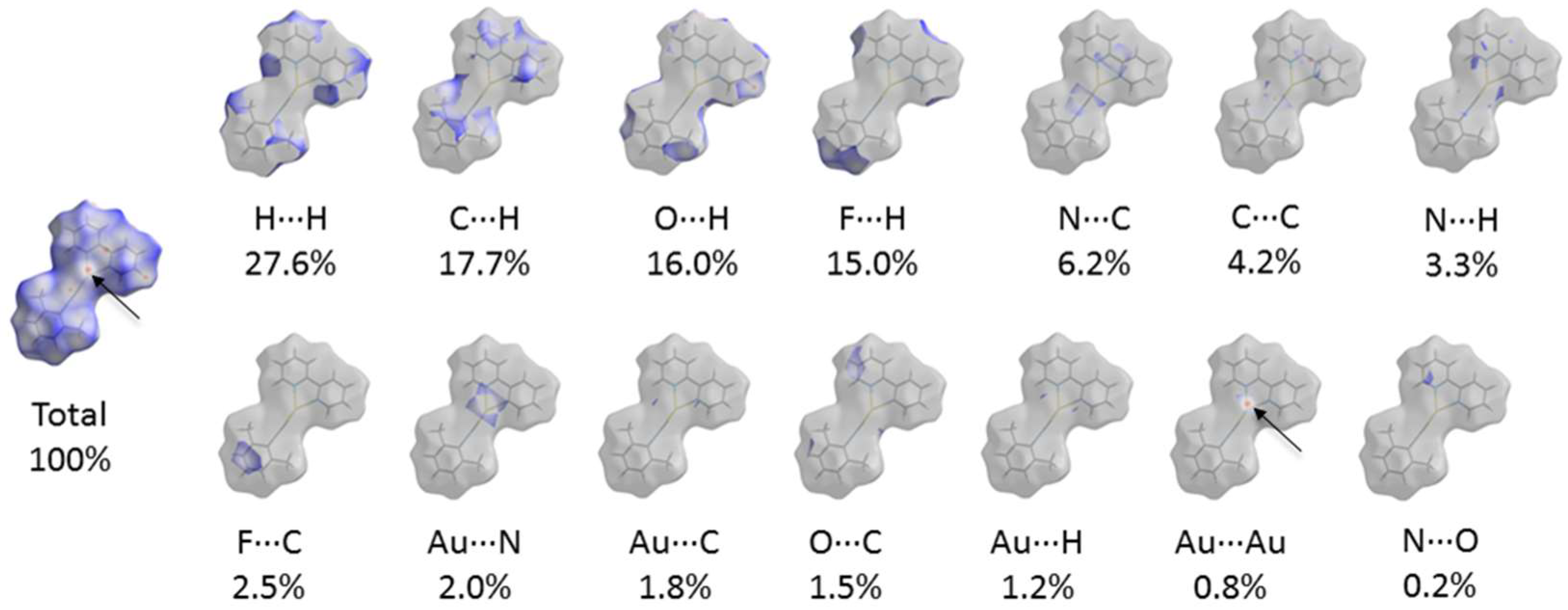
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vickery, J.C.; Olmstead, M.M.; Fung, E.Y.; Balch, A.L. Solvent-Stimulated Luminescence from the Supramolecular Aggregation of a Trinuclear Gold(I) Complex that Displays Extensive Intermolecular AuċAu Interactions. Angew. Chem. Int. Ed. Engl. 1997, 36, 1179–1181. [Google Scholar] [CrossRef]
- Wing-Wah Yam, V.; Kam-Wing Lo, K. Luminescent polynuclear d10 metal complexes. Chem. Soc. Rev. 1999, 28, 323–334. [Google Scholar] [CrossRef]
- Leznoff, D.B.; Xue, B.-Y.; Stevens, C.L.; Storr, A.; Thompson, R.C.; Patrick, B.O. Synthesis, structure and magnetic properties of 3D interpenetrating nets of M(pyrazine)[Au(CN)2]2 (M=Cu, Ni, Co) supported by aurophilic interactions. Polyhedron 2001, 20, 1247–1254. [Google Scholar] [CrossRef]
- Mitsumi, M.; Ueda, H.; Furukawa, K.; Ozawa, Y.; Toriumi, K.; Kurmoo, M. Constructing Highly Conducting Metal−Metal Bonded Solids by Electrocrystallization of [PtII2(RCS2)4] (RCS2− = Dithiocarboxylato, R = Methyl or Ethyl). J. Am. Chem. Soc. 2008, 130, 14102–14104. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, A.; Castillo, O.; Calzolari, A.; Miguel, P.J.S.; Gómez-García, C.J.; di Felice, R.; Zamora, F. Electrical Conductivity in Platinum-Dimer Columns. Inorg. Chem. 2008, 47, 9736–9738. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Ramnial, T.; Yu, H.-Z.; Leznoff, D.B. Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor. J. Am. Chem. Soc. 2008, 130, 10662–10673. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical Chemistry of Gold. Angew. Chem. Int. Ed. Engl. 2004, 43, 4412–4456. [Google Scholar] [CrossRef]
- White-Morris, R.L.; Olmstead, M.M.; Balch, A.L. Aurophilic Interactions in Cationic Gold Complexes with Two Isocyanide Ligands. Polymorphic Yellow and Colorless Forms of [(Cyclohexyl Isocyanide)2AuI](PF6) with Distinct Luminescence. J. Am. Chem. Soc. 2003, 125, 1033–1040. [Google Scholar] [CrossRef]
- Luong, L.M.C.; Malwitz, M.A.; Moshayedi, V.; Olmstead, M.M.; Balch, A.L. Role of Anions and Mixtures of Anions on the Thermochromism, Vapochromism, and Polymorph Formation of Luminescent Crystals of a Single Cation, [(C6H11NC)2Au]+. J. Am. Chem. Soc. 2020, 142, 5689–5701. [Google Scholar] [CrossRef]
- Seki, T.; Sakurada, K.; Ito, H. Controlling Mechano- and Seeding-Triggered Single-Crystal-to-Single-Crystal Phase Transition: Molecular Domino with a Disconnection of Aurophilic Bonds. Angew. Chem. Int. Ed. Engl. 2013, 52, 12828–12832. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kurenuma, S.; Ito, H. Luminescence Color-Tuning through Polymorph Doping: Preparation of a White-Emitting Solid from a Single Gold(I)–Isocyanide Complex by Simple Precipitation. Chem.-A Eur. J. 2013, 19, 16214–16220. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kobayashi, K.; Ito, H. Low-temperature-selective luminescent mechanochromism of a thienyl gold isocyanide complex. Chem. Commun. 2017, 53, 6700–6703. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Barros, C.L.; de Oliveira, P.J.P.; Jorge, F.E.; Canal Neto, A.; Campos, M. Gaussian basis set of double zeta quality for atoms Rb through Xe: Application in non-relativistic and relativistic calculations of atomic and molecular properties. Mol. Phys. 2010, 108, 1965–1972. [Google Scholar] [CrossRef]
- Canal Neto, A.; Jorge, F.E. All-electron double zeta basis sets for the most fifth-row atoms: Application in DFT spectroscopic constant calculations. Chem. Phys. Lett. 2013, 582, 158–162. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, WA, Australia, 2017. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii of metals in covalent compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L.; Kukushkin, V.Y. Metal-mediated [2+3] cycloaddition of nitrones to palladium-bound isonitriles. Chem.-A Eur. J. 2009, 15, 5969–5978. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Kuznetsov, M.L.; Sorokoumov, V.N.; Balova, I.A.; Haukka, M.; Kukushkin, V.Y. Substituent R-dependent regioselectivity switch in nucleophilic addition of N-phenylbenzamidine to PdII-and PtII-complexed isonitrile RN-C giving aminocarbene-like species. Organometallics 2011, 30, 863–874. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Dolgushin, F.M.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L.; Kukushkin, V.Y. Novel reactivity mode of metal diaminocarbenes: Palladium(II)-mediated coupling between acyclic diaminocarbenes and isonitriles leading to dinuclear species. Organometallics 2011, 30, 3362–3370. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; Carias, M.C.; Guedes da Silva, M.F.C.; Pombeiro, A.J.; Kukushkin, V.Y. Novel Metal-mediated (M = Pd, Pt) coupling between isonitriles and benzophenone hydrazone as a route to aminocarbene complexes exhibiting high catalytic activity (M = Pd) in the Suzuki-Miyaura reaction. Organometallics 2009, 28, 6559–6566. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Liakhov, D.M.; Tskhovrebov, A.G.; Balova, I.A. Polystyrene-supported acyclic diaminocarbene palladium complexes in Sonogashira cross-coupling: Stability vs. catalytic activity. Catalysts 2018, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Haukka, M.; Kukushkin, V.Y. Synthesis and characterization of cis-(RNC)2PtII species useful as synthons for generation of various (aminocarbene)Pt II complexes. J. Chem. Crystallogr. 2012, 42, 1170–1175. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Novikov, A.S.; Melnik, M.V.; Tskhovrebov, A.G.; Balova, I.A. Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes. J. Organomet. Chem. 2020, 912, 121174. [Google Scholar] [CrossRef]
- Grudova, M.V.; Kubasov, A.S.; Tskhovrebov, A.G. Synthesis and Crystal Structure of the Dirhodium Tetraacetate-Di(Cyclohexylisocyanide) Complex. J. Struct. Chem. 2021, 62, 1795–1800. [Google Scholar] [CrossRef]
- Buslov, I.V.; Novikov, A.S.; Khrustalev, V.N.; Grudova, M.V.; Kubasov, A.S.; Matsulevich, Z.V.; Borisov, A.V.; Lukiyanova, J.M.; Grishina, M.M.; Kirichuk, A.A.; et al. 2-Pyridylselenenyl versus 2-Pyridyltellurenyl Halides: Symmetrical Chalcogen Bonding in the Solid State and Reactivity towards Nitriles. Symmetry 2021, 13, 2350. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Grishina, M.M.; Matsulevich, Z.V.; Lukiyanova, J.M.; Borisova, G.N.; Osmanov, V.K.; Novikov, A.S.; Kirichuk, A.A.; Borisov, A.V.; Solari, E.; et al. Novel cationic 1,2,4-selenadiazoles: Synthesis via addition of 2-pyridylselenyl halides to unactivated nitriles, structures and four-center Se⋯N contacts. Dalt. Trans. 2021, 50, 10689–10691. [Google Scholar] [CrossRef]
- Grudova, M.V.; Khrustalev, V.N.; Kubasov, A.S.; Strashnov, P.V.; Matsulevich, Z.V.; Lukiyanova, J.M.; Borisova, G.N.; Kritchenkov, A.S.; Grishina, M.M.; Artemjev, A.A.; et al. Adducts of 2-Pyridylselenenyl Halides and Nitriles as Novel Supramolecular Building Blocks: Four-Center Se···N Chalcogen Bonding versus Other Weak Interactions. Cryst. Growth Des. 2022, 22, 313–322. [Google Scholar] [CrossRef]
- Grudova, M.V.; Kubasov, A.S.; Khrustalev, V.N.; Novikov, A.S.; Kritchenkov, A.S.; Nenajdenko, V.G.; Borisov, A.V.; Tskhovrebov, A.G. Exploring Supramolecular Assembly Space of Cationic 1,2,4-Selenodiazoles: Effect of the Substituent at the Carbon Atom and Anions. Molecules 2022, 27, 1029. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Bokach, N.A.; Haukka, M.; Kukushkin, V.Y. Different routes for amination of platinum(II)-bound cyanoguanidine. Inorg. Chem. 2009, 48, 8678–8688. [Google Scholar] [CrossRef] [PubMed]
- Astafiev, A.A.; Shakhov, A.M.; Kritchenkov, A.S.; Khrustalev, V.N.; Shepel, D.V.; Nadtochenko, V.A.; Tskhovrebov, A.G. Femtosecond laser synthesis of nitrogen-doped luminescent carbon dots from acetonitrile. Dye. Pigment. 2021, 188, 109176. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Kritchenkov, A.S.; Khrustalev, V.N.; Haukka, M. Attractive halogen···halogen interactions in crystal structure of trans-dibromogold(III) complex. Z. Für Krist. -Cryst. Mater. 2020, 25, 477–480. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Halogenated Diazabutadiene Dyes: Synthesis, Structures, Supramolecular Features, and Theoretical Studies. Molecules 2020, 25, 5013. [Google Scholar] [CrossRef] [PubMed]
- Repina, O.V.; Novikov, A.S.; Khoroshilova, O.V.; Kritchenkov, A.S.; Vasin, A.A.; Tskhovrebov, A.G. Lasagna-like supramolecular polymers derived from the PdII osazone complexes via C(sp2)–H⋯Hal hydrogen bonding. Inorg. Chim. Acta 2020, 502, 119378. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Vasileva, A.A.; Goddard, R.; Riedel, T.; Dyson, P.J.; Mikhaylov, V.N.; Serebryanskaya, T.V.; Sorokoumov, V.N.; Haukka, M. Palladium(II)-Stabilized Pyridine-2-Diazotates: Synthesis, Structural Characterization, and Cytotoxicity Studies. Inorg. Chem. 2018, 57, 930–934. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Odintsova, O.V.; Mikhaylov, V.N.; Sorokoumov, V.N.; Serebryanskaya, T.V.; Starova, G.L. Supramolecular polymers derived from the PtII and PdII schiff base complexes via C(sp2)–H … Hal hydrogen bonding: Combined experimental and theoretical study. J. Organomet. Chem. 2019, 886, 71–75. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Tupertsev, B.S.; Nazarov, A.A.; Antonets, A.A.; Astafiev, A.A.; Kritchenkov, A.S.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N. Azoimidazole Gold(III) Complexes: Synthesis, Structural Characterization and Self-Assembly in the Solid State. Inorg. Chim. Acta 2021, 522, 120373. [Google Scholar] [CrossRef]
- Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Tsyrenova, B.D.; Nenajdenko, V.G.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Supramolecular organic frameworks derived from bromoaryl-substituted dichlorodiazabutadienes via Cl···Br halogen bonding. Mendeleev Commun. 2021, 31, 191–193. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Savchenko, A.O.; Zhukova, A.I.; Chernikova, N.Y.; Kurykin, M.A.; Novikov, A.S.; Tskhovrebov, A.G. Attractive fluorine···fluorine interactions between perfluorinated alkyl chains: A case of perfluorinated Cu(II) diiminate Cu[C2F5-C(NH)-CF=C(NH)-CF3]2. Z. Für Krist. -Cryst. Mater. 2021, 236, 117–122. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Goddard, R.; Fürstner, A. Two Amphoteric Silver Carbene Clusters. Angew. Chem. Int. Ed. 2018, 57, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Lingnau, J.B.; Fürstner, A. Gold Difluorocarbenoid Complexes: Spectroscopic and Chemical Profiling. Angew. Chem.-Int. Ed. 2019, 58, 8834–8838. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalt. Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Andrusenko, E.V.; Kabin, E.V.; Novikov, A.S.; Bokach, N.A.; Starova, G.L.; Kukushkin, V.Y. Metal-mediated generation of triazapentadienate-terminated di- and trinuclear μ2-pyrazolate NiII species and control of their nuclearity. New J. Chem. 2017, 41, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Novikov, A.S. Strong metallophilic interactions in nickel coordination compounds. Inorg. Chim. Acta 2018, 483, 21–25. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Novikov, A.S.; Suslonov, V.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-mediated reactions between dialkylcyanamides and acetamidoxime generate unusual (nitrosoguanidinate)nickel(ii) complexes. Dalt. Trans. 2017, 46, 10090–10101. [Google Scholar] [CrossRef] [Green Version]
- Shmelev, N.Y.; Okubazghi, T.H.; Abramov, P.A.; Komarov, V.Y.; Rakhmanova, M.I.; Novikov, A.S.; Gushchin, A.L. Intramolecular aurophilic interactions in dinuclear gold(i) complexes with twisted bridging 2,2′-bipyridine ligands. Dalt. Trans. 2021, 50, 12448–12456. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X-H⋯F-Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| ρ(r) | ∇2ρ(r) | λ2 | Hb | V(r) | G(r) |
|---|---|---|---|---|---|
| 0.023 | 0.058 | –0.023 | –0.003 | –0.020 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudova, M.V.; Novikov, A.S.; Kubasov, A.S.; Khrustalev, V.N.; Kirichuk, A.A.; Nenajdenko, V.G.; Tskhovrebov, A.G. Aurophilic Interactions in Cationic Three-Coordinate Gold(I) Bipyridyl/Isocyanide Complex. Crystals 2022, 12, 613. https://doi.org/10.3390/cryst12050613
Grudova MV, Novikov AS, Kubasov AS, Khrustalev VN, Kirichuk AA, Nenajdenko VG, Tskhovrebov AG. Aurophilic Interactions in Cationic Three-Coordinate Gold(I) Bipyridyl/Isocyanide Complex. Crystals. 2022; 12(5):613. https://doi.org/10.3390/cryst12050613
Chicago/Turabian StyleGrudova, Mariya V., Alexander S. Novikov, Alexey S. Kubasov, Victor N. Khrustalev, Anatoly A. Kirichuk, Valentine G. Nenajdenko, and Alexander G. Tskhovrebov. 2022. "Aurophilic Interactions in Cationic Three-Coordinate Gold(I) Bipyridyl/Isocyanide Complex" Crystals 12, no. 5: 613. https://doi.org/10.3390/cryst12050613
APA StyleGrudova, M. V., Novikov, A. S., Kubasov, A. S., Khrustalev, V. N., Kirichuk, A. A., Nenajdenko, V. G., & Tskhovrebov, A. G. (2022). Aurophilic Interactions in Cationic Three-Coordinate Gold(I) Bipyridyl/Isocyanide Complex. Crystals, 12(5), 613. https://doi.org/10.3390/cryst12050613









