Synthesis of Needle-like Nanostructure Composite Electrode of Co3O4/rGO/NF for High-Performance Symmetric Supercapacitor
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
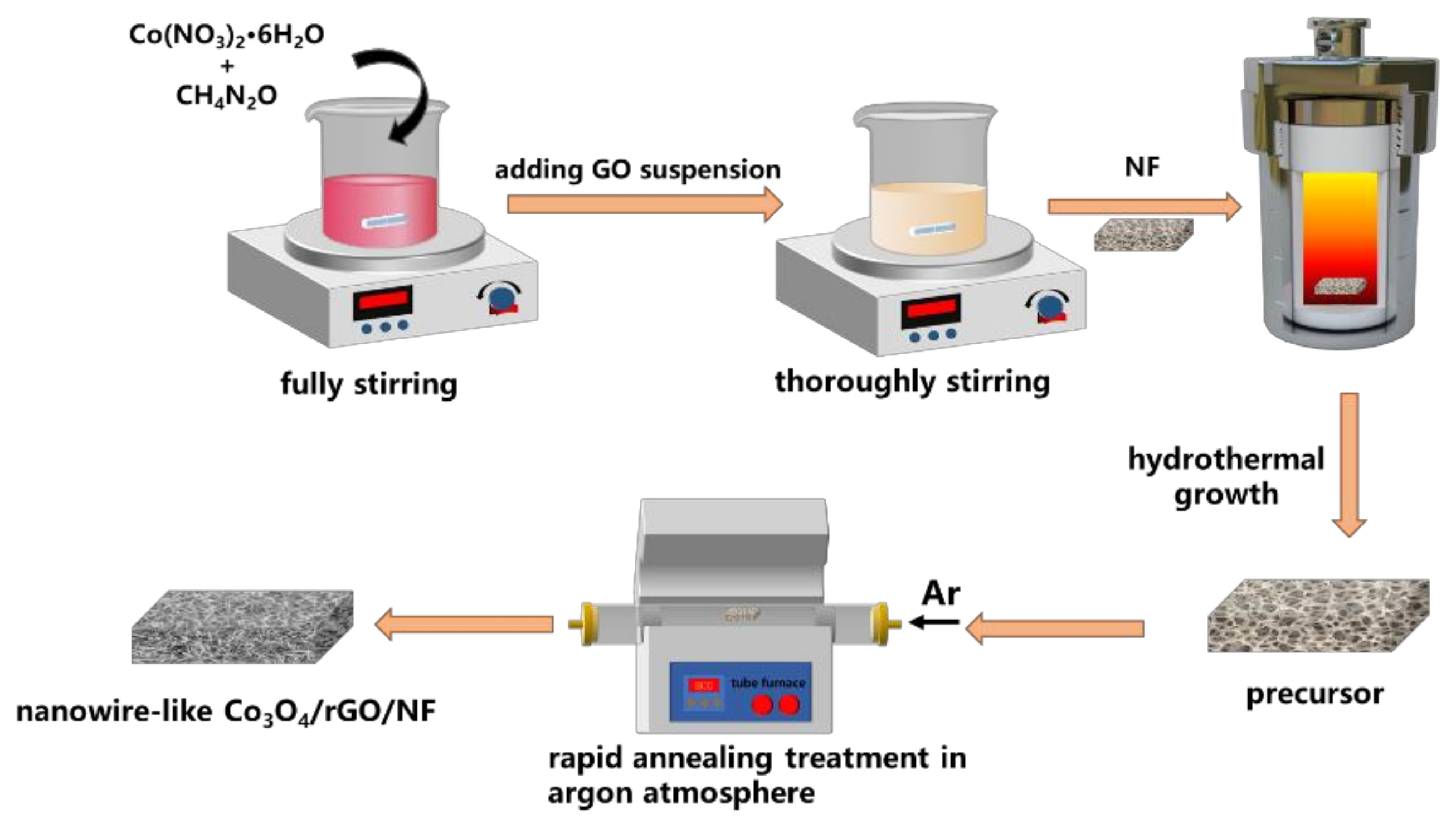
2.2. Preparation of Electrode Materials
2.2.1. Preparation of GO and NF
2.2.2. Preparation of Co3O4/rGO/NF Electrode Material
2.3. Structural Characterization
2.4. Electrochemical Characterization
3. Result and Discussion
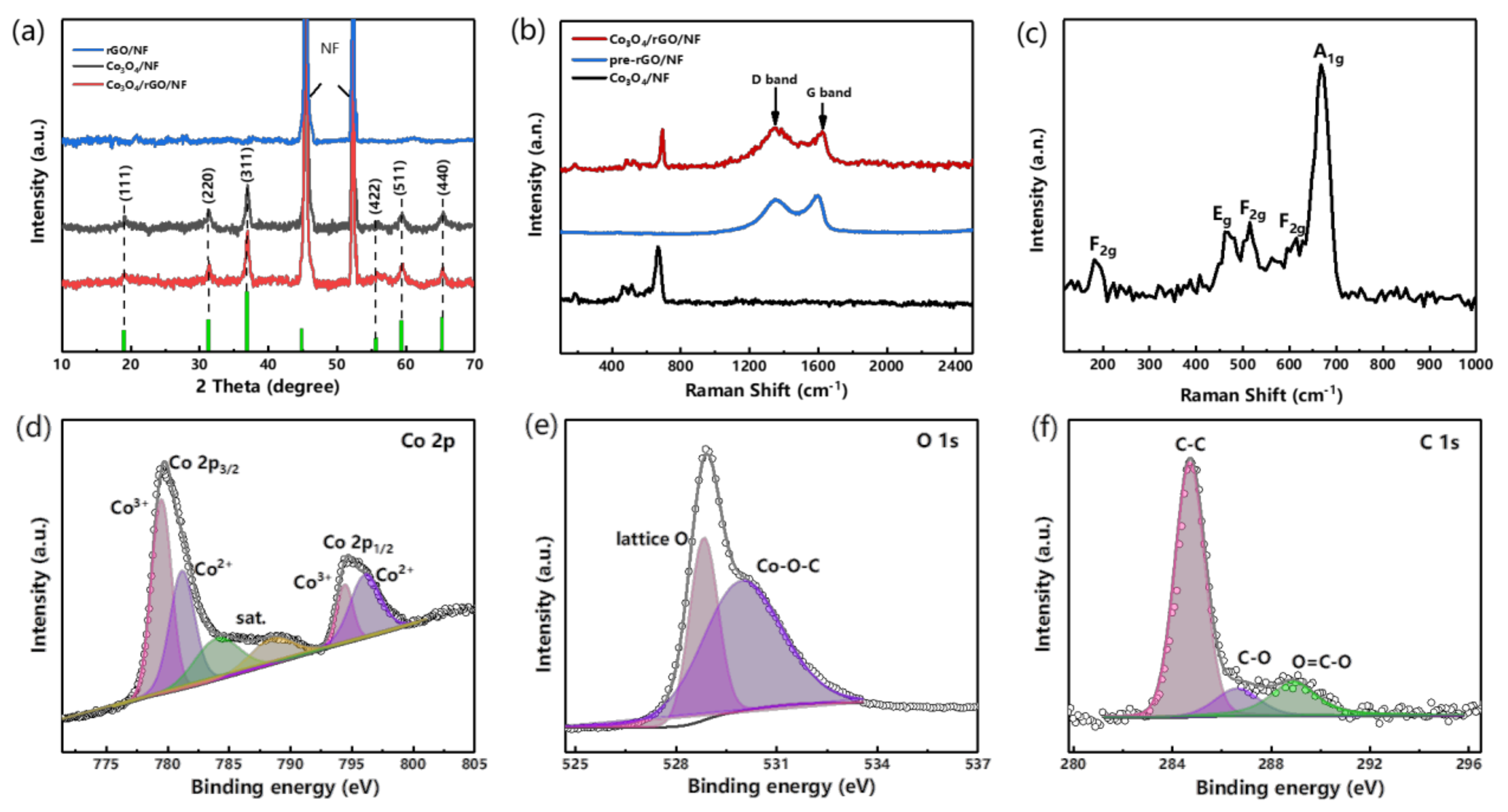
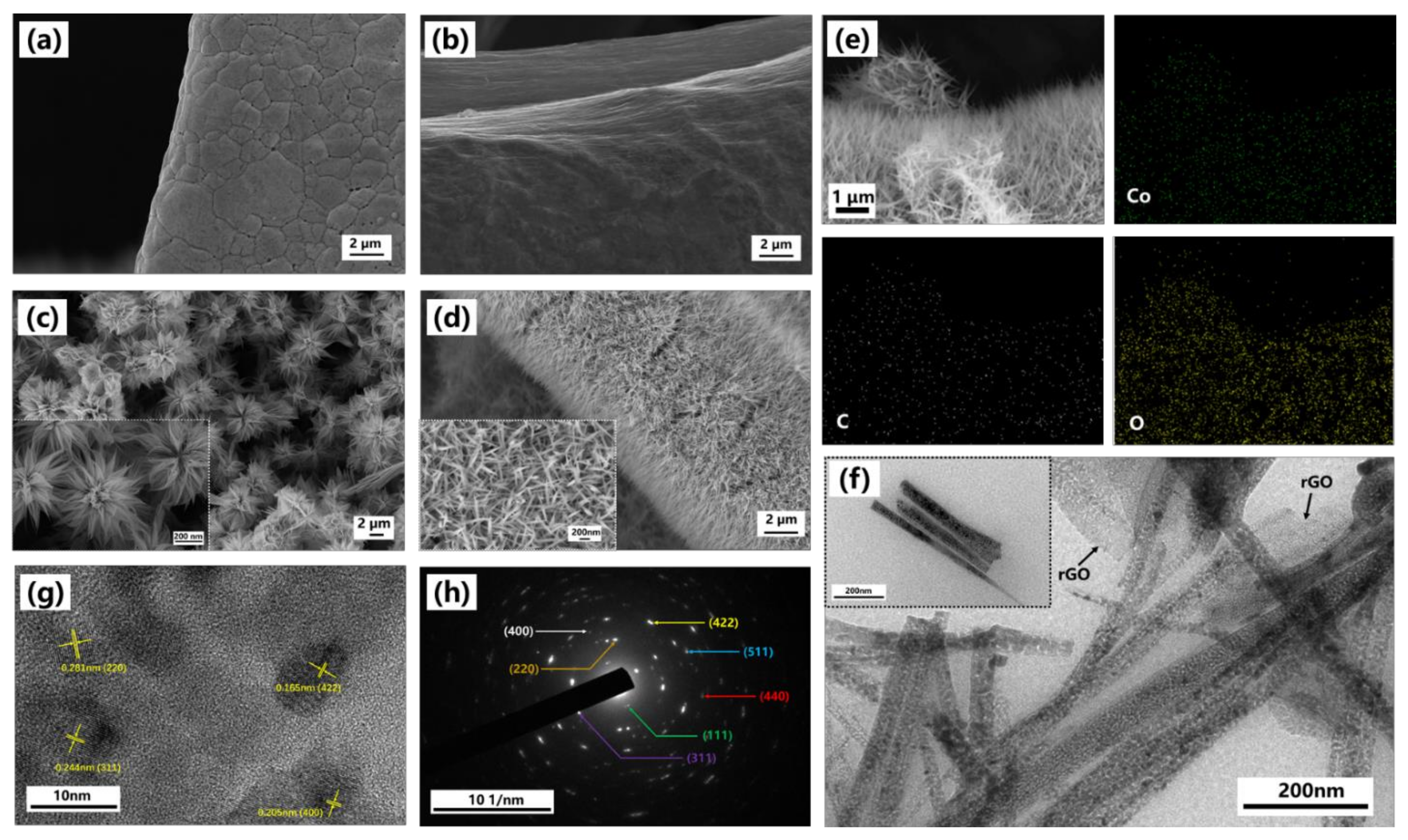
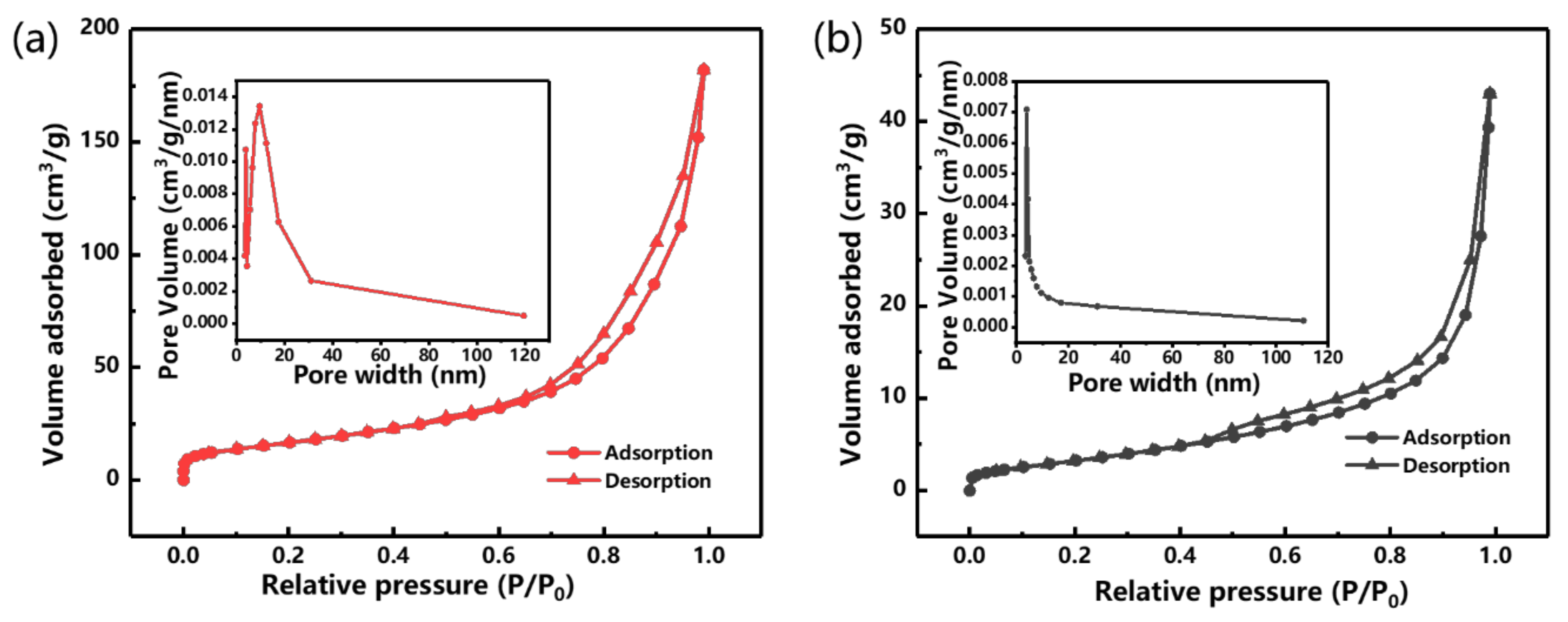
3.1. Morphology and Structural Composition
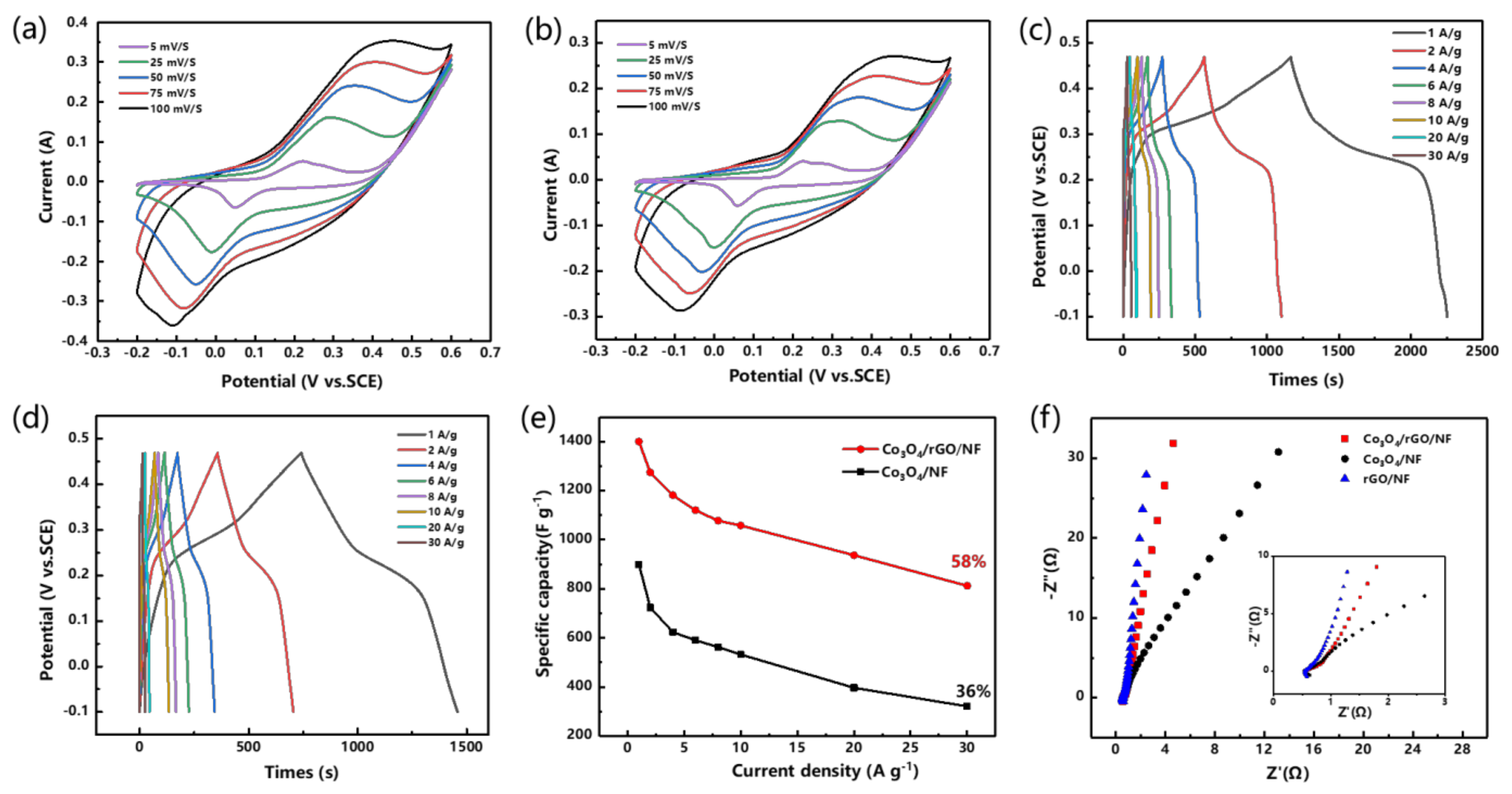
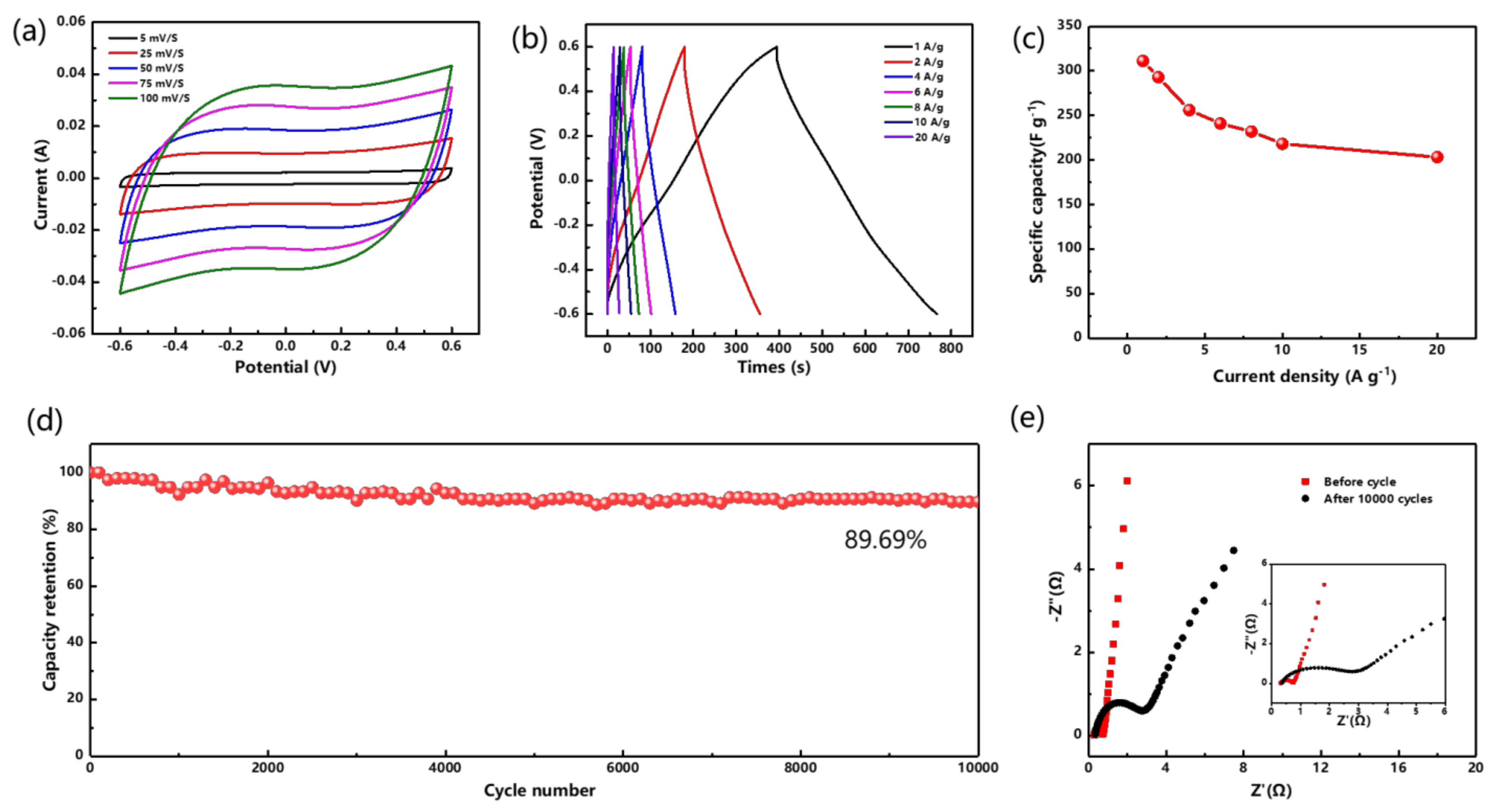
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, G.-R.; Wang, Z.-L.; Su, C.-Y.; Tong, Y.-X. Single-crystal ZnO nanorod/amorphous and nanoporous metal oxide shell composites: Controllable electrochemical synthesis and enhanced supercapacitor performances. Energy Environ. Sci. 2011, 4, 1288–1292. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Yu, F.; Wang, T.; Wen, Z.; Wang, H. High performance all-solid-state symmetric supercapacitor based on porous carbon made from a metal-organic framework compound. J. Power Sources 2017, 364, 9–15. [Google Scholar] [CrossRef]
- Yu, F.; Chang, Z.; Yuan, X.; Wang, F.; Zhu, Y.; Fu, L.; Chen, Y.; Wang, H.; Wu, Y.; Li, W. Ultrathin NiCo2S4@graphene with a core–shell structure as a high performance positive electrode for hybrid supercapacitors. J. Mater. Chem. A 2018, 6, 5856–5861. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, L.; You, T.; Zhu, L.; Liu, X.; Wen, Z. Preparation of Zn0.65Ni0.35O composite from metal-organic framework as electrode material for supercapacitor. Mater. Lett. 2017, 194, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Thomas, J. Energy storing electrical cables: Integrating energy storage and electrical conduction. Adv. Mater. 2014, 26, 4279–4285. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Z.; Zhou, R.; Tan, D.; Wang, H.; Wang, F. Pseudocapacitance contribution in boron-doped graphite sheets for anion storage enables high-performance sodium-ion capacitors. Mater. Horiz. 2018, 5, 529–535. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Yu, F.; Tiong, V.T.; Pang, L.; Zhou, R.; Wang, X.; Waclawik, E.R.; Ostrikov, K.; Wang, H. Flower-like Cu5Sn2S7/ZnS nanocomposite for high performance supercapacitor. Chin. Chem. Lett. 2019, 30, 1115–1120. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Yang, L.; Hu, M.; Lv, Q.; Zhang, H.; Yang, W.; Lv, R. Salt and sugar derived high power carbon microspheres anode with excellent low-potential capacity. Carbon 2020, 163, 288–296. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res. 2010, 3, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Pang, L.; Wang, H.-X. Preparation of mulberry-like RuO2 electrode material for supercapacitors. Rare Met. 2021, 40, 440–447. [Google Scholar] [CrossRef]
- Pore, O.C.; Fulari, A.V.; Shejwal, R.V.; Fulari, V.J.; Lohar, G.M. Review on recent progress in hydrothermally synthesized MCo2O4/rGO composite for energy storage devices. Chem. Eng. J. 2021, 426, 131544. [Google Scholar] [CrossRef]
- Kumar, M.; Subramania, A.; Balakrishnan, K. Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim. Acta 2014, 149, 152–158. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Asghari, A.; Kiani, M.A. High Performance Supercapacitors Based on the Electrodeposited Co3O4 Nanoflakes on Electro-etched Carbon Fibers. Electrochim. Acta 2014, 138, 9–14. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, J.; Zhou, L.; Shao, R.; Utetiwabo, W.; Tufail, M.K.; Wang, S.; Yang, W.; Zhang, J. Orderly defective superstructure for enhanced pseudocapacitive storage in titanium niobium oxide. Nano Res. 2022, 15, 1570–1578. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Xiao, X.; Xu, F.; Sun, Y.; Li, Z. Reduced graphene oxide/nickel cobaltite nanoflake composites for high specific capacitance supercapacitors. Electrochim. Acta 2013, 111, 937–945. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef]
- Xiang, K.; Xu, Z.; Qu, T.; Tian, Z.; Zhang, Y.; Wang, Y.; Xie, M.; Guo, X.; Ding, W.; Guo, X. Two dimensional oxygen-vacancy-rich Co3O4 nanosheets with excellent supercapacitor performances. Chem. Commun. 2017, 53, 12410–12413. [Google Scholar] [CrossRef]
- Yin, F.; Wu, S.; Wang, Y.; Wu, L.; Yuan, P.; Wang, X. Self-assembly of mildly reduced graphene oxide monolayer for enhanced Raman scattering. J. Solid State Chem. 2016, 237, 57–63. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Ma, Y.; Yao, J.; Li, X.; Sun, Y.; Xiong, Z.; Li, D. RF magnetron sputtering synthesis of three-dimensional graphene@Co3O4 nanowire array grown on Ni foam for application in supercapacitors. J. Alloys Compd. 2018, 740, 174–179. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Liu, Y.; Ding, Y.; Du, X.; Song, F.; Xiang, R.; Ma, B. A mononuclear cobalt complex with an organic ligand acting as a precatalyst for efficient visible light-driven water oxidation. Chem. Commun. 2014, 50, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Shin, D.; Jeong, H.; Kim, B.-S.; Han, J.W.; Lee, H. Highly Water-Resistant La-Doped Co3O4 Catalyst for CO Oxidation. ACS Catal. 2019, 9, 10093–10100. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, N.; Zhao, D.; Wang, X.; Zhang, J.; Liu, S.; Guo, L.; Sun, X. Microwave-assisted in-situ isomorphism via introduction of Mn into CoCo2O4 for battery-supercapacitor hybrid electrode material. Chem. Eng. J. 2022, 430, 132729. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, P.; Wang, X.; Wang, P.; Gao, D.; Luo, C. Enhanced water oxidation of CoP/CNTs via interfacial charge transfer induced by poly dimethyl diallyl ammonium chloride. New J. Chem. 2020, 44, 20520–20524. [Google Scholar] [CrossRef]
- Hou, Z.; Shu, C.; Hei, P.; Yang, T.; Zheng, R.; Ran, Z.; Long, J. A 3D free-standing Co doped Ni2P nanowire oxygen electrode for stable and long-life lithium-oxygen batteries. Nanoscale 2020, 12, 6785–6794. [Google Scholar] [CrossRef]
- Titkov, A.I.; Salanov, A.N.; Koscheev, S.V.; Boronin, A.I. Mechanisms of Pd(110) surface reconstruction and oxidation: XPS, LEED and TDS study. Surf. Sci. 2006, 600, 4119–4125. [Google Scholar] [CrossRef]
- Natile, M.M.; Galenda, A.; Glisenti, A. From La2O3 To LaCoO3: XPS Analysis. Surf. Sci. Spectra 2008, 15, 1–13. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhong, J.; Wang, H.; Fu, M.; Ye, D.; Hu, Y. Synergistic effect of tunable oxygen-vacancy defects and graphene on accelerating the photothermal degradation of methanol over Co3O4/rGO nanocomposites. Chem. Eng. J. 2021, 425, 131658. [Google Scholar] [CrossRef]
- Nie, R.; Shi, J.; Du, W.; Ning, W.; Hou, Z.; Xiao, F.-S. A sandwich N-doped graphene/Co3O4 hybrid: An efficient catalyst for selective oxidation of olefins and alcohols. J. Mater. Chem. A 2013, 1, 9037–9045. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Tang, S.; Deng, M.; Du, Y. High-energy all-solid-state symmetric supercapacitor based on Ni3S2 mesoporous nanosheet-decorated three-dimensional reduced graphene oxide. ACS Energy Lett. 2017, 2, 759–768. [Google Scholar] [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lu, S.; Xu, W. Synthesis of Needle-like Nanostructure Composite Electrode of Co3O4/rGO/NF for High-Performance Symmetric Supercapacitor. Crystals 2022, 12, 664. https://doi.org/10.3390/cryst12050664
Wang X, Lu S, Xu W. Synthesis of Needle-like Nanostructure Composite Electrode of Co3O4/rGO/NF for High-Performance Symmetric Supercapacitor. Crystals. 2022; 12(5):664. https://doi.org/10.3390/cryst12050664
Chicago/Turabian StyleWang, Xiaoyan, Shixiang Lu, and Wenguo Xu. 2022. "Synthesis of Needle-like Nanostructure Composite Electrode of Co3O4/rGO/NF for High-Performance Symmetric Supercapacitor" Crystals 12, no. 5: 664. https://doi.org/10.3390/cryst12050664
APA StyleWang, X., Lu, S., & Xu, W. (2022). Synthesis of Needle-like Nanostructure Composite Electrode of Co3O4/rGO/NF for High-Performance Symmetric Supercapacitor. Crystals, 12(5), 664. https://doi.org/10.3390/cryst12050664




