Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Applied Printing Parameters
2.2. Heat Treatment
2.3. Thermal Conductivity and Microstructural Characterizations
3. Results and Discussion
3.1. Manufacturing Characteristics
3.2. XRD Analysis
3.3. Microstructural Characterization
3.4. Thermal Conductivity
4. Conclusions
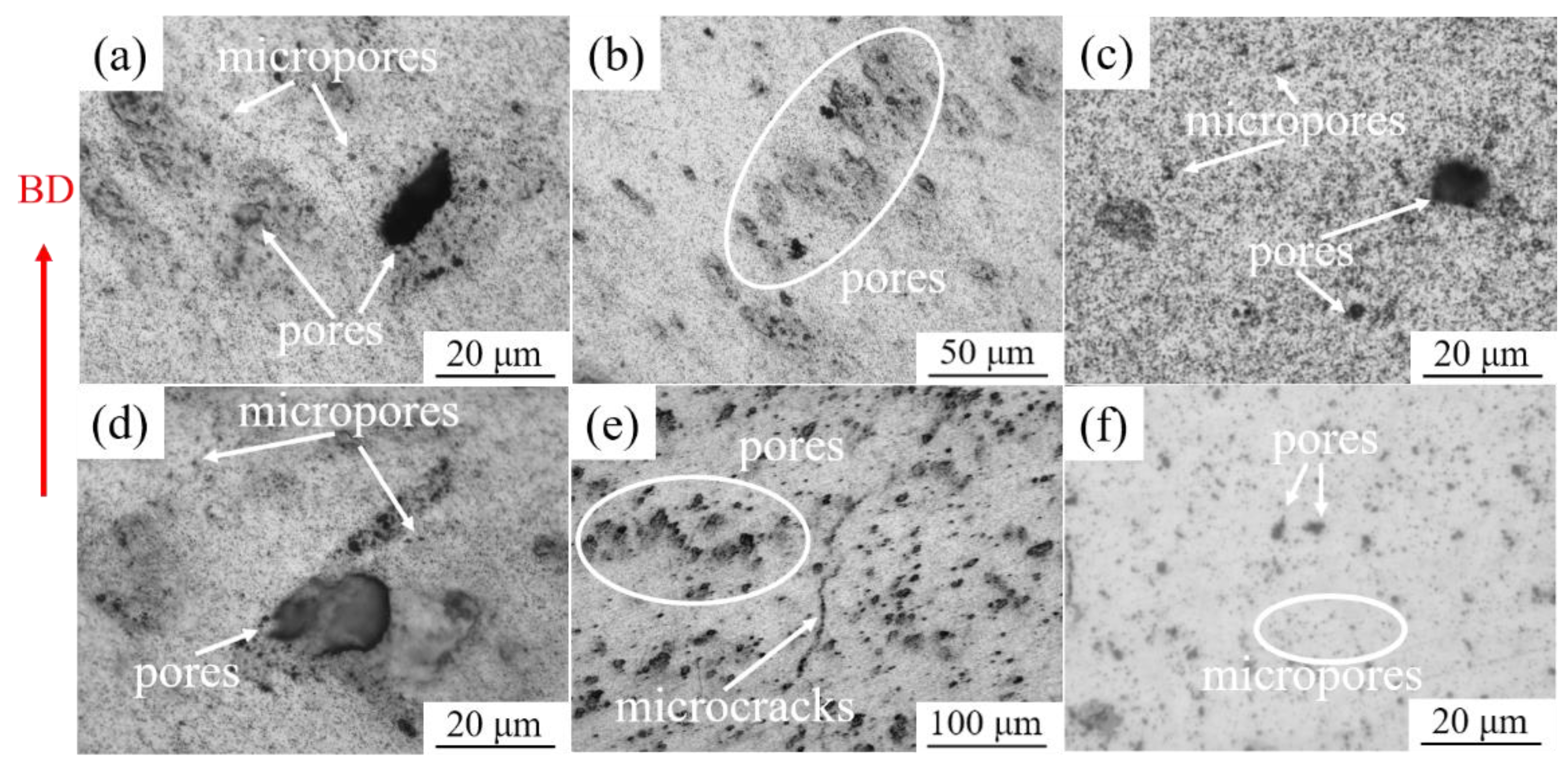
- When the applied energy densities were 447.92 J/mm3 and 333.33 J/mm3, respectively, the obtained relative densities of SLM-processed Ag (91.06%) and 925Ag (96.56%) parts were the highest. As-built Ag and 925Ag parts showed irregular pores and micron-sized pores formed by non-molten particles, which is the direct reason for their low relative density.
- The SLMed Ag exhibited fine equiaxed grains, while both equiaxed grains and elongated lath grains existed in the SLMed 925Ag parts. The annealed Ag and solution-treated 925Ag exhibited large equiaxed grains. Irregular macropores and micropores still existed in the heat-treated parts, indicating that the applied heat treatments could not reduce the porosity of SLMed parts.
- The existence of pores hinders the lattice vibration. The electron is heated to create thermal motion, though this motion is scattered at the grain boundaries. Pores and grain size of parts processed in this study are the main reasons for the observation of their low thermal conductivities, as compared to as-cast parts. The comparison between the thermal conductivities of Ag and 925Ag shows that the material nature has a greater influence on the thermal conductivity than the porosity level of parts processed in this study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lian, C.; Yong, H.; Yingxin, Y.; Shiwei, N.; Haitao, R. The research status and development trend of additive manufacturing technology. Int. J. Adv. Manuf. Technol. 2017, 89, 3651–3660. [Google Scholar]
- Ma, W.L.; Tao, F.H.; Jia, C.Z.; Yang, J.C. Research Status and Application Analysis of 3D Printing Technology. Appl. Mech. Mater. 2014, 529, 697–700. [Google Scholar] [CrossRef]
- Guoqing, W.; Yingzhou, H.; Weinan, Z.; Chenguang, S.; Hongsong, H. Research Status and Development Trend of Laser Additive Manufacturing Technology. In Proceedings of the 2017 4th International Conference on Information Science and Control Engineering (ICISCE), Changsha, China, 21–23 July 2017; IEEE: Changsha, China, 2017; pp. 1210–1213. [Google Scholar]
- Yang, Y.; Wang, D.; Wu, W. Research progress of direct manufacturing of metal parts by selective laser melting. Chin. J. Lasers 2011, 38, 0601007. [Google Scholar] [CrossRef]
- Zitelli, C.; Folgarait, P.; Di Schino, A. Laser Powder Bed Fusion of Stainless Steel Grades: A Review. Met. Open Access Metall. J. 2019, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, K.; Guo, W.; Gangil, N.; Konovalov, S.V. The fabrication of NiTi shape memory alloy by selective laser melting: A review. Rapid Prototyp. J. 2019, 25, 1421–1432. [Google Scholar] [CrossRef]
- Junfeng, L.; Zhengying, W.; Bingheng, L. Research Progress on Technology of Selective Laser Melting of Titanium and Titanium Alloys. Laser Optoelectron. Prog. 2018, 55, 011403. [Google Scholar] [CrossRef]
- Microstructure and compressive properties of Al-Si10-Mg lattice structures manufactured using selective laser melting. Mater. Und Werkst. 2021, 52, 762–771. [CrossRef]
- Wang, P.; Eckert, J.; Prashanth, K.G.; Wu, M.W.; Kaban, I.; Xi, L.X.; Scudino, S. A review of particulate-reinforced aluminum matrix composites fabricated by selective laser melting. Trans. Nonferrous Met. Soc. China 2020, 30, 2001–2034. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H. A review of SLMed magnesium alloys: Processing, properties, alloying elements and postprocessing. Met. Open Access Metall. J. 2020, 10, 1073. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Gupta, M. Selective Laser Melting of Magnesium and Magnesium Alloy Powders: A Review. Met. Open Access Metall. J. 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Zai, L.; Zhang, C.; Wang, Y.; Guo, W.; Tian, Y. Laser powder bed fusion of precipitation-hardened martensitic stainless steels: A review. Met. Open Access Metall. J. 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Zhibo, L.; Jianguang, C.; Lining, D.; Jian, D.; Zhenhan, B. Research on application of high thermal conductivity materials for spacecraft thermal management. China’s Mater. Prog. 2018, 37, 9. [Google Scholar]
- Zhihong, J. Quick Check Manual for Chinese and Foreign Brands of Casting Metal Materials; Chemical Industry Press: Beijing, China, 2008; p. 95. [Google Scholar]
- Robinson, J.; Stanford, M.; Arjunan, A. Stable formation of powder bed laser fused 99.9% silver. Mater. Today Commun. 2020, 24, 101195. [Google Scholar] [CrossRef]
- Robinson, J.; Stanford, M.; Arjunan, A. Correlation between selective laser melting parameters, pore defects and tensile properties of 99.9% silver. Mater. Today Commun. 2020, 25, 101550. [Google Scholar] [CrossRef]
- Momen, H.M. Effects of particle size and laser wavelength on heating of silver nanoparticles under laser irradiation in liquid. Pramana J. Phys. 2016, 87, 26. [Google Scholar] [CrossRef]
- Xiong, W.; Hao, L.; Li, Y.; Tang, D.; Cui, Q.; Feng, Z.; Yan, C. Effect of selective laser melting parameters on morphology, microstructure, densification and mechanical properties of supersaturated silver alloy. Mater. Des. 2019, 170, 10769. [Google Scholar] [CrossRef]
- Bajaj, P.; Wright, J.; Todd, I.; Jägle, E.A. Predictive process parameter selection for Selective Laser Melting Manufacturing: Applications to high thermal conductivity alloys. Addit. Manuf. 2018, 27, 246–258. [Google Scholar] [CrossRef]
- Seol, H.J.; Park, Y.G.; Hoon Kwon, Y.; Takada, Y.; Kim, H.I. Age-hardening behaviour and microstructure of a silver alloy with high Cu content for dental application. J. Mater. Sci. Mater. Med. 2005, 16, 977–983. [Google Scholar] [CrossRef]
- Vikram, R.; Kollo, L.; Prashanth, K.; Suwas, S. Investigating the Structure, Microstructure, and Texture in Selective Laser-Melted Sterling Silver 925. Metall. Mater. Trans. A 2021, 52, 5329–5341. [Google Scholar] [CrossRef]
- Ruinan, G.; WONG Kam, S.; Ming, Y. Laser Additive Manufacturing of Typical High Reflectivity Materials Such As Gold, Silver and Copper. Sci. China Phys. Mech. Astron. 2020, 50, 14. [Google Scholar]
- Tanaka, Y.; Udoh, K.; Hisatsune, K.; Sakurai, T. Distribution of niobium in an Fe–Pt–Nb magnet. Mater. Sci. Eng. A 1998, 250, 164–168. [Google Scholar] [CrossRef]
- Seol, H.J.; Shiraishi, T.; Tanaka, Y.; Miura, E.; Takuma, Y.; Hisatsune, K. Partial phase diagram for the AuCu–Zn pseudobinary system. J. Alloys Compd. 2002, 339, 144–148. [Google Scholar] [CrossRef]
- Butler, C.; Babu, S.; Lundy, R.; Meehan, R.; Punch, J.; Jeffers, N. Effects of processing parameters and heat treatment on thermal conductivity of additively manufactured AlSi10Mg by selective laser melting. Mater. Charact. 2021, 173, 110945. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, M.; Li, Y.; Zhang, W.; Yang, C.; Kollo, L.; Eckert, J.; Prashanth, K.G. Premature failure of an additively manufactured material. NPG Asia Mater. 2020, 12, 30. [Google Scholar] [CrossRef]
- Snow, Z.; Nassar, A.R.; Reutzel, E.W. Invited Review Article: Review of the formation and impact of flaws in powder bed fusion additive manufacturing. Addit. Manuf. 2020, 36, 101457. [Google Scholar] [CrossRef]
- Li, J.; Gong, S.; Liu, K.; Qi, W.; Tian, J.; Shan, F. Formation mechanism and mechanical properties of the selective laser melting Ni/Co base alloy. J. Alloy. Compd. 2019, 777, 963–967. [Google Scholar] [CrossRef]
- Ren, K.; Chew, Y.; Zhang, Y.; Fuh, J.; Bi, G. Thermal field prediction for laser scanning paths in laser aided additive manufacturing by physics-based machine learning. Comput. Methods Appl. Mech. Eng. 2020, 362, 112734. [Google Scholar] [CrossRef]
- Chen, F.; Yan, W. High-fidelity modelling of thermal stress for additive manufacturing by linking thermal-fluid and mechanical models. Mater. Des. 2020, 196, 109185. [Google Scholar] [CrossRef]
- Shuang, L. Study on the Microstructure and Properties of New High Conductivity Ag-Au Alloy. Ph.D. Thesis, Chongqing University of Technology, Chongqing, China, 2020. [Google Scholar]
- Greil, S.; Edtmaier, C.; Haubner, R.; Lauter, L. Metallographic investigations of silver alloys used for minting. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2017; Volume 891, pp. 89–94. [Google Scholar]
- Dingjie, S. Effect of Heat Treatment Process on Microstructure and Hardness of 925 Silver Alloy. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2007. [Google Scholar]
- Qingqing, Y. Study on Strengthening Mechanism and Anti-Corrosion Behavior of High-Performance Gold and 925 Silver. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2007. [Google Scholar]
- Skelton, J.M. Approximate models for the lattice thermal conductivity of alloy thermoelectrics. J. Mater. Chem. C 2021, 9, 11772–11787. [Google Scholar] [CrossRef]
- Jihui, N.; Raghavan, R.; Zhi, L.; Pawel, K. Structural vs. compositional disorder in thermal conductivity reduction of SiGe alloys. J. Appl. Phys. 2017, 122, 045104. [Google Scholar]
- Simmons, J.C.; Chen, X.; Azizi, A.; Daeumer, M.A.; Zavalij, P.Y.; Zhou, G.; Schiffres, S.N. Influence of processing and microstructure on the local and bulk thermal conductivity of selective laser melted 316L stainless steel. Addit. Manuf. 2020, 32, 100996. [Google Scholar] [CrossRef]
- Tomanek, L.B.; Stutts, D.S.; Pan, T.; Liou, F. Influence of porosity on the thermal, electrical, and mechanical performance of selective laser melted stainless steel. Addit. Manuf. 2021, 39, 101886. [Google Scholar] [CrossRef]
- Li, B.; Hou, L.; Wu, R.; Zhang, J.; Li, X.; Zhang, M.; Dong, A.; Sun, B. Microstructure and thermal conductivity of Mg-2Zn-Zr alloy. J. Alloys Compd. 2017, 722, 772–777. [Google Scholar] [CrossRef]
- Robinson, J.; Arjunan, A.; Stanford, M.; Lyall, I.; Williams, C. Effect of silver addition in copper-silver alloys fabricated by laser powder bed fusion in situ alloying. J. Alloys Compd. 2021, 857, 157561. [Google Scholar] [CrossRef]








| Serial Number | Laser Power (w) | Laser Scanning Speed (mm/s) | Layer Thickness (mm) | Hatch Spacing (mm) |
|---|---|---|---|---|
| 1 | 400 | 200 | 0.025 | 0.100 |
| 2 | 400 | 300 | 0.025 | 0.100 |
| 3 | 400 | 400 | 0.025 | 0.100 |
| 4 | 430 | 300 | 0.025 | 0.130 |
| 5 | 430 | 350 | 0.025 | 0.130 |
| 6 | 430 | 400 | 0.025 | 0.130 |
| 7 | 400 | 200 | 0.025 | 0.130 |
| 8 | 400 | 300 | 0.025 | 0.130 |
| 9 | 400 | 400 | 0.025 | 0.130 |
| 10 | 430 | 400 | 0.030 | 0.080 |
| Material | Heat Treatment Mode | Heat Treatment Situation |
|---|---|---|
| Ag | Annealing | 700 °C/2 h/furnace cooling (FC) |
| 925Ag | Solution treatment | 700 °C/2 h/water cooling (WC) |
| Material | Laser Power (w) | Scanning Speed (mm/s) | Layer Thickness (mm) | Scanning Spacing (mm) | Energy Density (J/mm3) | Density (g/mm3) | Relative Density |
|---|---|---|---|---|---|---|---|
| Ag | 430 | 400 | 0.030 | 0.080 | 447.922 | 9.552 ± 0.079 | 91.06% |
| 925Ag | 180 | 600 | 0.030 | 0.060 | 333.333 | 10.043 ± 0.091 | 96.56% |
| Density (g/cm3) | Thermal Diffusion Coefficient (mm2/s) | Specific Heat Capacity (J/(g·K)) | Thermal Conductivity (W/(m·K)) | |
|---|---|---|---|---|
| As-cast Ag | 10.490 | 176.270 | 0.232 | 429.000 |
| As-built Ag | 9.552 ± 0.079 | 41.336 ± 0.162 | 0.255 ± 0.011 | 100.879 ± 0.011 |
| Annealed Ag | 56.319 ± 0.797 | 0.209 ± 0.007 | 112.329 ± 1.589 | |
| As-cast 925Ag | 10.400 | 93.656 | 0.232 | 228.000 |
| As-built 925Ag | 10.042 ± 0.052 | 21.224 ± 0.024 | 0.261 ± 0.009 | 55.708 ± 0.063 |
| Solution-treated 925Ag | 20.235 ± 0.205 | 0.227 ± 0.002 | 46.159 ± 0.469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wei, Y.; Wei, X.; Khanlari, K.; Wang, Z.; Feng, Y.; Yang, X. Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity. Crystals 2022, 12, 480. https://doi.org/10.3390/cryst12040480
Wang D, Wei Y, Wei X, Khanlari K, Wang Z, Feng Y, Yang X. Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity. Crystals. 2022; 12(4):480. https://doi.org/10.3390/cryst12040480
Chicago/Turabian StyleWang, Di, Yang Wei, Xiongmian Wei, Khashayar Khanlari, Zhi Wang, Yongwei Feng, and Xusheng Yang. 2022. "Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity" Crystals 12, no. 4: 480. https://doi.org/10.3390/cryst12040480
APA StyleWang, D., Wei, Y., Wei, X., Khanlari, K., Wang, Z., Feng, Y., & Yang, X. (2022). Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity. Crystals, 12(4), 480. https://doi.org/10.3390/cryst12040480









