Abstract
Using the homovalent cation substitution strategy, a new sulfate, Cs2Ca3(SO4)4, was successfully prepared using the spontaneous crystallization technique. A single-crystal structure measurement suggested that it crystallizes in space group P21/c, with lattice parameters and molecules per unit cell of a = 9.9153(8), b = 9.3760(6), c = 9.8044(9), β = 118.365(3)°, V = 802.04(11), and Z = 2. In the structure of Cs2Ca3(SO4)4, CaO6 octahedra and SO4 tetrahedra are interconnected via a corner-sharing mode to form a three-dimensional framework comprising large cavities filled with Cs+ cations. First-principles calculations and diffuse reflectance spectra indicated that Cs2Ca3(SO4)4 has a large energy band gap. Moreover, structural comparisons with similar compounds were conducted to explain the role of cations in tuning structural symmetry and birefringence. This paper helps to explain the size effect of cations on structural evolution.
1. Introduction
Sulfates have been used for a wide range of purposes since ancient times. For example, potassium sulfate can be used as a chemical fertilizer for plants, promoting plant growth and crop yield. More recently, sulfates have been considered suitable for use as ultraviolet birefringent materials based on certain merits, including that they are environmentally friendly raw chemical materials, have a wide transparency window, and easily grow as crystals. A series of excellent birefringent materials have been reported so far, including NH4NaLi2(SO4)2 [1], ASbF2SO4 (A = Rb, Cs) [2,3], K2Bi2(SO4)2Cl4 [4], Nb2O3(IO3)2(SO4) [5], and Ce(SO4)F2 [6]. Aside from changing the cation type, introducing organic π-conjugated units into sulfates is also an effective way to produce new materials possessing high birefringence. For example, using a combination of inorganic sulfates and neutral thiourea molecules, a birefringence of 0.21 at 546.1 nm in Te(CS(NH2)2)4SO4·2H2O [7] and 0.16 at 554 nm in Zn[CS(NH2)2]3SO4 can be achieved [8]. However, most of these sulfates have relatively long UV absorption edges, which is not conducive to application in the UV region. Therefore, the search for short-wave UV sulfates is still ongoing.
Since the SO4 groups in sulfates exist only as monomers, cations play an important role in the synthesis of new compounds. In order to obtain sulfates with short UV absorption edges, the choice of counterpart cation is best confined to those metal elements in the first or second main group [9,10,11]. Numerous experiments have confirmed that metal cations without d–d or f–f electron transitions barely damage the UV absorption edge of the [SO4]2− anionic group [12,13,14]. Based on the abovementioned idea, sulfates of single/mixed alkali metals were synthesized using the slow aqueous evaporation technique. Moreover, NH4+ cations were introduced into the sulfates to tune the structure and the optical properties [1,4,15,16,17]. Pure alkali metal sulfates exhibit low thermal stability, which can be elevated by introducing alkali earth metal elements into the sulfates [14,18,19]. Because alkali earth sulfates have low solubility in water, they are often prepared using a traditional high-temperature molten method, such as A2M2(SO4)3 (A = Rb, Cs; M = Mg, Ca) and Cs2Mg3(SO4)4 [14,18,19,20].
According to the cation substitution strategy, in which cations in the same family have a similar coordination environment, we intended to synthesize a new compound, Cs2Ca3(SO4)4, by substituting Ca2+ for Mg2+ cations in Cs2Mg3(SO4)4 [18]. By changing the ratio of raw materials, Cs2Ca3(SO4)4 crystals were successfully prepared using the spontaneous crystallization technique. First-principles calculations demonstrated that Cs2Ca3(SO4)4 showed a greatly enhanced birefringence compared to Cs2Mg3(SO4)4, which was analyzed using detailed structural comparisons. Both the UV–vis–NIR diffuse reflectance spectrum and electronic band structure verified that Cs2Ca3(SO4)4 has a large energy band gap. This work may serve as a useful reference for understanding the role of cations in regulating crystal structures and optical properties.
2. Materials and Methods
Single crystals of Cs2Ca3(SO4)4 were grown in an open environment using the spontaneous crystallization technique. First, the raw materials of Cs2SO4 and CaSO4 at a molar ratio of 1:3 were finely ground and then left at 973 K for 3 days to obtain the nominal Cs2Ca3(SO4)4 polycrystalline product. Second, Cs2Ca3(SO4)4 powder was mixed with Cs2SO4 and then melted in a program-controlled muffle furnace at 1273 K for 2 days. Finally, Cs2Ca3(SO4)4 single crystals with a maximum size of 0.5 mm × 0.7 mm × 0.3 mm were obtained by slowly cooling the muffle furnace. The purity of the obtained crystals was checked by powder X-ray diffraction (XRD) data collected on a Rigaku MiniFlex II diffractometer (Cu Kα radiation). A small crystal with dimensions of 0.08 mm × 0.06 mm × 0.06 mm was selected for single-crystal structure measurements, which were performed on a Bruker D8 with graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å). The structure was solved using SHELXS and SHELXL [21], and further checked using the PLATON program [22]. Detailed crystallographic information is given in Supplementary Tables S1–S4. CCDC 2126379 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving.html, accessed on 14 December 2021, (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk). The UV–vis–NIR diffuse reflectance spectrum was measured on a PerkinElmer Lamda-950 spectrophotometer. The first-principles calculations were performed using the CASTEP suite in Material Studio [23,24]. The Perdew–Burke–Ernzerhof functional and generalized gradient approximation [25,26] was selected [27]. The kinetic energy absorption, k-point sampling, and pseudopotential used for calculating the electronic band structure and density of states were 300 eV, 1 × 1 × 1, and ultrasoft, respectively. Detailed birefringence calculation settings can be found in the Supplementary Materials.
3. Results and Discussion
3.1. Powder XRD
Figure 1 shows the XRD patterns of the synthesized Cs2Ca3(SO4)4 crystals. The black pattern represents the experimental powder XRD, and the red pattern represents the XRD calculated from the single-crystal structure. The experimental XRD pattern basically reflects that of our obtained compound, but with additional minor miscellaneous peaks (marked with asterisks in Figure 1), which led us to compare the XRD patterns of the raw reagents (Cs2SO4 and CaSO4) with the title compound. As shown in Supplementary Figure S1, the pattern of Cs2Ca3(SO4)4 is distinct from the patterns of the raw reagents, indicating that the additional peaks are caused by other compounds. Considering our previously reported compound, Cs2Ca2(SO4)3, was prepared using the same raw reagents, it is imperative that its characteristic peaks are properly distinguished from those corresponding to Cs2Ca3(SO4)4, newly synthesized here. In conclusion, the impurity peaks indicated in Figure 1 could be caused by Cs2Ca2(SO4)3 [14].
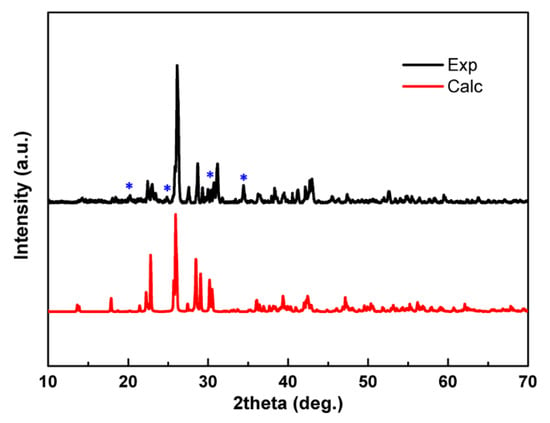
Figure 1.
Calculated and experimental XRD patterns for Cs2Ca3(SO4)4.
3.2. Crystal Structure
Crystal data for Cs2Ca3(SO4)4 (M = 770.30 g/mol) are as follows: monoclinic, space group P21/c (no. 14), a = 9.9153(8) Å, b = 9.3760(6) Å, c = 9.8044(9) Å, β = 118.365(3)°, V = 802.04(11) Å3, Z = 2, T = 299(2) K, μ(MoKα) = 6.104 mm−1, Dcalc = 3.190 g/cm3, 9439 reflections measured (2.33° ≤ 2Θ ≤ 27.45°), 1635 of them unique (Rint = 0.0506, Rsigma = 0.0359), which were used in all calculations. The final R1 was 0.0438 (I > 2σ(I)) and wR2 was 0.0864 (all data). The fundamental building units of Cs2Ca3(SO4)4 were CaO6 octahedra and SO4 tetrahedra. These groups are interconnected by sharing vertex oxygen atoms to form a three-dimensional framework with large cavities filled by Cs+ cations (Figure 2). Each Cs+ cation is coordinated to eight oxygen atoms to form a CsO8 polyhedron, with Cs–O bond distances ranging from 3.026(5) to 3.354(5) Å (Supplementary Figure S2), because the next closest atom is not oxygen but sulfur, with a Cs–S bond distance of 3.6568(15) Å. The S–O bond lengths are in the range of 1.453(5)–1.478(5) Å and O–S–O bond angles vary from 105.4(4) to 112.3(3)°. Each Ca atom is bound to six O atoms to generate a CaO6 octahedron, with Ca–O bond lengths ranging from 2.276(5) to 2.526(5) Å. These bond distances and angles appear reasonable when compared with those reported for other sulfates [14,18,20].
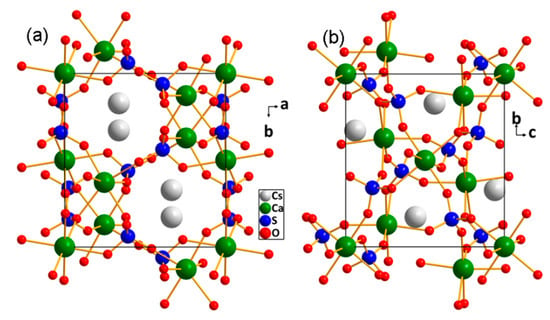
Figure 2.
Crystal structure of Cs2Ca3(SO4)4 viewed along (a) c-axis and (b) a-axis.
After comparing the structures of Cs2Mg3(SO4)4, Cs2Ca3(SO4)4, and Rb2Mg3(SO4)4, some interesting things were discovered [18]. First, Cs2Mg3(SO4)4 and Cs2Ca3(SO4)4 have distinct lattice types. The structure of the former belongs to a non-centrosymmetric P212121 space group, while the latter crystallizes in a centrosymmetric P21/c space group. Although they have similar three-dimensional frameworks made up of alkaline earth metal cation-centered polyhedra and SO4 tetrahedra, the connection mode is quite different. Figure 3 shows a structural comparison with regard to the coordination environment of alkali earth metal cations (Mg2+ vs. Ca2+). In Cs2Mg3(SO4)4, each Mg2+ cation is 4/5-fold coordinated to form MgO5 or MgO6 polyhedron, which is connected to five SO4 groups (Figure 3c,d). Interestingly, corner-sharing mode is used when the Mg2O6 octahedra link with four SO4 groups, and the linking with the additional SO4 group (five altogether) occurs through edge-sharing mode. In addition, the Mg1 atom connects to one Mg2 atom via a common oxygen atom (Figure 3d). When Mg2+ cations are replaced by Ca2+ cations of large radii in constructing the new compound Cs2Ca3(SO4)4, CaO6 octahedra connect to adjacent SO4 groups exclusively by corner-sharing mode because of the extended Ca–O bond distances. Moreover, the Ca2 atom in Cs2Ca3(SO4)4 bridges two adjacent Ca atoms, which is not observed in Cs2Mg3(SO4)4. The longer Ca–O bond makes the SO4 groups arrange more uniformly than those in Cs2Mg3(SO4)4. Second, Cs2Ca3(SO4)4 is isomorphous to Rb2Mg3(SO4)4, in which MO6 (M = Ca, Mg) octahedra connect to adjacent SO4 tetrahedra via corner-sharing mode (Figure 3e,f). The only difference is that MO6 octahedra have a different distortion, resulting from uneven bond distances. In Rb2Mg3(SO4)4, one Mg1–O bond distance (2.510 Å) is apparently longer than the other Mg–O bonds, which induces a large distortion of Mg1O6 octahedra. Such distortion is beneficial to the polarization anisotropy. In addition, the cation radius ratio of Cs/Ca, Rb/Mg, and Cs/Mg is 1.707, 2.27, and 2.60, respectively, indicating that the larger the difference in cation radius, the more easily the non-centrosymmetric structure is formed.
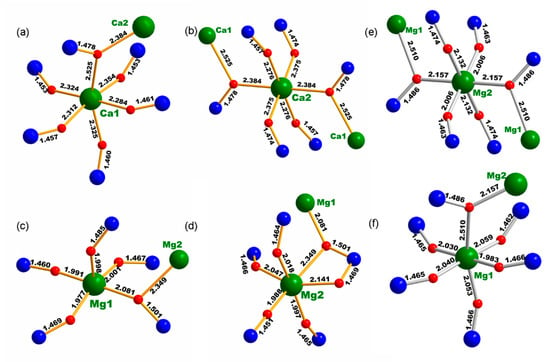
Figure 3.
Connection mode between (a,b) Ca and S atoms in Cs2Ca3(SO4)4 and between Mg and S atoms in (c,d) Cs2Mg3(SO4)4 and (e,f) Rb2Mg3(SO4)4.
3.3. UV–vis–NIR Diffuse Reflectance Spectroscopy
When light is projected onto a rough surface, it is reflected in all directions, which is referred to as diffuse reflectance. The measured UV–vis–NIR diffuse reflectance spectrum is depicted in Figure 4. Apparently, the reflectance of Cs2Ca3(SO4)4 is close to 100% in the wavelength range of 400–800 nm and higher than 60% in the range of 200–400 nm. Although the synthesized samples had low levels of Cs2Ca2(SO4)3, this impurity exhibited a higher reflectance than our measured value and, thus, the measured spectrum essentially shows reflectance due to Cs2Ca3(SO4)4. Therefore, it can be inferred that the energy band gap is greater than 6.2 eV, suggesting that Cs2Ca3(SO4)4 has a wide UV light window of penetration and may have a potential application as a window material.
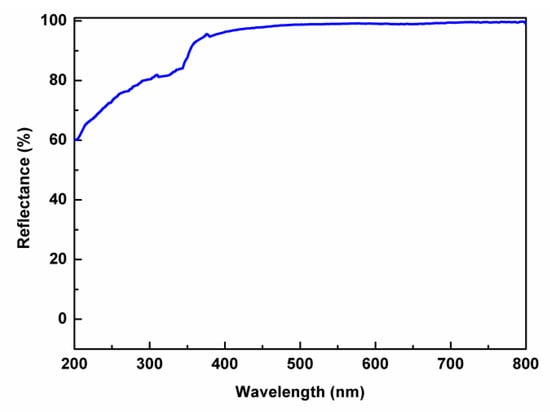
Figure 4.
Diffuse reflectance spectrum of Cs2Ca3(SO4)4.
3.4. Electronic Structure
Electronic structures can be used to verify diffuse reflectance spectra and understand the origin of optical properties. The energy band structure calculation demonstrates that the title compound exhibits a large band gap of 5.453 eV. Although the calculated value is slightly smaller than the experimental one, it basically verifies that Cs2Ca3(SO4)4 has a high reflectance in the UV region (Figure 5a). The total density of states (DOS) and partial DOS are shown in Figure 5b. In the energy region from −20 to −15 eV, the contribution of Ca-3p, Cs-5s, S-2p, and O-1s states dominates (Figure 5b). In the energy range from −10 to −5 eV, the DOS is mainly composed of Cs-6p, S-2p, and O-2p states. In the energy range from −5 to 0 eV, only O-2p states contribute. In the energy range from 5 to 10 eV, Cs-6s and Cs-6p states take up most of the DOS, while the contribution from S, O, and Ca elements gradually decreases. As we know, the optical absorption edge is closely related to the energy band gap determined by the states around the Fermi level [28]. From the above analysis, in the range of −5 to 10 eV, the elements S, O, and Cs make the largest contribution and, thus, determine the energy band gap of the title compound. In other words, SO4 groups and Cs+ cations represent the main contribution in determining the optical properties of Cs2Ca3(SO4)4.
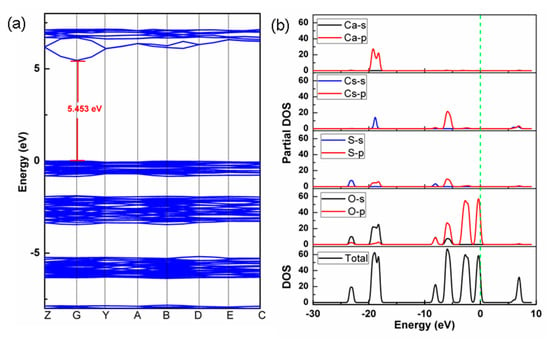
Figure 5.
(a) Electronic band structure; (b) DOS and partial DOS plots.
The birefringence of Cs2Ca3(SO4)4 and two similar compounds was calculated. As shown in Figure 6, the birefringence of the three compounds increases with the decreased incident wavelength, and the values are 0.0016 at 534 nm for Cs2Mg3(SO4)4, 0.0043 at 534 nm for Cs2Ca3(SO4)4, and 0.0175 at 534 nm for Rb2Mg3(SO4)4. Apparently, Cs2Ca3(SO4)4 has a birefringence higher than that of Cs2Mg3(SO4)4 but lower than that of Rb2Mg3(SO4)4, which results from the distinct anisotropy polarization of cationic polyhedra and the arrangement of SO4 groups. It is well known that SO4 groups in sulfates exist only as isolated units, so cations have a dominant role in controlling their arrangement.
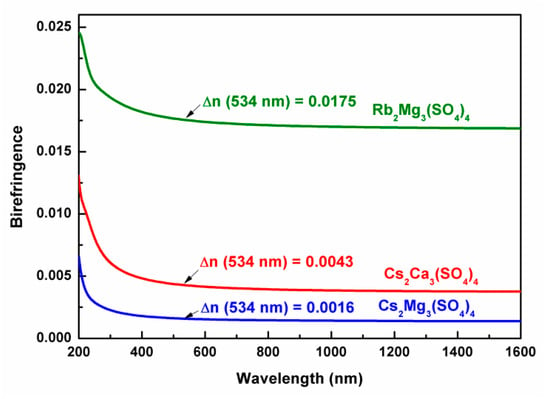
Figure 6.
Calculated birefringence for Cs2Ca3(SO4)4, Cs2Mg3(SO4)4, and Rb2Mg3(SO4)4.
4. Conclusions
Using an element substitution strategy with a template compound of Cs2Mg3(SO4)4, Cs2Ca3(SO4)4 crystals were synthesized using the spontaneous crystallization technique. The crystal structure has fundamental building units of CaO6 octahedra and SO4 groups, which are connected to each other through a corner-sharing mode. Structural comparisons indicate that the SO4 groups in Cs2Ca3(SO4)4 are more uniformly arranged than those in Cs2Mg3(SO4)4, which may be the reason for the sharply enhanced birefringence. The measured UV–vis–NIR diffuse reflectance spectrum shows that the title compound has a wide transparency window in the UV optical region. The first-principles calculations suggest that Cs+ cations and SO4 groups are responsible for the large energy band gap of Cs2Ca3(SO4)4. This work may provide a useful reference for understanding the role of cations in regulating crystal structures and optical properties.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst12020126/s1. Figure S1: Comparison of XRD patterns with raw reagents of Cs2SO4 and CaSO4, Figure S2: Ball-and-stick representation of CsO8 polyhedron in Cs2Ca3(SO4)4, Table S1: Crystal data and structure refinement for Cs2Ca3(SO4)4, Table S2: Atom coordinates and equivalent isotropic displacement parameters for Cs2Ca3(SO4)4, Table S3: Selected bond distances and angles for Cs2Ca3(SO4)4, Table S4: Anisotropic displacement parameters for Cs2Ca3(SO4)4.
Author Contributions
Writing—original draft preparation, P.F.; investigation, W.T.; writing—review and editing, Y.S.; conceptualization, J.H.; methodology, Y.S. and J.J.; software, Y.L.; supervision, Y.S.; funding acquisition, Y.S. and J.H.; P.F. and W.T. contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Start-up Funds for Introducing Talents, Minjiang University, Fujian Province, China, grant numbers MJY21029 and MJY19014; the Natural Science Foundation, Fujian Province, China, grant numbers 2021J011029 and 2019J01762; and the Middle-aged and Young Teachers’ Project, Fujian Province, China, grant number JAT200407.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the support from theoretical calculations conducted by Sangen Zhao at the Fujian Institute of Material Structure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Liang, F.; Zhao, S.; Li, L.; Wu, Z.; Ding, Q.; Liu, S.; Lin, Z.; Hong, M.; Luo, J. Two non-π-conjugated deep-UV nonlinear optical sulfates. J. Am. Chem. Soc. 2019, 141, 3833–3837. [Google Scholar] [CrossRef]
- Dong, X.; Huang, L.; Hu, C.; Zeng, H.; Lin, Z.; Wang, X.; Ok, K.M.; Zou, G. CsSbF2SO4: An excellent ultraviolet nonlinear optical sulfate with a KTiOPO4 (KTP)-type structure. Angew. Chem. 2019, 131, 6598–6604. [Google Scholar] [CrossRef]
- Yang, F.; Huang, L.; Zhao, X.; Huang, L.; Gao, D.; Bi, J.; Wang, X.; Zou, G. An energy band engineering design to enlarge the band gap of KTiOPO4 (KTP)-type sulfates via aliovalent substitution. J. Mater. Chem. C 2019, 7, 8131–8138. [Google Scholar] [CrossRef]
- Chen, K.C.; Yang, Y.; Peng, G.; Yang, S.D.; Yan, T.; Fan, H.X.; Lin, Z.S.; Ye, N. A2Bi2(SO4)2Cl4 (A = NH4, K, Rb): Achieving a subtle balance of the large second harmonic generation effect and sufficient birefringence in sulfate nonlinear optical materials. J. Mater. Chem. C 2019, 7, 9900–9907. [Google Scholar] [CrossRef]
- Tang, H.X.; Zhang, Y.X.; Zhuo, C.; Fu, R.B.; Lin, H.; Ma, Z.J.; Wu, X.T. A niobium oxyiodate sulfate with a strong second-harmonic-generation response built by rational multi-component design. Angew. Chem. Int. Ed. 2019, 58, 3824–3828. [Google Scholar] [CrossRef]
- Wu, C.; Wu, T.H.; Jiang, X.X.; Wang, Z.J.; Sha, H.Y.; Lin, L.; Lin, Z.S.; Huang, Z.P.; Long, X.F.; Humphrey, M.G.; et al. Large second-harmonic response and giant birefringence of CeF2(SO4) induced by highly polarizable polyhedra. J. Am. Chem. Soc. 2021, 143, 4138–4142. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.Y.; Lin, C.S.; Peng, G.; Fan, H.X.; Zhao, X.; Chen, K.C.; Luo, M.; Ye, N. Te(CS(NH2)2)4SO4·2H2O: A three-in-one semiorganic nonlinear optical crystal with an unusual quadrilateral (TeS4)6− chromophore. Cryst. Growth Des. 2021, 21, 2596–2601. [Google Scholar] [CrossRef]
- Ramajothi, J.; Dhanuskodi, S.; Nagarajan, K. Crystal growth, thermal, optical and microhardness studies of tris (thiourea) zinc sulphate—A semiorganic NLO material. Cryst. Res. Technol. 2004, 39, 414–420. [Google Scholar] [CrossRef]
- Peng, G.; Lin, C.-S.; Yang, Y.; Zhao, D.; Lin, Z.; Ye, N.; Huang, J.-S. Y2(CO3)3·H2O and (NH4)2Ca2Y4(CO3)9·H2O: Partial aliovalent cation substitution enabling evolution from centrosymmetry to noncentrosymmetry for nonlinear optical response. Chem. Mater. 2019, 31, 52–56. [Google Scholar] [CrossRef]
- Lu, J.; Yue, J.-N.; Xiong, L.; Zhang, W.-K.; Chen, L.; Wu, L.-M. Uniform alignment of non-π-conjugated species enhances deep ultraviolet optical nonlinearity. J. Am. Chem. Soc. 2019, 141, 8093–8097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, D.H.; Hu, C.; Chen, X.L.; Yang, Z.H.; Pan, S.L. Rational design via synergistic combination leads to an outstanding deep-ultraviolet birefringent Li2Na2B2O5 material with an unvalued B2O5 functional gene. J. Am. Chem. Soc. 2019, 141, 3258–3264. [Google Scholar] [CrossRef]
- Yang, Y.C.; Liu, X.; Lu, J.; Wu, L.M.; Chen, L. [Ag(NH3)2]2SO4: A strategy for the coordination of cationic moieties to design nonlinear optical materials. Angew. Chem. Int. Ed. 2021, 60, 21216–21220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, S.; Shan, P.; Li, X.; Ding, Q.; Liu, S.; Wu, Z.; Wang, S.; Li, L.; Luo, J. Li8NaRb3(SO4)6·2H2O as a new sulfate deep-ultraviolet nonlinear optical material. J. Mater. Chem. C 2018, 6, 12240–12244. [Google Scholar] [CrossRef]
- Shen, Y.G.; Xue, X.L.; Tu, W.Y.; Liu, Z.Q.; Yan, R.W.; Zhang, H.; Jia, J.R. Synthesis, crystal structure, and characterization of a noncentrosymmetric sulfate Cs2Ca2(SO4)3. Eur. J. Inorg. Chem. 2020, 2020, 854–858. [Google Scholar] [CrossRef]
- Sudhakar, K.; Nandhini, S.; Muniyappan, S.; Arumanayagam, T.; Vivek, P.; Murugakoothan, P. Synthesis, crystal growth, optical, thermal, and mechanical properties of a nonlinear optical single crystal: Ammonium sulfate hydrogen sulphamate (ASHS). Appl. Phys. A Mater. 2018, 124, 334. [Google Scholar] [CrossRef]
- He, F.F.; Wang, Q.; Hu, C.F.; He, W.; Luo, X.Y.; Huang, L.; Gao, D.J.; Bi, J.; Wang, X.; Zou, G.H. Centrosymmetric (NH4)2SbCl(SO4)2 and non-centrosymmetric NH4SbCl2SO4: Synergistic effect of hydrogen-bonding interactions and lone-pair cations on the framework structures and macroscopic centricities. Cryst. Growth Des. 2018, 18, 6239–6247. [Google Scholar] [CrossRef]
- He, F.F.; Wang, L.; Hu, C.F.; Zhou, J.; Li, Q.; Huang, L.; Gao, D.J.; Bi, J.; Wang, X.; Zou, G.H. Cation-tuned synthesis of the A2SO4·SbF3 (A = Na+, NH4+, K+, Rb+) family with nonlinear optical properties. Dalton Trans. 2018, 47, 17486–17492. [Google Scholar] [CrossRef]
- Wang, M.; Wei, D.Q.; Liang, L.J.; Yan, X.; Lv, K.K. Centrosymmetric Rb2Mg3(SO4)4 and non-centrosymmetric Cs2Mg3(SO4)4 with a phase-matching nonlinear optical response. Inorg. Chem. Commun. 2019, 107, 107486. [Google Scholar] [CrossRef]
- Shen, Y.G.; Xue, X.L.; Yan, R.W.; Lin, H. Synthesis, characterizations and theoretical analysis of a noncentrosymmetric sulfate. Inorg. Chem. Commun. 2020, 116, 107899. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Wu, E.Q.; Yang, S.D.; Wang, H.H.; Lin, X.X.; Shen, Y.G. Optical properties and thermal stability of a cubic sulfate Rb2Ca2(SO4)3. Chin. J. Struc. Chem. 2021, 40, 949–954. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sec. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Ceperley, D.M.; Alder, B.J. Ground-state of the electron-gas by a stochastic method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef] [Green Version]
- Rappe, A.M.; Rabe, K.M.; Kaxiras, E.; Joannopoulos, J.D. Optimized pseudopotentials. Phys. Rev. B 1990, 41, 1227–1230. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, C.H.; Jan, J.H. Band-resolved analysis of nonlinear optical properties of crystalline and molecular materials. Phys. Rev. B 2004, 70, 235110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).