Changes in the Optical Properties of an M-Doped (M = Pt, Ti) hBN Sheet and CO2 Capturing
Abstract
1. Introduction
2. Materials and Methods
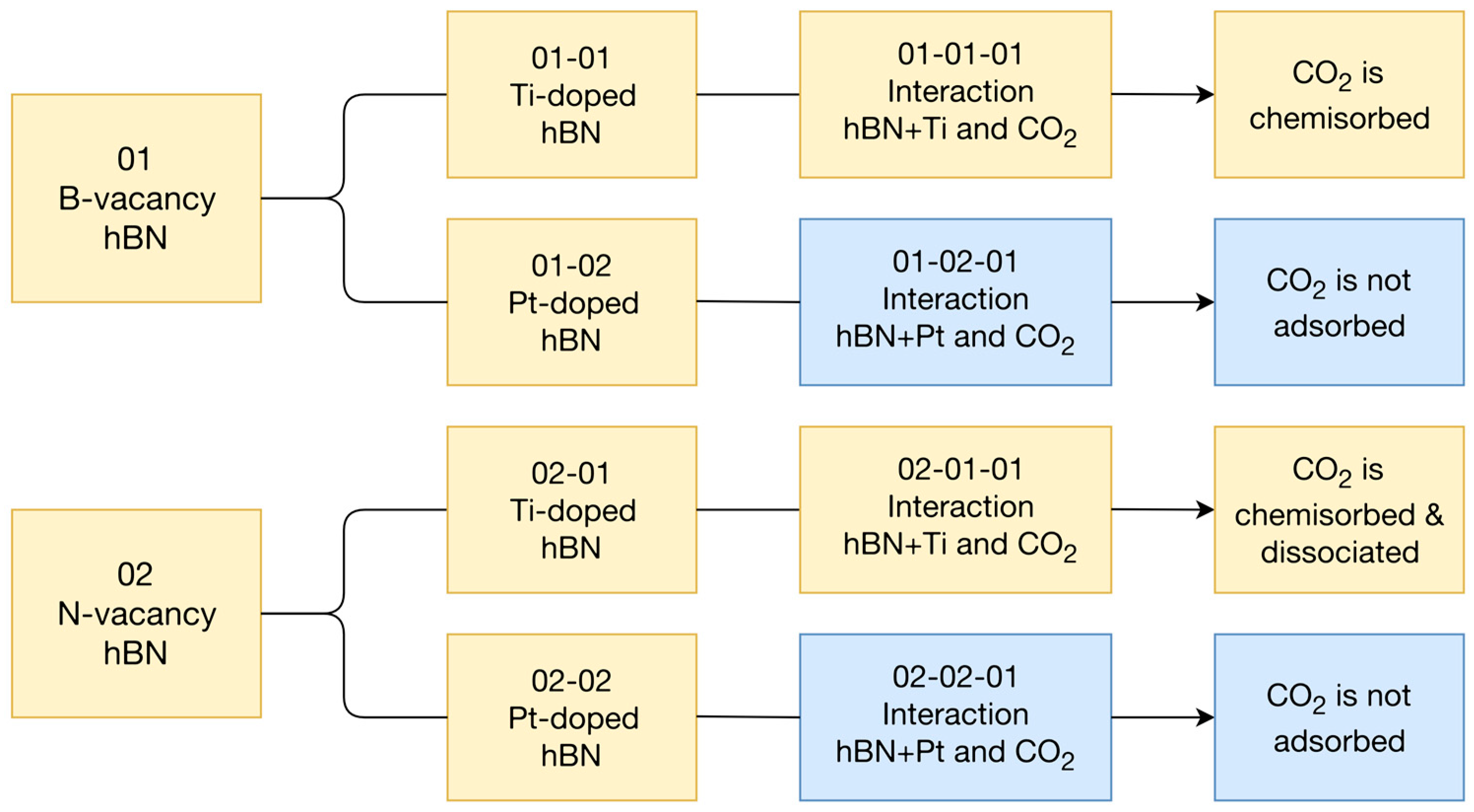
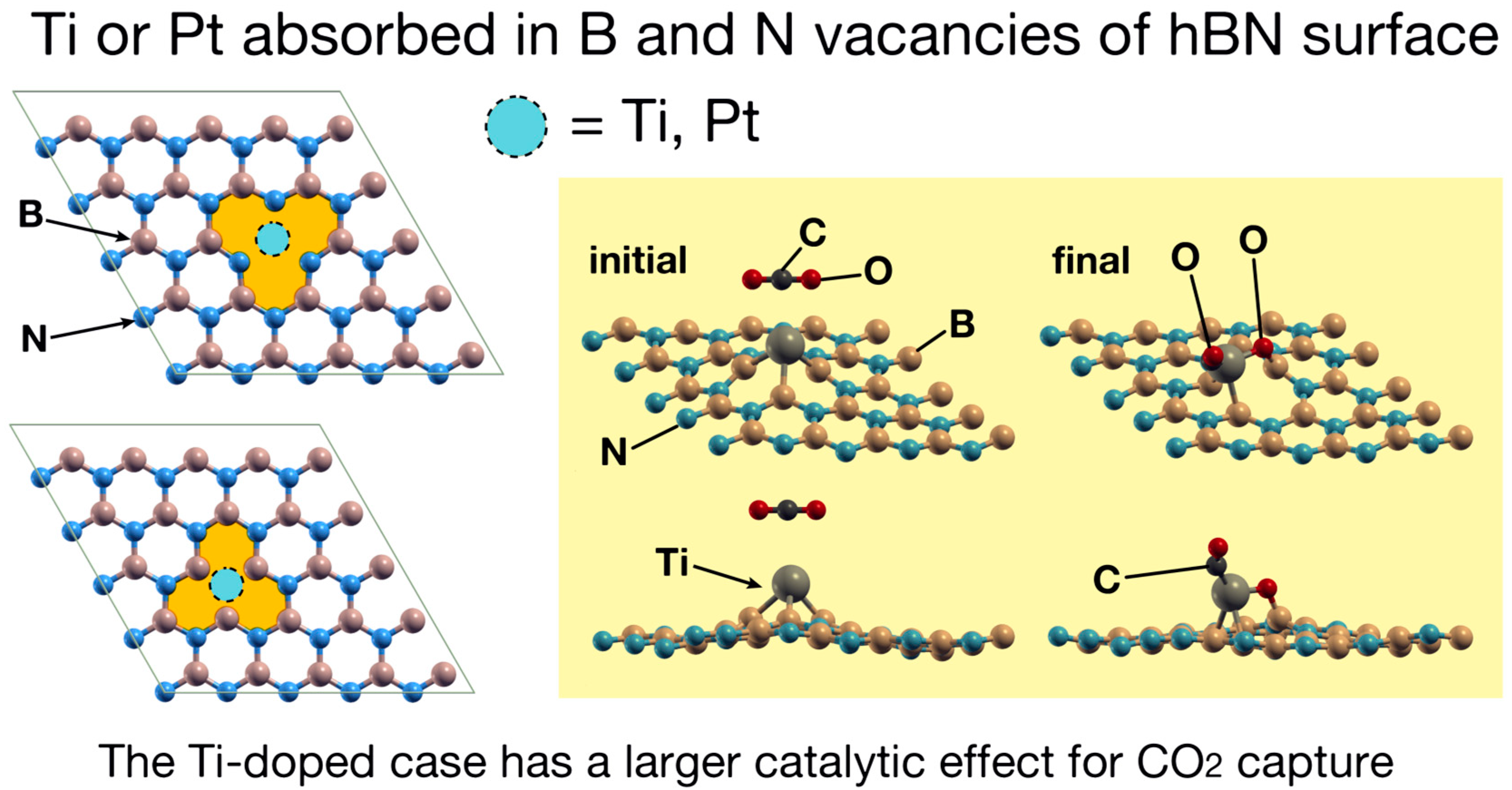
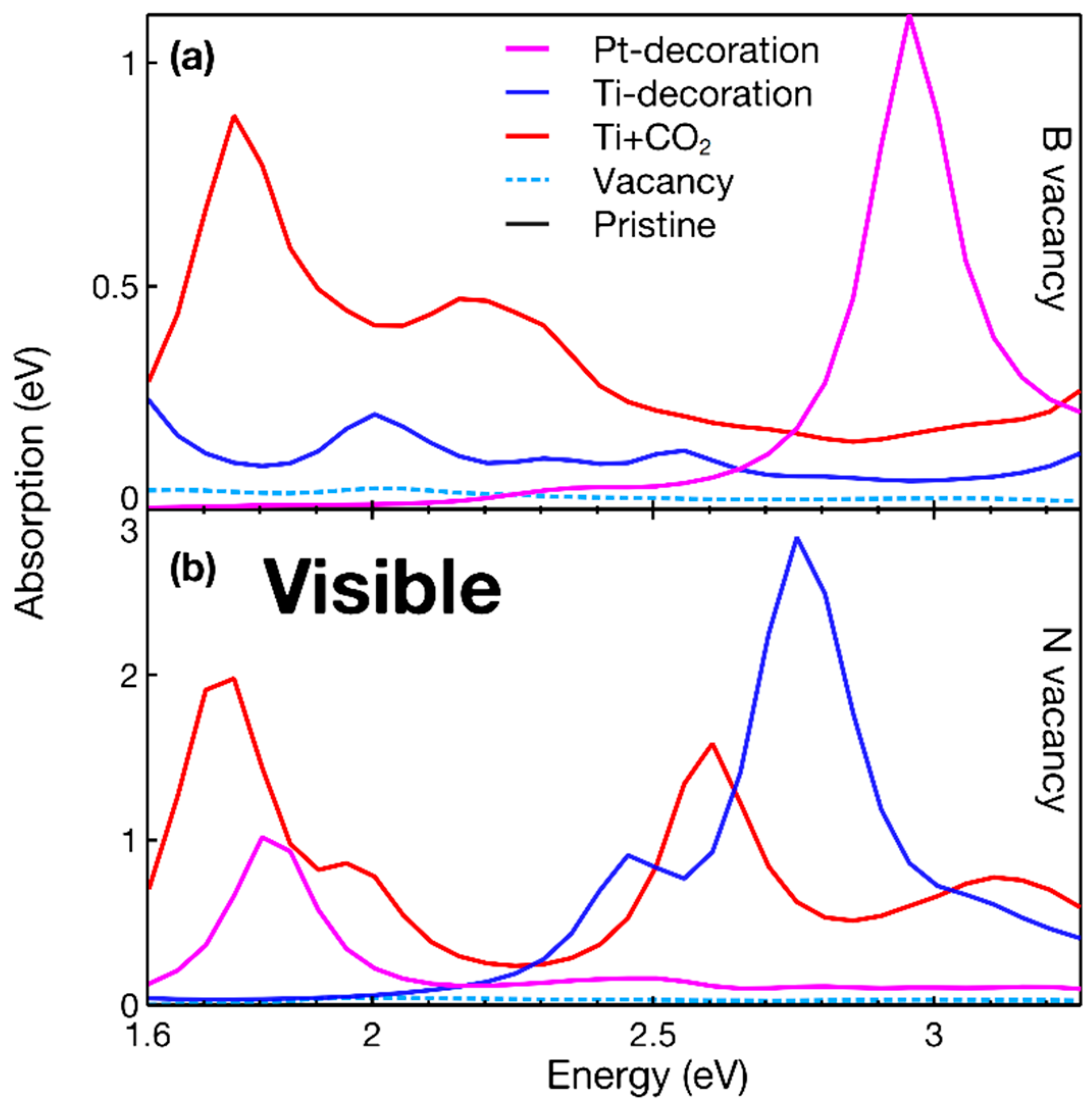
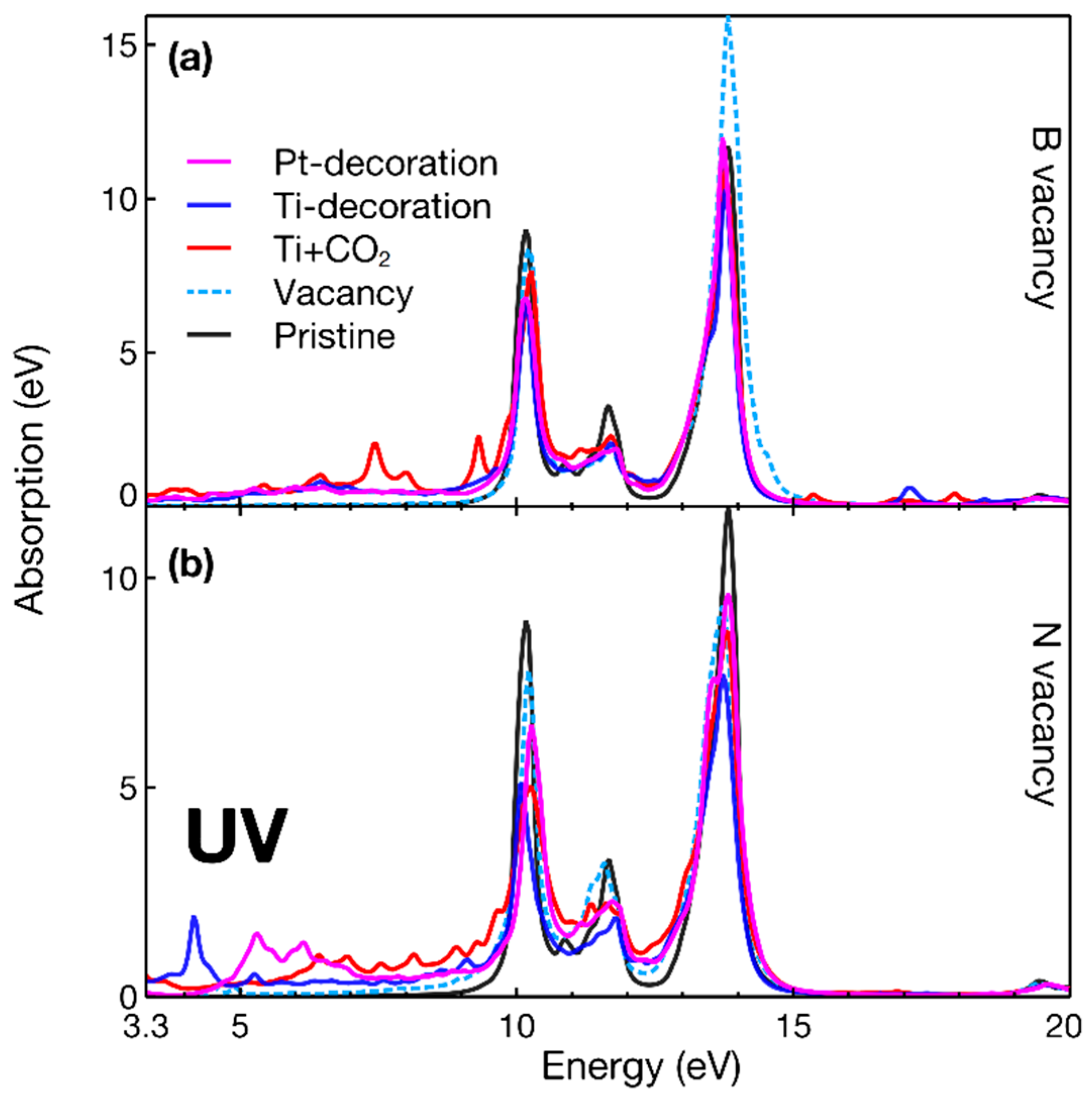
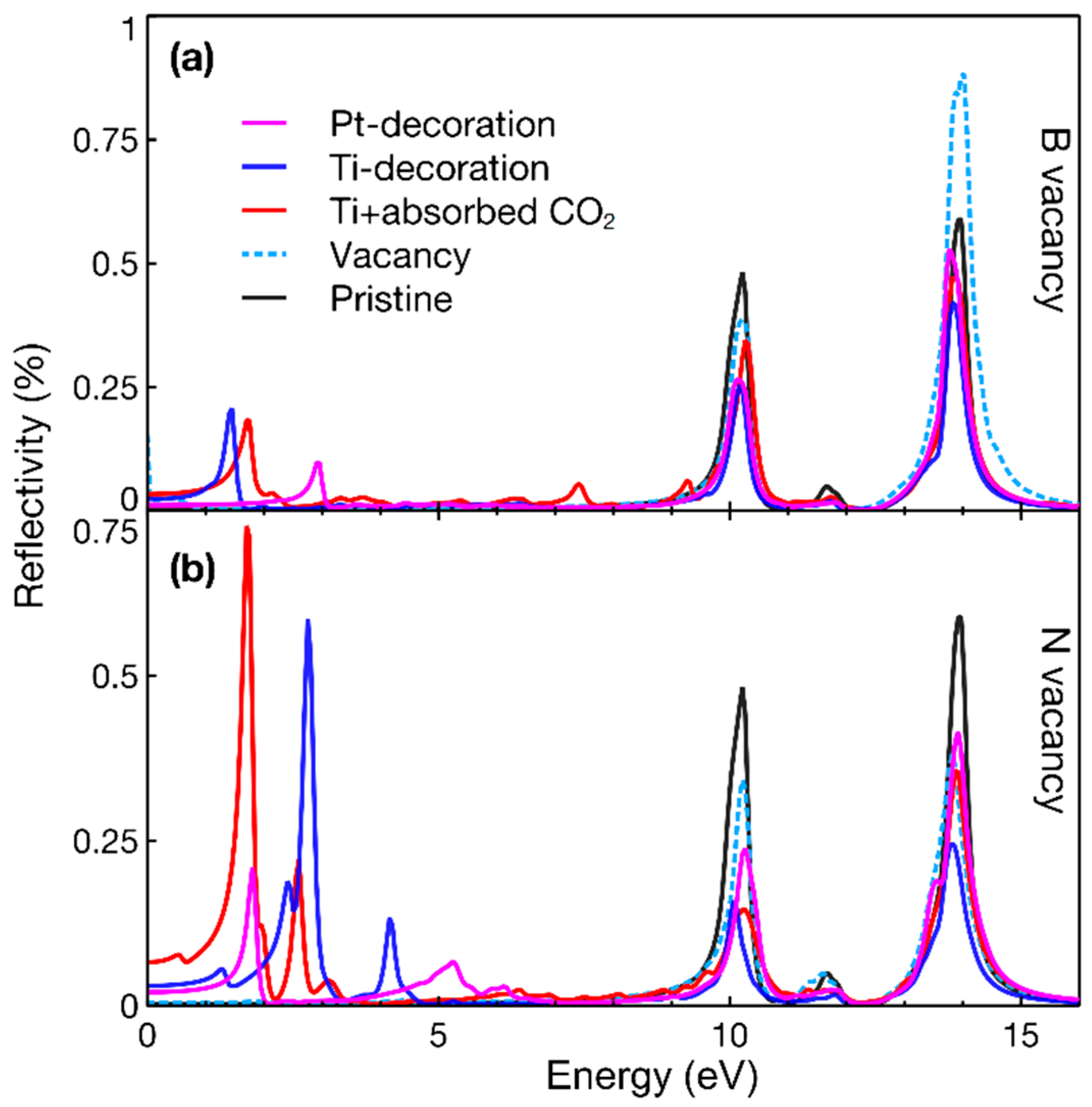
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edgar, J.H.; Hoffman, T.B.; Clubine, B.; Currie, M.; Du, X.Z.; Lin, J.Y.; Jiang, H.X. Characterization of Bulk Hexagonal Boron Nitride Single Crystals Grown by the Metal Flux Technique. J. Cryst. Growth 2014, 403, 110–113. [Google Scholar] [CrossRef]
- Cao, X.K.; Clubine, B.; Edgar, J.H.; Lin, J.Y.; Jiang, H.X. Two-Dimensional Excitons in Three-Dimensional Hexagonal Boron Nitride. Appl. Phys. Lett. 2013, 103, 191106. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Y.; Wang, F.; Yang, Z.; Wang, J. Two Dimensional Hexagonal Boron Nitride (2D-HBN): Synthesis, Properties and Applications. J. Mater. Chem. C 2017, 5, 11992–12022. [Google Scholar] [CrossRef]
- Pease, R.S. Crystal Structure of Boron Nitride. Nature 1950, 165, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Pease, R.S. An X-Ray Study of Boron Nitride. Acta Crystallogr. 1952, 5, 356–361. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Will, G.; Elf, F. Isothermal Compression of Hexagonal Graphite-like Boron Nitride up to 12 GPa. Solid State Commun. 1995, 96, 1–3. [Google Scholar] [CrossRef]
- Kim, K.K.; Kim, S.M.; Lee, Y.H. A New Horizon for Hexagonal Boron Nitride Film. J. Korean Phys. Soc. 2014, 64, 1605–1616. [Google Scholar] [CrossRef]
- Maestre, C.; Toury, B.; Steyer, P.; Garnier, V.; Journet, C. Hexagonal Boron Nitride: A Review on Selfstanding Crystals Synthesis towards 2D Nanosheets. J. Phys. Mater. 2021, 4, 044018. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-Bandgap Properties and Evidence for Ultraviolet Lasing of Hexagonal Boron Nitride Single Crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef]
- Said, A.; Debbichi, M.; Said, M. Theoretical Study of Electronic and Optical Properties of BN, GaN and BxGa1−xN in Zinc Blende and Wurtzite Structures. Optik 2016, 127, 9212–9221. [Google Scholar] [CrossRef]
- Henriques, J.C.G.; Ventura, G.B.; Fernandes, C.D.M.; Peres, N.M.R. Optical Absorption of Single-Layer Hexagonal Boron Nitride in the Ultraviolet. J. Phys. Condens. Matter 2020, 32, 025304. [Google Scholar] [CrossRef] [PubMed]
- Uosaki, K.; Elumalai, G.; Noguchi, H.; Masuda, T.; Lyalin, A.; Nakayama, A.; Taketsugu, T. Boron Nitride Nanosheet on Gold as an Electrocatalyst for Oxygen Reduction Reaction: Theoretical Suggestion and Experimental Proof. J. Am. Chem. Soc. 2014, 136, 6542–6545. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Jamali, S.S.; Lyalin, A.; Molino, P.J.; Jiang, L.; Liu, H.K.; Taketsugu, T.; Huang, Z. Atomically Thin Hexagonal Boron Nitride Nanofilm for Cu Protection: The Importance of Film Perfection. Adv. Mater. 2017, 29, 1603937. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Veettil Vineesh, T.; Sekar, A.; Narayanaru, S.; Sahoo, M.; Nayak, S.; Chakraborty, S.; Narayanan, T.N. Mechanistic Insight into Enhanced Hydrogen Evolution Reaction Activity of Ultrathin Hexagonal Boron Nitride-Modified Pt Electrodes. ACS Catal. 2018, 8, 6636–6644. [Google Scholar] [CrossRef]
- Han, R.; Liu, F.; Wang, X.; Huang, M.; Li, W.; Yamauchi, Y.; Sun, X.; Huang, Z. Functionalised Hexagonal Boron Nitride for Energy Conversion and Storage. J. Mater. Chem. A 2020, 8, 14384–14399. [Google Scholar] [CrossRef]
- Hasan Khan, M.S.; Mime, F.I.; Islam, M.R. Electronic and Optical Properties of Sn Doped Hexagonal BN Monolayer: A First-Principles Study. In Proceedings of the 2020 IEEE Region 10 Symposium (TENSYMP), Dhaka, Bangladesh, 5–7 June 2020; pp. 230–233. [Google Scholar]
- Ramirez-de-Arellano, J.M.; Jiménez, G.A.F.; Magaña, L.F. Catalytic Effect of Ti or Pt in a Hexagonal Boron Nitride Surface for Capturing CO2. Crystals 2021, 11, 662. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A–Z Guide to the Elements; Reprinted with Corrections; Oxford University Press: Oxford, UK, 2003; ISBN 978-0-19-850340-8. [Google Scholar]
- Deng, C.; He, R.; Shen, W.; Li, M. Theoretical Analysis of Oxygen Reduction Reaction Activity on Single Metal (Ni, Pd, Pt, Cu, Ag, Au) Atom Supported on Defective Two-Dimensional Boron Nitride Materials. Phys. Chem. Chem. Phys. 2019, 21, 18589–18594. [Google Scholar] [CrossRef]
- Deng, C.; He, R.; Wen, D.; Shen, W.; Li, M. Theoretical Study on the Origin of Activity for the Oxygen Reduction Reaction of Metal-Doped Two-Dimensional Boron Nitride Materials. Phys. Chem. Chem. Phys. 2018, 20, 10240–10246. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Cottenier, S. Density Functional Theory and the Family of (L)APW-Methods: A Step-by-Step Introduction, 2nd ed.; Center for Molecular Modeling (CMM) & Department of Materials Science and Engineering (DMSE), Ghent University: Ghent, Belgium, 2013. [Google Scholar]
- Troullier, N.; Martins, J. A Straightforward Method for Generating Soft Transferable Pseudopotentials. Solid State Commun. 1990, 74, 613–616. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Harrison, N.M.; Gillan, M.J.; White, J.A. First-Principles Spin-Polarized Calculations on the Reduced and Reconstructed TiO2 (110) Surface. Phys. Rev. B 1997, 55, 15919–15927. [Google Scholar] [CrossRef]
- Bohm, D.; Pines, D. A Collective Description of Electron Interactions: III. Coulomb Interactions in a Degenerate Electron Gas. Phys. Rev. 1953, 92, 609–625. [Google Scholar] [CrossRef]
- Lucarini, V. (Ed.) Kramers-Kronig Relations in Optical Materials Research; Springer Series in Optical Sciences; Springer: New York, NY, USA, 2005; ISBN 978-3-540-23673-3. [Google Scholar]
- Jiménez-González, A.F.; Ramírez-de-Arellano, J.M.; Magaña, L.F. Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change. Int. J. Mol. Sci. 2021, 22, 6832. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—A New Program for Displaying Crystalline Structures and Electron Densities. J. Mol. Graph. Model. 1999, 17, 176–179. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-de-Arellano, J.M.; Jiménez-González, A.F.; Magaña, L.F. Changes in the Optical Properties of an M-Doped (M = Pt, Ti) hBN Sheet and CO2 Capturing. Crystals 2022, 12, 1773. https://doi.org/10.3390/cryst12121773
Ramírez-de-Arellano JM, Jiménez-González AF, Magaña LF. Changes in the Optical Properties of an M-Doped (M = Pt, Ti) hBN Sheet and CO2 Capturing. Crystals. 2022; 12(12):1773. https://doi.org/10.3390/cryst12121773
Chicago/Turabian StyleRamírez-de-Arellano, Juan Manuel, Ali Fransuani Jiménez-González, and Luis Fernando Magaña. 2022. "Changes in the Optical Properties of an M-Doped (M = Pt, Ti) hBN Sheet and CO2 Capturing" Crystals 12, no. 12: 1773. https://doi.org/10.3390/cryst12121773
APA StyleRamírez-de-Arellano, J. M., Jiménez-González, A. F., & Magaña, L. F. (2022). Changes in the Optical Properties of an M-Doped (M = Pt, Ti) hBN Sheet and CO2 Capturing. Crystals, 12(12), 1773. https://doi.org/10.3390/cryst12121773








