Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results
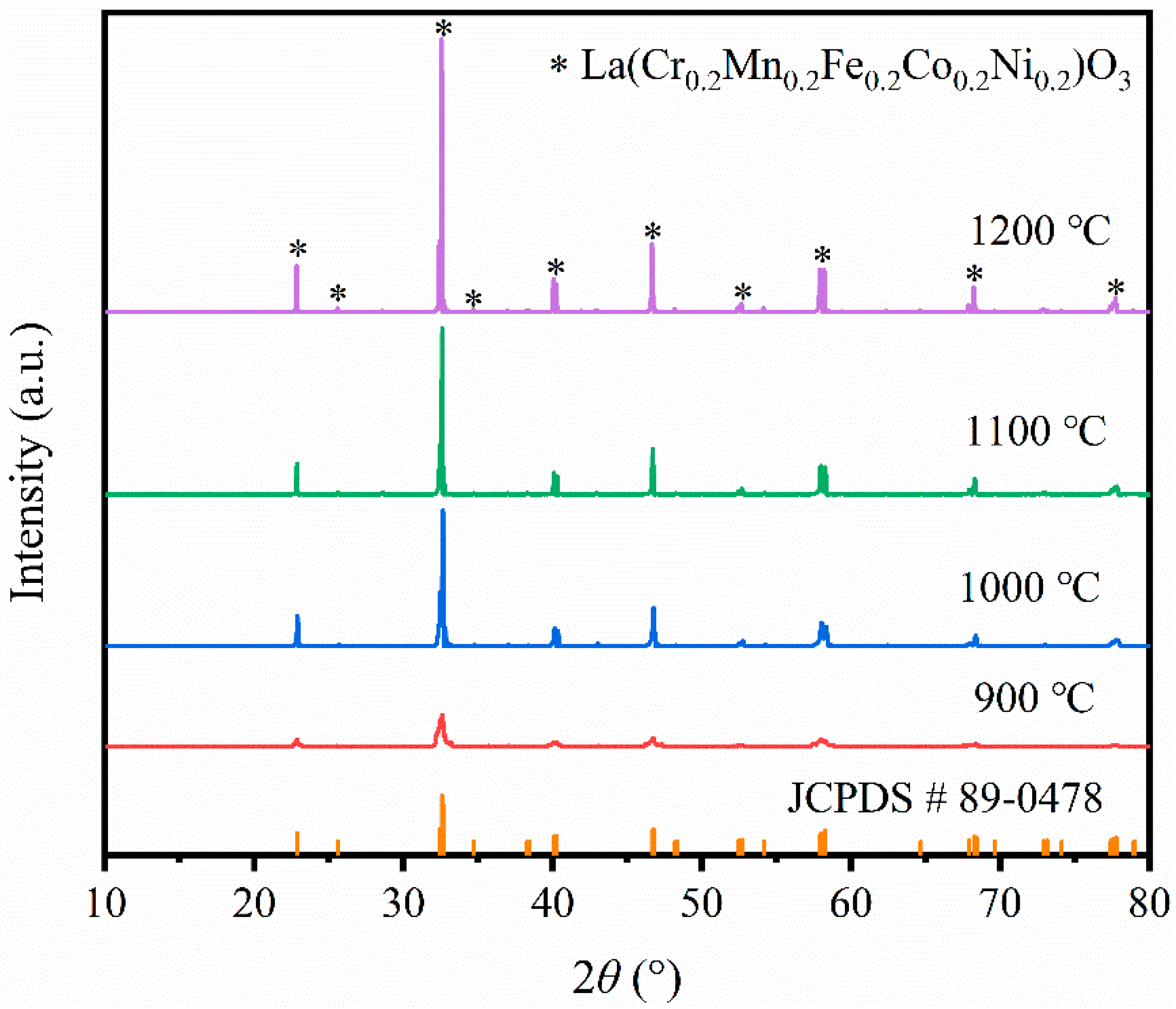
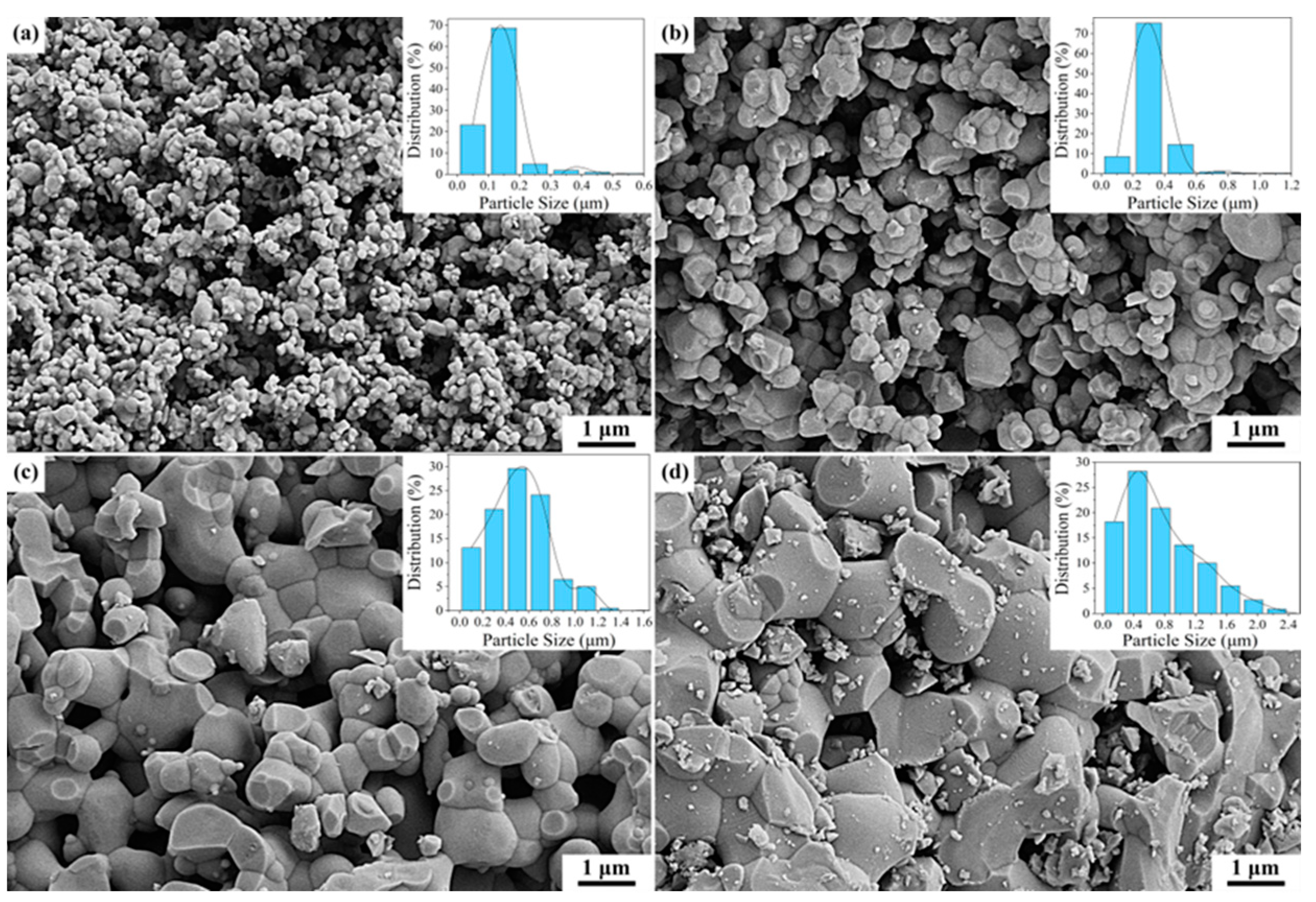
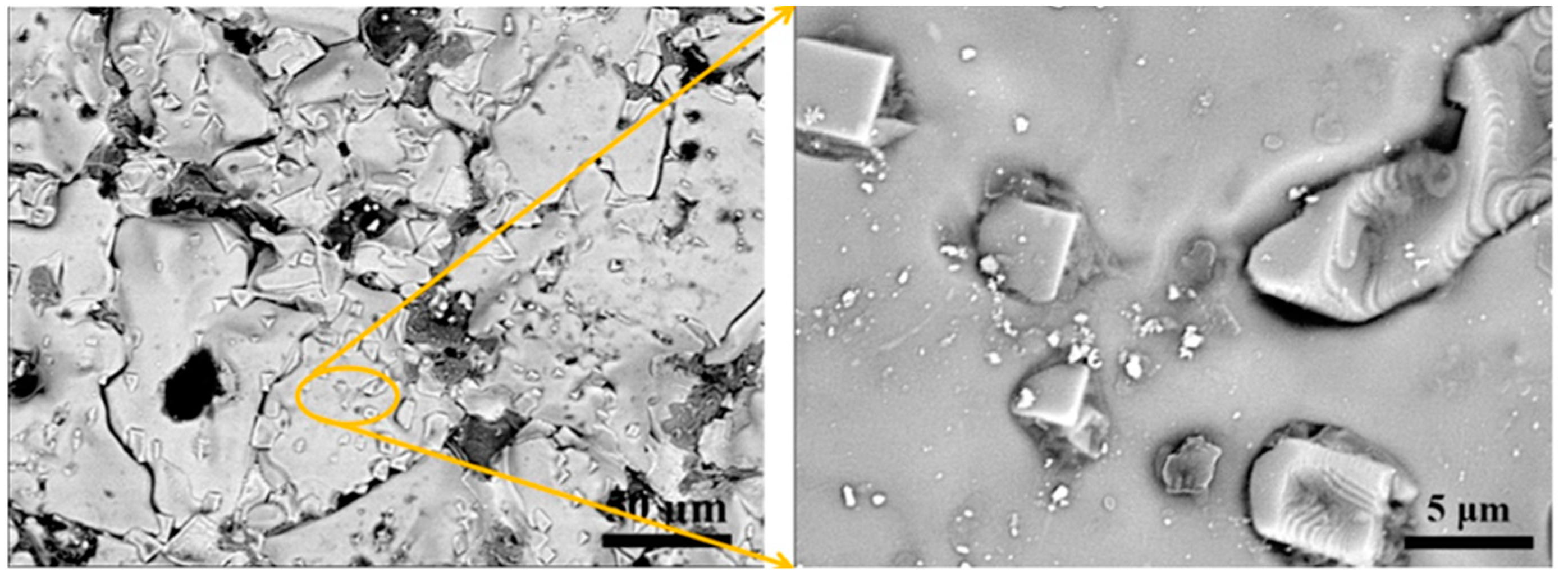
3.1. Phase Composition and Microstructural Analysis
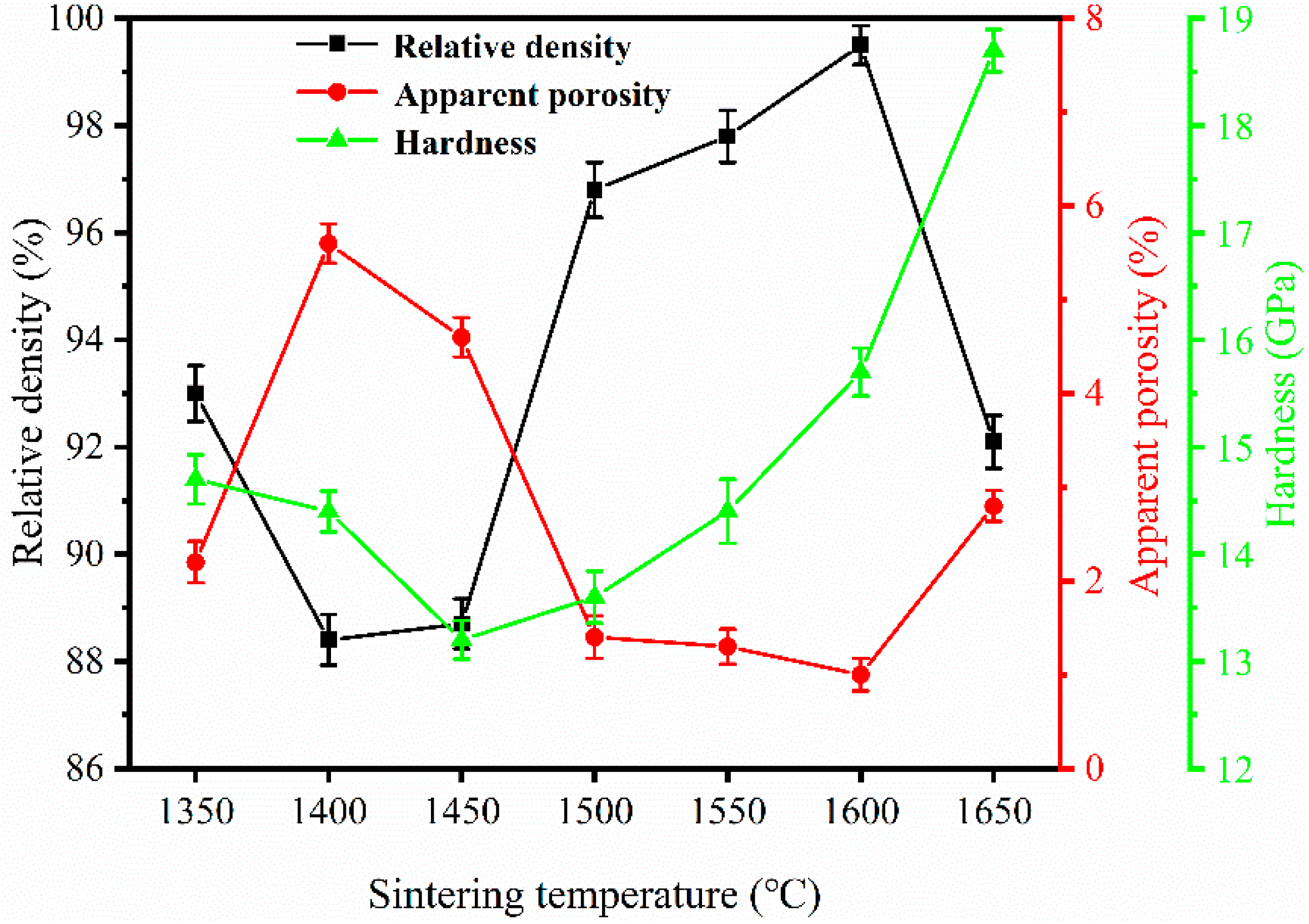
3.2. Density, Apparent Porosity, and Hardness
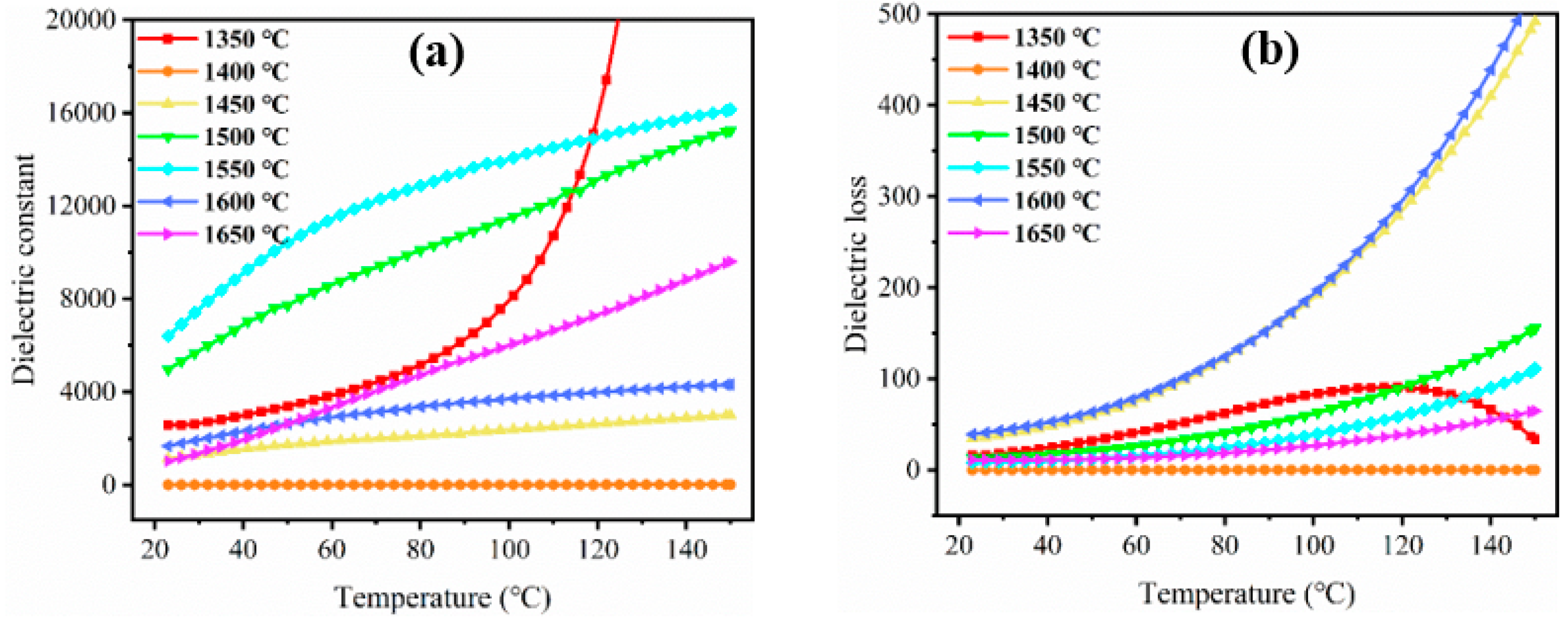
3.3. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156–158. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Hu, C.; Yeh, Y.; Chen, R.; Luo, J. Single-phase duodenary high-entropy fluorite/pyrochlore oxides with an order-disorder transition. Acta Mater. 2021, 211, 116858. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xu, J.; Zhang, P.; Lou, Z.; Reece, M.J.; Gao, F. Ultra-low thermal conductivity and enhanced mechanical properties of high-entropy rare earth niobates (RE3NbO7, RE = Dy, Y., Ho, Er, Yb). J. Eur. Ceram. Soc. 2021, 41, 1052–1057. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. J. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, M.; Zhang, W.; Yi, D.; Lan, J.; Nan, C.; Lin, Y. Electrical and thermal transport behaviours of high-entropy perovskite thermoelectric oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Guo, J.; Zhu, Y.; Feng, J. High-entropy perovskite RETa3O9 ceramics for high-temperature environmental/thermal barrier coatings. J. Adv. Ceram. 2022, 11, 556–569. [Google Scholar] [CrossRef]
- Wang, J.; Stenzel, D.; Azmi, R.; Najib, S.; Breitung, B. Spinel to Rock-Salt Transformation in High Entropy Oxides with Li Incorporation. Electrochem 2020, 1, 60–74. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, B.; Xiang, H.; Dai, F.Z.; Liu, Y.; Zhang, R.; Zhou, Y. High-entropy spinel ferrites MFe2O4 (M = Mg, Mn, Fe, Co, Ni, Cu, Zn) with tunable electromagnetic properties and strong microwave absorption. J. Adv. Ceram. 2022, 11, 754–768. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, H.; Ning, S.; Chu, Y. Chrysanthemum-like high-entropy diboride nanoflowers: A new class of high- entropy nanomaterials. J. Adv. Ceram. 2020, 9, 339–348. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, J.; Zhang, Y.; Shao, G.; Wang, H.; Li, M.; Liu, W.; Fan, B.; Xu, H.; Lu, H.; et al. A novel high-entropy monoboride (Mo0.2Ta0.2Ni0.2Cr0.2W0.2)B with superhardness and low thermal conductivity. Ceram. Int. 2020, 46, 26626–26631. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980–4989. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Low-temperature sintering of single-phase, high-entropy carbide ceramics. J. Am. Ceram. Soc. 2019, 102, 7217–7224. [Google Scholar] [CrossRef]
- Wei, X.; Liu, J.; Li, F.; Qin, Y.; Liang, Y.; Zhang, G. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc. 2019, 39, 2989–2994. [Google Scholar] [CrossRef]
- Jin, T.; Sang, X.; Unocic, R.R.; Kinch, R.T.; Liu, X.; Hu, J.; Liu, H.; Dai, S. Mechanochemical-Assisted Synthesis of High-Entropy Metal Nitride via a Soft Urea Strategy. Adv. Mater. 2018, 30, 1707512. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.E.; Vecchio, K.; Luo, J. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.; Li, F.; Wei, X.; Wu, H.; Zhang, G.J. A high entropy silicide by reactive spark plasma sintering. J. Adv. Ceram. 2019, 8, 148–152. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Schwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An innovative approach to design SOFC air electrode materials: High entropy La1−xSrx(Co,Cr,Fe,Mn, Ni)O3−δ (x=0, 0.1, 0.2, 0.3) perovskites synthesized by the sol–gel method. J. Mater. Chem. A 2020, 8, 24455–24468. [Google Scholar] [CrossRef]
- Wang, N.; Cao, M.; He, Z.; Diao, C.; Zhang, Q.; Zhang, Y.; Dai, J.; Zeng, F.; Hao, H.; Yao, Z. Structural and dielectric behavior of giant permittivity SrNbxTi1−xO3 ceramics sintered in nitrogen atmosphere. Ceram. Int. 2016, 42, 13593–13600. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, M.; Zhang, Q.; Hao, H.; Yao, Z. Dielectric Relaxation in Zr-Doped SrTiO3 Ceramics Sintered in N2 with Giant Permittivity and Low Dielectric Loss. J. Am. Ceram. Soc. 2015, 98, 476–482. [Google Scholar] [CrossRef]
- Wang, K.F.; Liu, J.M.; Ren, Z.F. ChemInform Abstract: Multiferroicity—The Coupling Between Magnetic and Polarization Orders. Adv. Phys. 2009, 58, 321–448. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and Magnetoelectric Materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Bokov, A.A.; Ye, Z.G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Chem. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Yokokawa, H.; Sakai, N.; Kawada, T.; Dokiya, M. Chemical thermodynamic considerations in sintering of LaCrO3-based perovskites. J. Electrochem. Soc. 1991, 138, 1018–1027. [Google Scholar] [CrossRef]
- Graham, H.C.; Davis, H.H. Oxidation/vaporization kinetics of Cr2O3. J. Am. Ceram. Soc. 1971, 54, 89–93. [Google Scholar] [CrossRef]
- Mori, M.; Yamamoto, T.; Ichikawa, T.; Takeda, Y. Dense sintered conditions and sintering mechanisms for alkaline earth metal (Mg, Ca and Sr)-doped LaCrO3 perovskites under reducing atmosphere. Solid State Ion. 2002, 148, 93–101. [Google Scholar] [CrossRef]
- Bhatt, H.; Bahadur, J.; Deo, M.N.; Ramanathan, S.; Pandey, K.K.; Sen, D.; Mazumder, S.; Sharma, S.M. Effects of calcination on microscopic and mesoscopic structures in Ca- and Sr-doped nano-crystalline lanthanum chromites. J. Solid. State. Chem. 2011, 184, 204–213. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Gao, L.; Guo, L. Effects of cation substitution on thermal expansion and electrical properties of lanthanum chromites. J. Alloys Compd. 2006, 425, 318–322. [Google Scholar] [CrossRef]
- Simner, S.P.; Hardy, J.S.; Stevenson, J.W. Sintering and properties of mixed lanthanide chromites. J. Electrochem. Soc. 2001, 148, A351. [Google Scholar] [CrossRef]
- Ga, A.; Yin, X.L.; Zhao, Q.; He, D.L.; Zhao, Y.; Chang, A.M. A study based on MgAl2O4-LaCrO3 composite ceramics for high temperature NTC thermistors. J. Mater. Sci. Mater. Electron. 2019, 30, 11117–11122. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, B.; Zhao, Q.; He, D.L.; Chang, A.M. Electrical characterization of 0.6MgAl(2)O(4)-0.4La(1−x)Sr(x)CrO3 high temperature NTC thermistors. J. Mater. Sci. Mater. Electron. 2017, 28, 16036–16043. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, F.; Miao, Y.; Liu, Y.; Gao, F. Preparation and Electrical Properties of High Entropy La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Perovskite Ceramics Powder. J. Inorg. Mater. 2021, 36, 431–435. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Pashkeev, I.Y.; Vinnik, D.A. Polysubstituted High-Entropy [LaNd](Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Perovskites: Correlation of the Electrical and Magnetic Properties. Nanomaterials 2021, 11, 1014. [Google Scholar] [CrossRef]
- Joly, V.L.J.; Joy, P.A.; Date, S.K.; Gopinath, C.S. Two ferromagnetic phases with different spin states of Mn and Ni in LaMn0.5Ni0.5O3. Phys. Rev. B 2002, 65, 184416. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, R.K.; Sinha, A.; Singh, P. Effect of isovalent ion substitution on electrical and dielectric properties of LaCrO3. J. Alloys Compd. 2013, 576, 154–160. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Moldschmidt, G.V. Die Gsetze der Krystallochemie. Naturwissienschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Ramadass, N. ABO3-type oxides—Their structure and properties—A bird’s eye view. J. Mater. Sci. Technol. 1978, 36, 231–239. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Kolisetty, A.; Fu, Z.; Koc, R. Development of La(CrCoFeNi)O3 system perovskites as interconnect and cathode materials for solid oxide fuel cells. Ceram. Int. 2017, 43, 7647–7652. [Google Scholar] [CrossRef]
- Koc, R.; Anderson, H.U. Liquid phase sintering of LaCrO3. J. Eur. Ceram. Soc. 1992, 9, 285–292. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, Q.; Li, R.; Chen, M.; Du, X.; Zhou, S. Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic. Appl. Phys. Lett. 2019, 115, 223901. [Google Scholar] [CrossRef]
- Kar, B.S.; Goswami, M.N.; Jana, P.C. Effects of lanthanum dopants on dielectric and multiferroic properties of BiFeO3–BaTiO3 ceramics. J. Alloys Compd. 2021, 861, 157960. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, F.Z.; Xiang, H.; Zhao, B.; Wang, X.; Ni, N.; Karre, R.; Wu, S.; Zhou, Y. Enabling highly efficient and broadband electromagnetic wave absorption by tuning impedance match in high-entropy transition metal diborides (HE TMB2). J. Adv. Ceram. 2021, 10, 1299–1316. [Google Scholar] [CrossRef]
- Ni, B.; Zhang, X.; Zhen, R.; Qi, X. Dielectric and ferroelectric properties of A-site disordered high-entropy chalcogenide oxides. J. Chin. Ceram. Soc. 2022, 50, 1475–1480. [Google Scholar]
- Boudad, L.; Taibi, M.; Belayachi, A.; Abd-lefdil, M. Elaboration, characterization, and giant dielectric permittivity in solid state synthesized Fe half-doped LaCrO3 perovskite. Mater. Today Proc. 2022, 58, 1108–1113. [Google Scholar] [CrossRef]
- Coşkun, M.; Polat, Ö.; Coşkun, F.M.; Durmuş, Z.; Çağlar, M.; Türüt, A. Frequency and temperature dependent electrical and dielectric properties of LaCrO3 and Ir doped LaCrO3 perovskite compounds. J. Alloys Compd. 2018, 740, 1012–1023. [Google Scholar] [CrossRef]
- RJD. Tilley. Understanding Solids: The Science of Materials; John Wiley & Sons: Chichester, UK, 2004; pp. 256–616. [Google Scholar]
- Quan, B.; Shi, W.; Ong, S.J.H.; Lu, X.; Wang, P.L.; Ji, G.; Guo, Y.; Zheng, L.; Xu, Z.J. Defect engineering in two common types of dielectric materials for electromagnetic absorption applications. Adv. Funct. Mater. 2019, 29, 1901236. [Google Scholar] [CrossRef]
- Aamir, M.; Bibi, I.; Ata, S.; Jilani, K.; Majid, F.; Kamal, S.; Alwadai, N.; Raza, M.A.S.; Bashir, M.; Iqbal, S.; et al. Ferroelectric, dielectric, magnetic, structural and photocatalytic properties of Co and Fe doped LaCrO3 perovskite synthesized via micro-emulsion route. Ceram. Int. 2021, 47, 16696–16707. [Google Scholar] [CrossRef]
- Zarrin, N.; Husain, S.; Gaur, D.D.; Somvanshi, A.; Fatema, M. Dopant incited alterations in structural, morphological, optical, and dielectric properties of Er-doped LaCrO3. J. Mater. Sci.-Mater Electron. 2020, 31, 3466–3478. [Google Scholar] [CrossRef]



| Element | Oxidation | CN | rc (Å) |
|---|---|---|---|
| La | +3 | XII | 1.36 |
| Cr | +3 | VI | 0.615 |
| Co | +3 | VI | 0.545 |
| Fe | +3 | VI | 0.55 |
| Mn | +4 | VI | 0.53 |
| Ni | +3 | VI | 0.56 |
| O | −2 | VI | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Zhang, Y.; Zhang, J.; Shi, P. Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics. Crystals 2022, 12, 1756. https://doi.org/10.3390/cryst12121756
Tian Z, Zhang Y, Zhang J, Shi P. Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics. Crystals. 2022; 12(12):1756. https://doi.org/10.3390/cryst12121756
Chicago/Turabian StyleTian, Zhifeng, Ying Zhang, Junzhan Zhang, and Peng Shi. 2022. "Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics" Crystals 12, no. 12: 1756. https://doi.org/10.3390/cryst12121756
APA StyleTian, Z., Zhang, Y., Zhang, J., & Shi, P. (2022). Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics. Crystals, 12(12), 1756. https://doi.org/10.3390/cryst12121756







