Aero-TiO2 Prepared on the Basis of Networks of ZnO Tetrapods
Abstract
1. Introduction
2. Materials and Methods
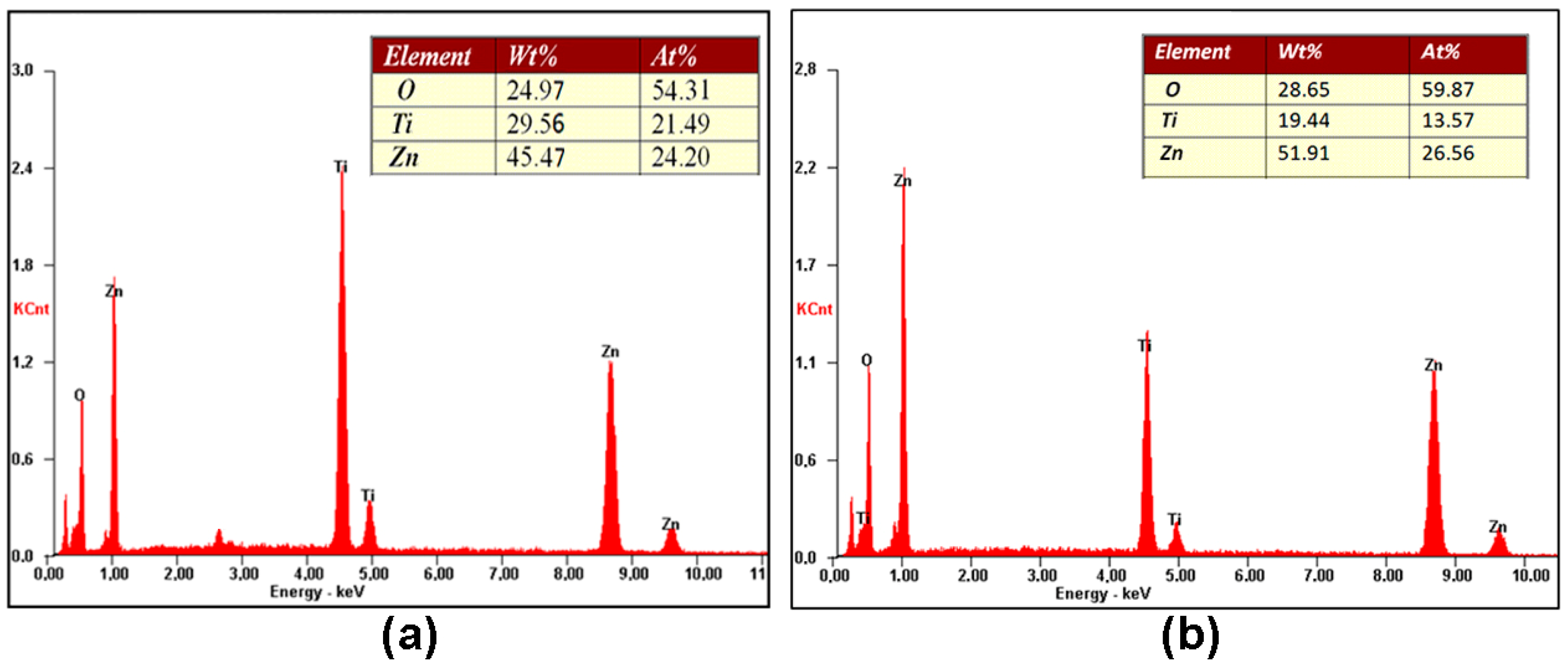
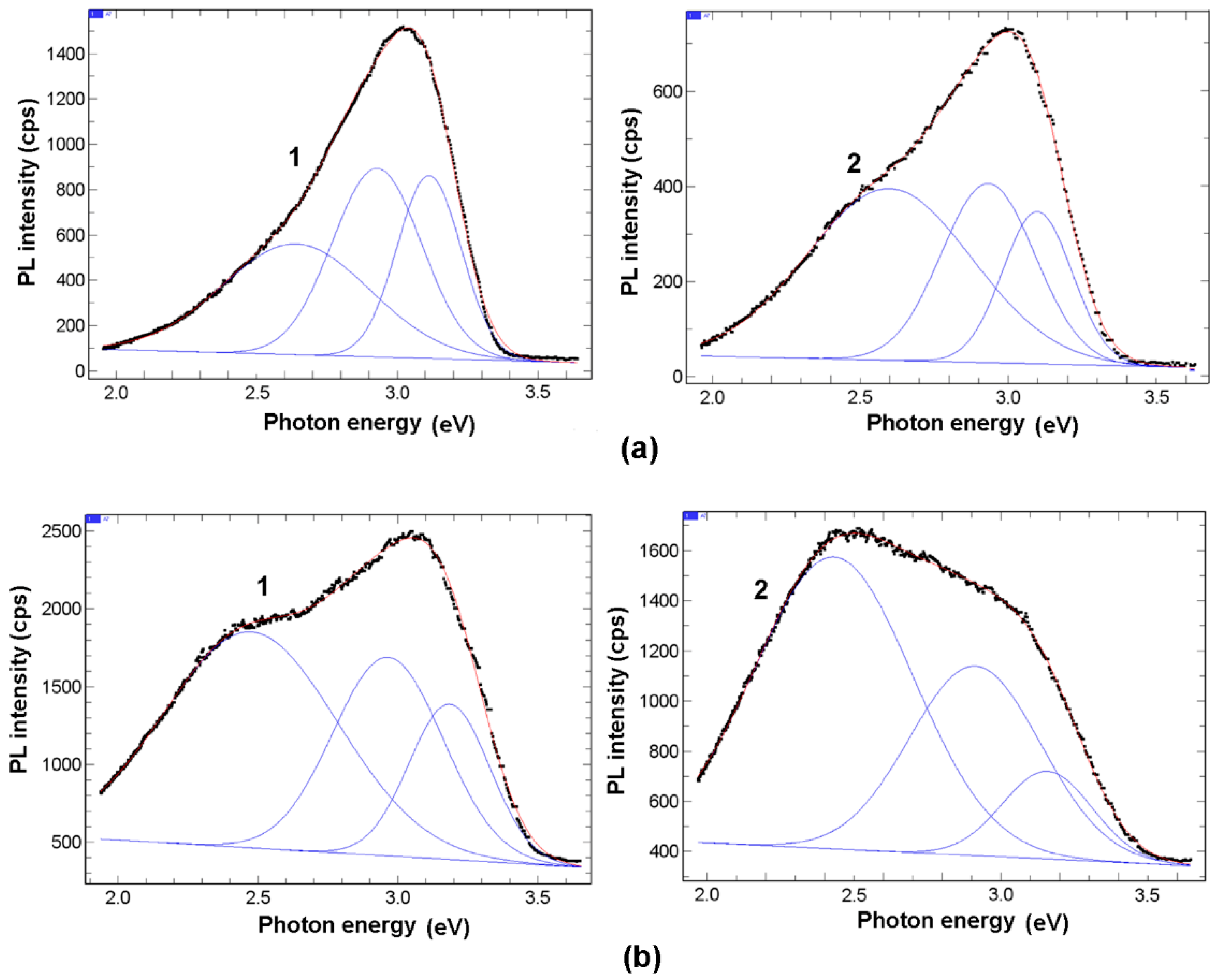
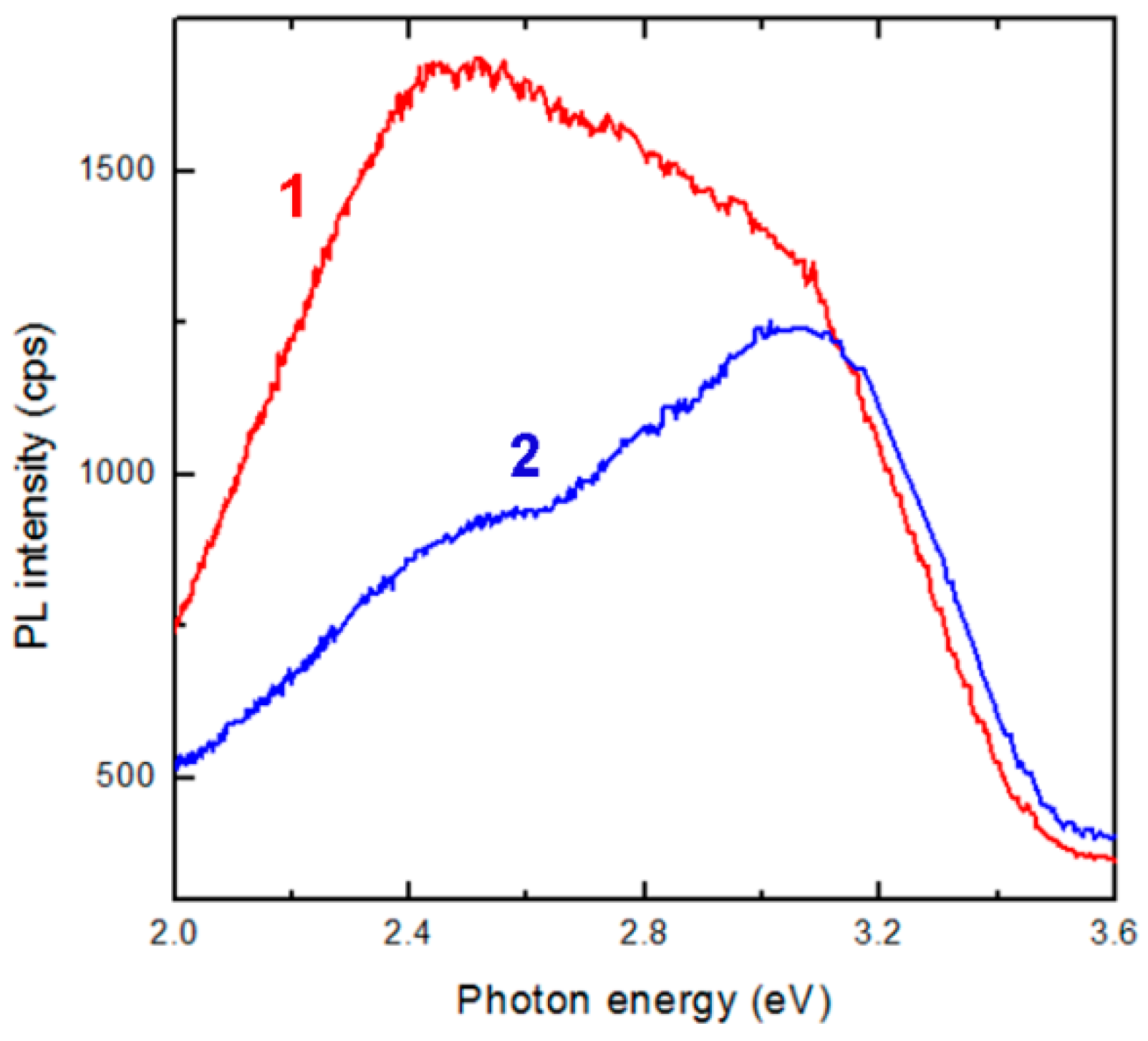
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, C.; Han, T.Y.-J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef] [PubMed]
- Moriche, R.; Prolongo, S.G.; Sanchez, M.; Jimenez-Suarez, A.; Campo, M.; Urena, A. Strain sensing based on multiscale composite materials reinforced with graphene nanoplatelets. JoVE 2016, 117, 54512. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, J.; Dai, Z.; Zhao, W.; Liu, P.; Zhao, L.; Guo, Y.; Yang, T.; Zou, L.; Jiang, K.; et al. Graphene welded carbon nanotube crossbars for biaxial strain sensors. Carbon 2017, 123, 786–793. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multi-functional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Ghimpu, L.; Grottrup, J.; Postolache, V.; Mecklenburg, M.; Stevens-Kalceff, M.A.; Ursaki, V.; Payami, N.; Feidenhansl, R.; Schulte, K.; et al. Strong light scattering and broadband (UV to IR) photoabsorption in stretchable 3D hybrid architectures based on Aerographite decorated by ZnO nanocrystallites. Sci. Rep. 2016, 6, 32913. [Google Scholar] [CrossRef]
- Schuchardt, A.; Braniste, T.; Mishra, Y.K.; Deng, M.; Mecklenburg, M.; Stevens-Kalceff, M.A.; Raevschi, S.; Schulte, K.; Kienle, L.; Adelung, R.; et al. Tree-Dimensional Aerographite-GaN hybrid networks: Single step fabrication of porous and mechanically fexible materials for multifunctional applications. Sci. Rep. 2015, 5, 8839. [Google Scholar] [CrossRef]
- Plesco, I.; Dragoman, M.; Strobel, J.; Ghimpu, L.; Schütt, F.; Dinescu, A.; Ursaki, V.; Kienle, L.; Adelung, R.; Tiginyanu, I. Flexible pressure sensor based on graphene aerogel microstructures functionalized with CdS nanocrystalline thin film. Superlattices Microstruct. 2018, 117, 418–422. [Google Scholar] [CrossRef]
- Plesco, I.; Strobel, J.; Schütt, F.; Himcinschi, C.; Sedrine, N.B.; Monteiro, T.; Rosário-Correia, M.; Gorceac, L.; Cinic, B.; Ursaki, V.; et al. Hierarchical Aerographite 3D fexible networks hybridized by InP micro/nanostructures for strain sensor applications. Sci. Rep. 2018, 8, 13880. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Kaps, S.; Schuchardt, A.; Paulowicz, I.; Jin, X.; Gedamu, D.; Freitag, S.; Claus, M.; Wille, S.; Kovalev, A.; et al. Fabrication of Macroscopically Flexible and Highly Porous 3D Semiconductor Networks from Interpenetrating Nanostructures by a Simple Flame Transport Approach. Part. Part. Syst. Charact. 2013, 30, 775–783. [Google Scholar] [CrossRef]
- Marx, J.; Smazna, D.; Adelung, R.; Schulte, K.; Fiedler, B. Processing, growth and thermodynamic calculations of carbon foam with a hollow tetrapodal morphology-Aerographite. Appl. Surf. Sci. 2018, 30, 535–542. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Braniste, T.; Smazna, D.; Deng, M.; Schütt, F.; Schuchardt, A.; Stevens-Kalceff, M.A.; Raevschi, S.; Schürmann, U.; Kienle, L.; et al. Self-organized and self-propelled aero-GaN with dual hydrophilic/hydrophobic behavior. Nano Energy 2019, 56, 759–769. [Google Scholar] [CrossRef]
- Plesco, I.; Braniste, T.; Wolff, N.; Gorceac, L.; Duppel, V.; Cinic, B.; Mishra, Y.K.; Sarua, A.; Adelung, R.; Kienle, L.; et al. Aero-ZnS architectures with dual hydrophilic—Hydrophobic properties for microfluidic applications. APL Mater. 2020, 8, 061105. [Google Scholar] [CrossRef]
- Wolff, N.; Ciobanu, V.; Enachi, M.; Kamp, M.; Braniste, T.; Duppel, V.; Shree, S.; Raevschi, S.; Medina-Sánchez, M.; Adelung, R.; et al. Advanced Hybrid GaN/ZnO Nanoarchitectured Microtubes for Fluorescent Micromotors Driven by UV Light. Small 2020, 16, 1905141. [Google Scholar] [CrossRef]
- Dragoman, M.; Braniste, T.; Iordanescu, S.; Aldrigo, M.; Raevschi, S.; Shree, S.; Adelung, R.; Tiginyanu, I. Electromagnetic interference shielding in X-band with aero-GaN. Nanotechnology 2019, 30, 34LT01. [Google Scholar] [CrossRef]
- Braniste, T.; Zhukov, S.; Dragoman, M.; Alyabyeva, L.; Ciobanu, V.; Dragoman, D.; Iordanescu, S.; Shree, S.; Raevschi, S. Terahertz shielding properties of aero-GaN. Semicond. Sci. Technol. 2019, 34, 12LT02. [Google Scholar] [CrossRef]
- Dragoman, M.; Ciobanu, V.; Shree, S.; Dragoman, D.; Braniste, T.; Raevschi, S.; Dinescu, A.; Sarua, A.; Mishra, Y.K.; Pugno, N.; et al. Sensing up to 40 atm Using Pressure-Sensitive Aero-GaN. Phys. Status Solidi RRL 2019, 13, 1900012. [Google Scholar] [CrossRef]
- Braniste, T.; Dragoman, M.; Zhukov, S.; Aldrigo, M.; Ciobanu, V.; Iordanescu, S.; Alyabyeva, L.; Fumagalli, F.; Ceccone, G.; Raevschi, S.; et al. Aero-Ga2O3 Nanomaterial Electromagnetically Transpnarent from Microwaves to Terahertz for Internet of Things Applications. Nanomaterials 2020, 10, 1047. [Google Scholar] [CrossRef]
- Plesco, I.; Ciobanu, V.; Braniste, T.; Ursaki, V.; Rasch, F.; Sarua, A.; Raevschi, S.; Adelung, R.; Dutta, J.; Tiginyanu, I. Highly Porous and Ultra-Lightweight Aero-Ga2O3: Enhancement of Photocatalytic Activity by Noble Metals. Materials 2021, 14, 1985. [Google Scholar] [CrossRef]
- Crap, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of TiO2. Solid State Chem. 2004, 32, 133–177. [Google Scholar]
- Ijadpanah-Saravi, H.; Safari, M.; Khodadadi-Darban, A.; Rezaei, A. Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Degradation of Cyanide in Wastewater. Anal. Lett. 2014, 47, 1772–1782. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Kong, X.Z. Preparation of hollow TiO2 nanoparticles through TiO2 deposition on polystyrene latex particles and characterizations of their structure and photocatalytic activity. Nanoscale Res. Lett. 2012, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hu, C.W.; Chen, H.W.; Ho, K.C. Incorporating carbon nanotube in a low- temperature fabrication process for dye- sensitized TiO2 solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1628–1633. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fary, D.J.; Cordero-Cabrera, M.C. Sensor performance of nanostructured TiO2 thin films derived from particulate sol-gel route and polymeric fugitive agents. Sens. Actuators B-Chem. 2007, 124, 74–83. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, H.M.; Wang, R.H. Investigation of the interaction between colloidal TiO2 and bovine hemoglobin using spectral method. Colloids Surf. B Biointerfaces 2008, 65, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Haghi, M.M.; Hekmatafshar, M.B.; Janipour, S.S.; Gholizadeh, S.S.; Kazem, M.; Sayyadifar, F.; Ghaedi, M. Antibacterial effect of TiO2 nanoparticles on pathogenic strain of E. coli. Int. J. Adv. Biotechnol. Res. 2012, 3, 621–624. [Google Scholar]
- Zhang, Q.; Fan, W.; Gao, L. Anatase TiO2 nanoparticles immobilized on ZnO tetrapods as a highly efficient and easily recyclable photocatalyst. Appl. Catal. B Environ. 2007, 76, 168–173. [Google Scholar] [CrossRef]
- Lopera, A.A.; Velasquez, A.M.; Chavarriaga, E.A.; Ocampo, S.; Zaghete, M.A.; Graminha, M.A.; Garcia, C.P. Synthesis by combustion in solution of Zn2TiO4+Ag for photocatalytic and photodynamic applications in the visible. J. Phys. Conf. Ser. 2017, 935, 012013. [Google Scholar] [CrossRef]
- Qin, X.; Cui, L.; Shao, G. Preparation of ZnO-Zn2TiO4. Sol Composite Films and Its Photocatalytic Activities. J. Nanomater. 2013, 2013, 428419. [Google Scholar] [CrossRef]
- Khatua, L.; Sahoo, R.; Satapathy, P.; Panda, R.; Das, S.K. Visible light photocatalytic dye decomposition behaviour of solid state reaction grown Zn2TiO4 nanoparticles. J. Semicond. 2018, 39, 123002. [Google Scholar] [CrossRef]
- Khatua, L.; Panda, R.; Nayak, R.; Singh, A.; Sahoo, P.K.; Pradhan, D.; Singh, U.D.; Das, S.K. Efficient UV photocatalytic dye decomposition activity with cost effective solid state reaction grown Zinc Orthotitanate (Zn2TiO4) nanoparticles. J. Alloys Comp. 2018, 764, 895–900. [Google Scholar] [CrossRef]
- Mahajan, J.; Jeevanandam, P. Synthesis of Zn2TiO4@CdS Core-shell Heteronanostructures by Novel Thermal Decomposition Approach for Photocatalytic Application. Chem. Sel. 2019, 4, 12580–12591. [Google Scholar]
- Gaidan, I.; Brabazon, D.; Ahad, I.U. Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature. Sensors 2017, 17, 1995. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Wang, X.-Z.; Liu, F.-J.; Zhang, G.-S.; Song, X.-J.; Cui, H.-Z. Fabrication of porous Zn2TiO4—ZnO microtubes and analysis of their acetone gas sensing properties. Rare Met. 2021, 40, 1528–1535. [Google Scholar] [CrossRef]
- Wana, L.; Li, X.; Qu, Z.; Shi, Y.; Li, H.; Zhao, Q.; Chen, G. Facile synthesis of ZnO/Zn2TiO4 core/shell nanowires for photocatalytic oxidation of acetone. J. Hazard. Mater. 2010, 184, 864–868. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Shen, C.; Xu, X. Efficient Photocatalytic Hydrogen Production over Rh-Doped Inverse Spinel Zn2TiO4. ChemCatChem 2016, 8, 2289–2295. [Google Scholar] [CrossRef]
- Fu, Q.; Guo, L. Band-engineered Zn2TiO4 nanowires for hydrogen generation from water using visible light: A first-principles study. AIP Adv. 2022, 12, 015201. [Google Scholar] [CrossRef]
- Mrazek, J.; Spanhel, L.; Surynek, M.; Potel, M.; Matejec, V. Crystallization properties of RE-doped (RE = Eu, Er, Tm) Zn2TiO4 prepared by the sol-gel method. J. Alloy. Comp. 2011, 509, 4018–4024. [Google Scholar] [CrossRef]
- Li, J.; Wu, B.; Zhang, Q.; Wang, H.; Li, Y. Highly Luminescent Mesoporous Zn2TiO4:Eu3+ Material with Excellent Sensing and Removal Abilities for Heavy-Metal Ions. J. Nanosci. Nanotechnol. 2016, 16, 9568–9574. [Google Scholar] [CrossRef]
- Girish, K.M.; Naik, R.; Prashantha, S.C.; Nagabhushana, H.; Nagaswarupa, H.P.; Raju, K.S.A.; Premkumar, H.B.; Sharma, S.C.; Nagabhushana, B.M. Zn2TiO4:Eu3+ nanophosphor: Self explosive route and its near UV excited photoluminescence properties for WLEDs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.J.; Echeverria, E.; Wagle, P.; Mainali, P.; Meyers, D.; Gupta, A.K.; Sachan, R.; Prassana, S.; McIlroy, D.N. High Temperature Atomic Layer Deposition of GaN on 1D Nanostructures. Nanomaterials 2020, 10, 2434. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J. (Ed.) Atomic Layer Deposition in Energy Conversion Applications; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2017; p. 312. [Google Scholar]
- Sridharan, K.; Jang, E.; Park, Y.M.; Park, T.G. Superior Photostability and Photocatalytic Activity of ZnO Nanoparticles Coated with Ultrathin TiO2 Layers through AtomicLayer Deposition. Chem. Eur. J. 2015, 21, 19136–19141. [Google Scholar] [CrossRef] [PubMed]
- Katoch, A.; Kim, J.-H.; Kim, S.S. TiO2/ZnO Inner/Outer Double-Layer Hollow Fibers for Improved Detection of Reducing Gases. ACS Appl. Mater. Interfaces 2014, 6, 21494–21499. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-S.; Lee, H.-Y.; Huang, J.-M.; Lin, C.-M. Epitaxial growth of ZnO films at extremely low temperature by atomic layer deposition with interrupted flow. Mater. Chem. Phys. 2010, 120, 236–239. [Google Scholar] [CrossRef]
- Yang, J.; Bahrami, A.; Ding, X.; Lehmann, S.; Kruse, N.; He, S.; Wang, B.; Hantusch, M.; Nielsch, K. Characteristics of ALD-ZnO Thin Film Transistor Using H2O and H2O2 as Oxygen Sources. Adv. Mater. Int. 2022, 9, 2101953. [Google Scholar] [CrossRef]
- He, S.; Bahrami, A.; Zhang, X.; Martinez, I.G.; Lehmann, S.; Nielsh, K. Effect of Powder ALD Interface Modification on the Thermoelectric Performance of Bismuth. Adv. Mater. Technol. 2022, 7, 2100953. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Abd Majid, W.H.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar]
- Arin, J.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Characterization of ZnO-TiO2 and zinc titanate nanoparticles synthesized by hydrothermal process. Res. Chem. Intermed. 2017, 43, 3183–3195. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Fleuri, P.A.; Damen, T.C. Raman Syectra of TiO2, MgF, ZnF, FeFs, and MnF. Phys. Rev. 1967, 154, 522–524. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kurti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, S.; Cui, T.; Li, B.; Zhou, Q.; Yuan, H.; Xu, D. Temperature-dependent optical phonon behaviour of a spinel Zn2TiO4 single crystal grown by the optical floating zone method in argon atmosphere. RSC Adv. 2017, 7, 35477. [Google Scholar] [CrossRef]
- Dette, C.; Perez-Osorie, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2002, 78, 239–245. [Google Scholar] [CrossRef]
- Gallart, M.; Cottineau, T.; Honerlage, B.; Keller, N.; Keller, V.; Gilliot, P. Temperature dependent photoluminescence of anatase and rutile TiO2 single crystals: Polaron and self-trapped exciton formation. J. Appl. Phys. 2018, 124, 133104. [Google Scholar] [CrossRef]
- Ali, Z.; Ali, S.; Ahmad, S.I.; Khan, I.; Aliabad, H.A.R. Structural and optoelectronic properties of the zinc titanate perovskite and spinel by modified Becke-Johnson potential. Phys. B 2013, 420, 54–57. [Google Scholar] [CrossRef]
- Song, J.; Leng, M.; Xiao, H.; Zhang, L.; Qin, Y.; Hou, W.; Du, N.; Liu, J. Preparation of Single Phase Zn2TiO4 Spinel from a New ZnTi Layered Double Hydroxide Precursor. J. Nanosci. Nanotechnol. 2014, 14, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Mayen-Hernandez, S.A.; Torres-Delgado, G.; Castanedo-Perez, R.; Villarreal, M.G.; Cruz-Orea, A.; Alvarez, J.G.M.; Zelaya-Angel, O. Optical and structural properties of ZnO + Zn2TiO4 thin films prepared by the sol-gel method. J. Mater. Sci. Mater. Electron. 2007, 18, 1127–1130. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, V.; Ursaki, V.V.; Lehmann, S.; Braniste, T.; Raevschi, S.; Zalamai, V.V.; Monaico, E.V.; Colpo, P.; Nielsch, K.; Tiginyanu, I.M. Aero-TiO2 Prepared on the Basis of Networks of ZnO Tetrapods. Crystals 2022, 12, 1753. https://doi.org/10.3390/cryst12121753
Ciobanu V, Ursaki VV, Lehmann S, Braniste T, Raevschi S, Zalamai VV, Monaico EV, Colpo P, Nielsch K, Tiginyanu IM. Aero-TiO2 Prepared on the Basis of Networks of ZnO Tetrapods. Crystals. 2022; 12(12):1753. https://doi.org/10.3390/cryst12121753
Chicago/Turabian StyleCiobanu, Vladimir, Veaceslav V. Ursaki, Sebastian Lehmann, Tudor Braniste, Simion Raevschi, Victor V. Zalamai, Eduard V. Monaico, Pascal Colpo, Kornelius Nielsch, and Ion M. Tiginyanu. 2022. "Aero-TiO2 Prepared on the Basis of Networks of ZnO Tetrapods" Crystals 12, no. 12: 1753. https://doi.org/10.3390/cryst12121753
APA StyleCiobanu, V., Ursaki, V. V., Lehmann, S., Braniste, T., Raevschi, S., Zalamai, V. V., Monaico, E. V., Colpo, P., Nielsch, K., & Tiginyanu, I. M. (2022). Aero-TiO2 Prepared on the Basis of Networks of ZnO Tetrapods. Crystals, 12(12), 1753. https://doi.org/10.3390/cryst12121753





