Spark Plasma Sintering (SPS) of Multi-Principal Element Alloys of Copper-Niobium-Titanium-Di-Boride-Graphite, Investigation of Microstructures, and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Powder Mixing and Sintering Process
2.2.2. Micro-Hardness Test
2.2.3. Microstructure Test
2.2.4. Wear Test
2.2.5. Corrosion Test
3. Results and Discussions
3.1. Sintering Mode of CuNbTiB2C Multi-Principal Element Alloys
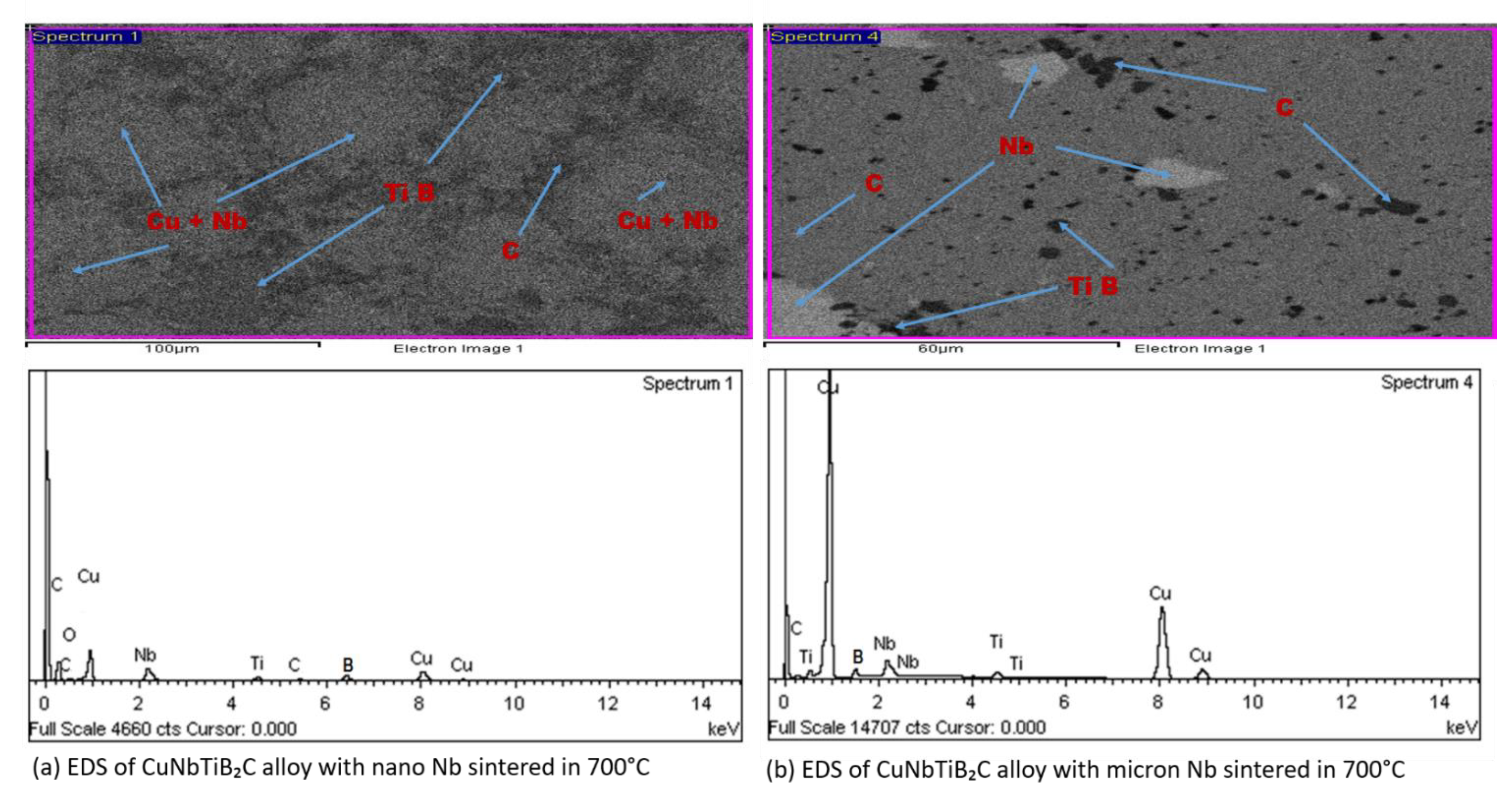
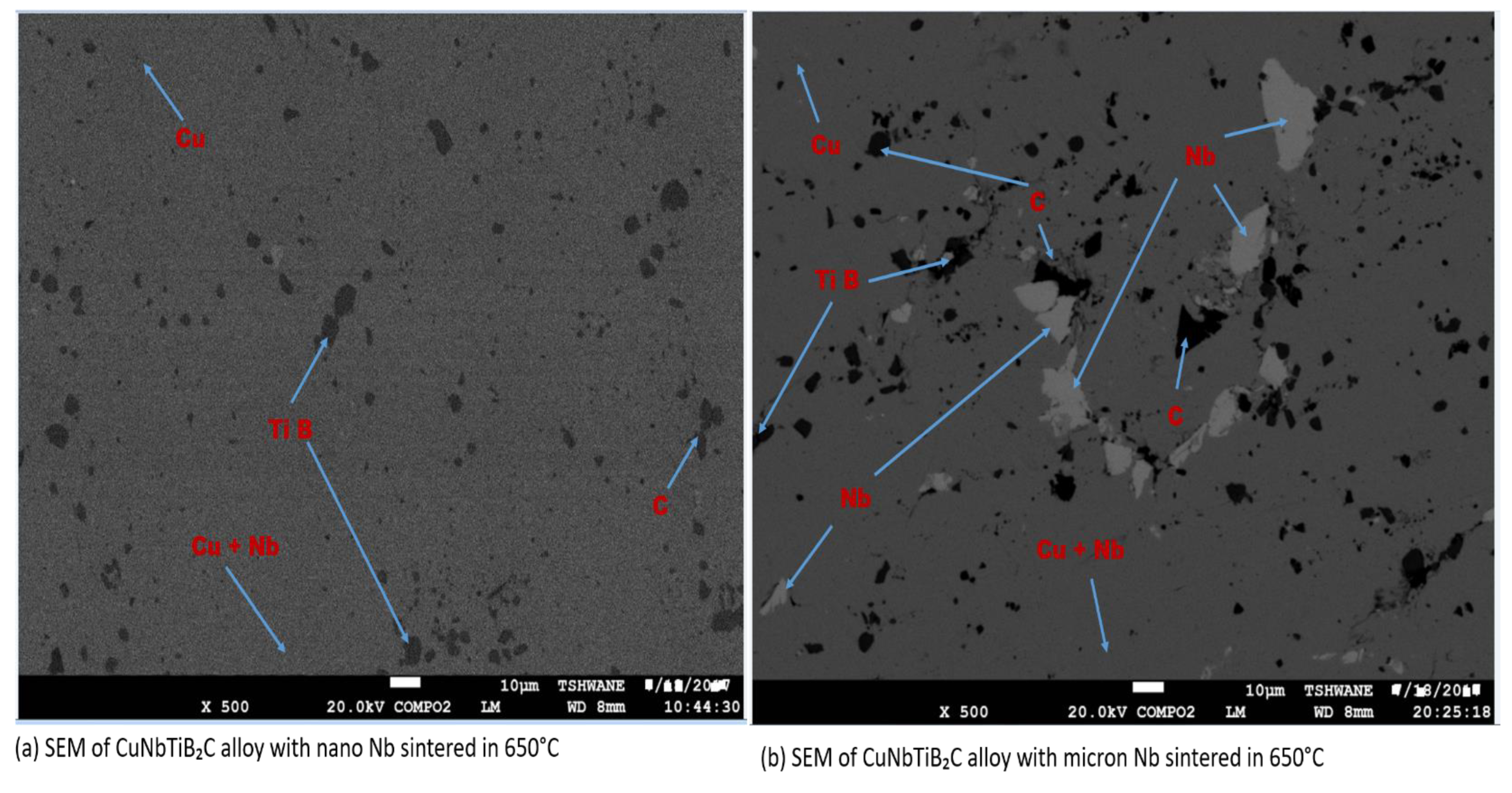
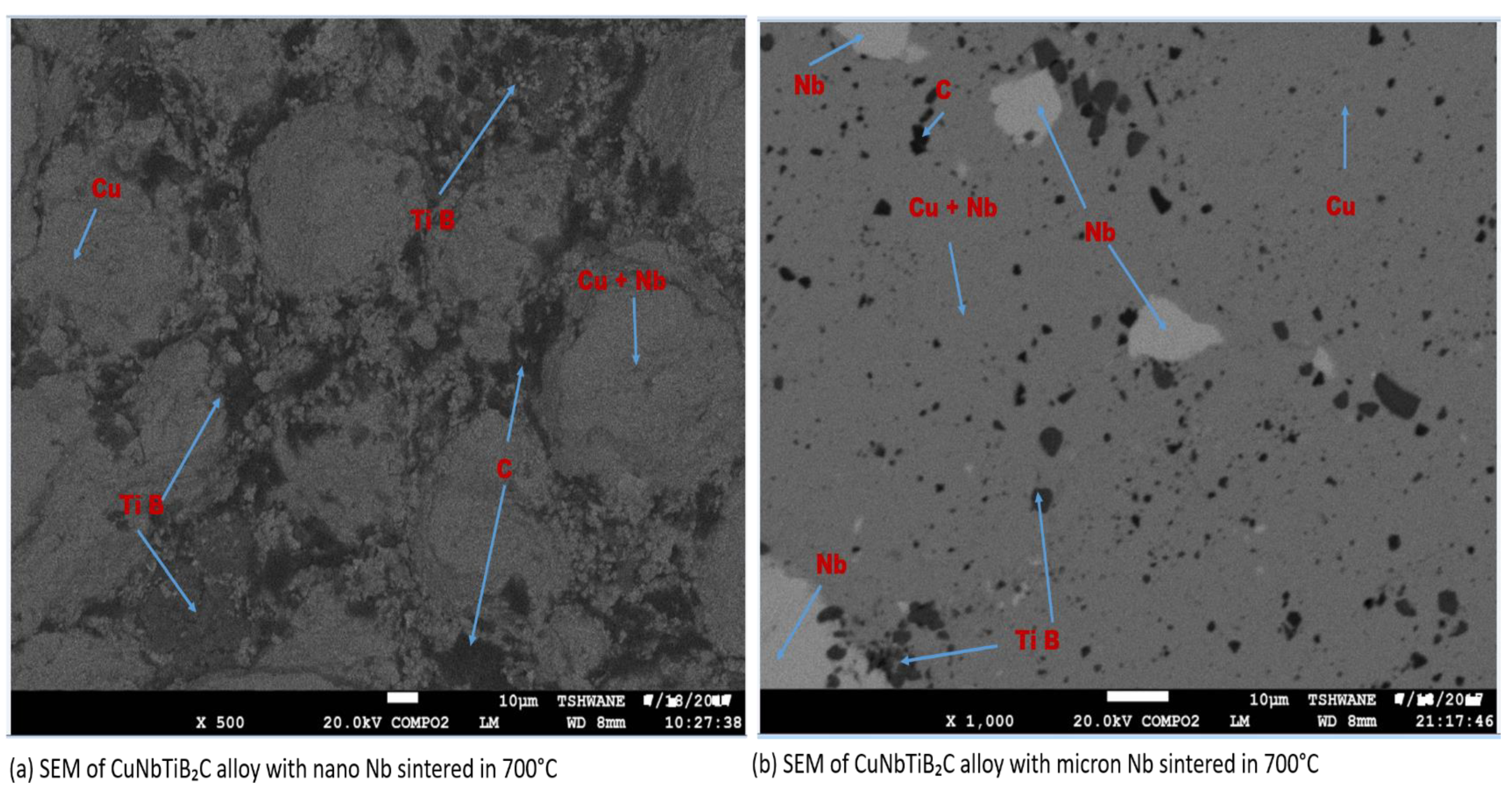
3.2. EDS Analysis, SEM, and Micro-Hardness, Density, and Relative Density of the Sintered Multi-Principal Element Alloys
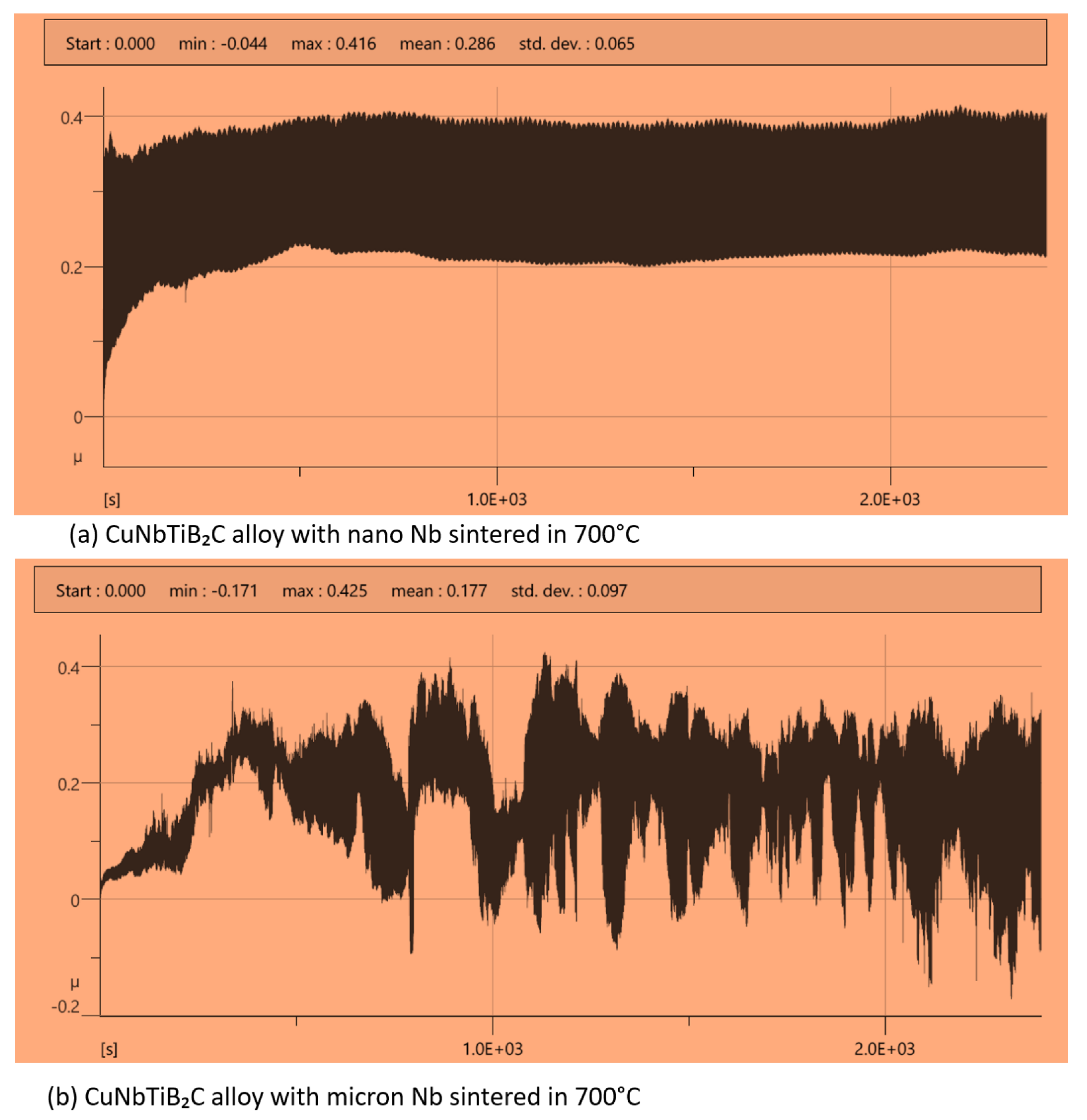
3.3. Wear Behavior of the Sintered Multi-Principal Element Alloys
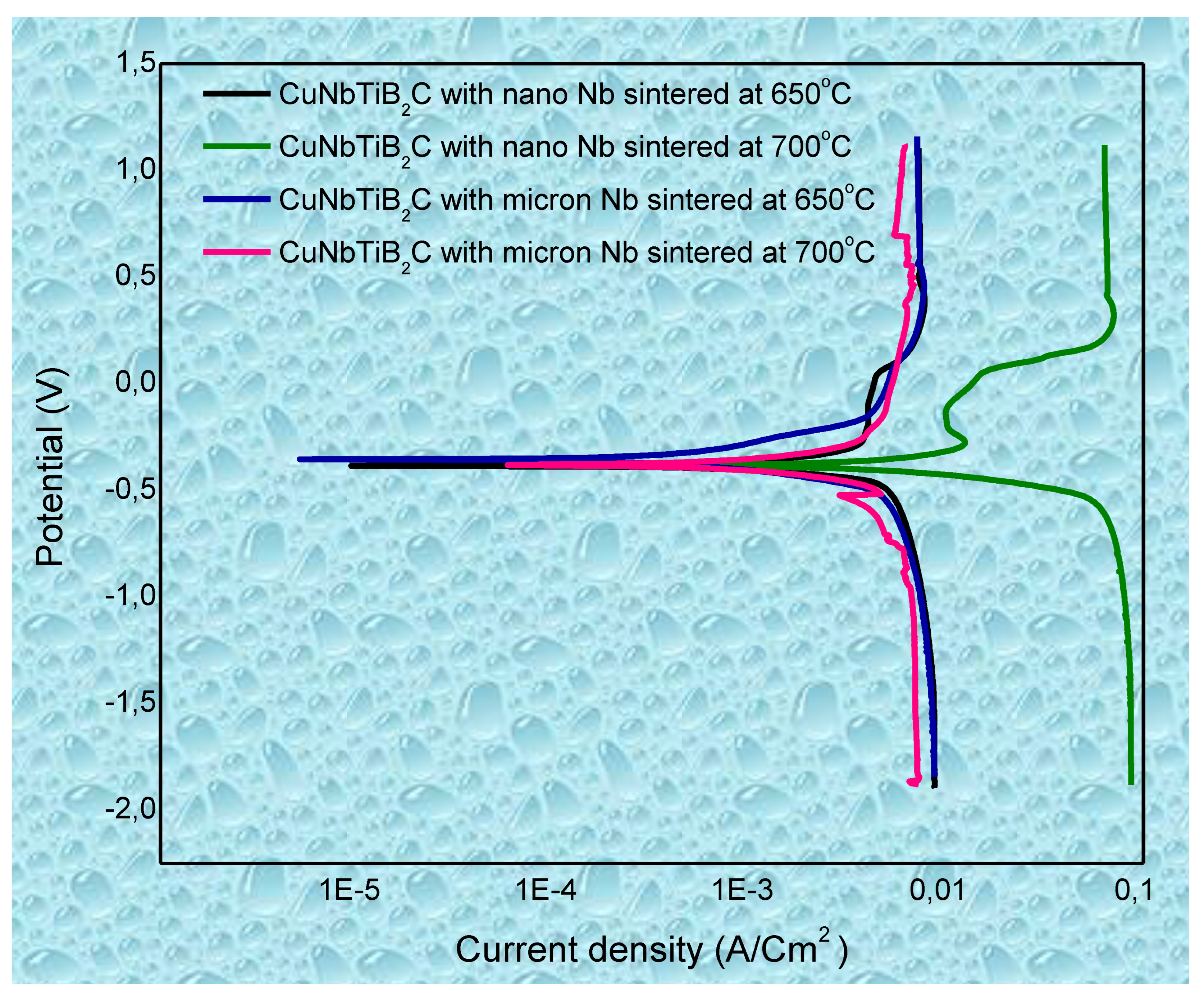
3.4. Corrosion Performance of the Multi-Principal Element Alloys of CuNbTiB2C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Nomoto, K.; Wang, Q.; Kong, C.; Sun, L.; Zhang, L.; Liang, S.; Lu, J.; Kruzic, J.J. A self-supported high-entropy metallic glass with a nanosponge architecture for efficient hydrogen evolution under alkaline and acidic conditions. Adv. Funct. Mater. 2021, 31, 2101586. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, T.; Sun, L.; Zhao, Y.; Li, W.; Luan, J.; Lyu, F.; Zhang, L.C.; Kruzic, J.J.; Kai, J.J.; et al. A novel multinary intermetallic as an active electrocatalyst for hydrogen evolution. Adv. Mater. 2020, 32, 2000385. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ferry, M.; Kruzic, J.J.; Li, X. Review: Multi-principal element alloys by additive manufacturing. J. Mater. Sci. 2022, 57, 9903–9935. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan,, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-entropy alloys: Potential candidates for high-temperature applications—An overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Tsao, T.-K.; Yeh, A.-C.; Kuo, C.-M.; Kakehi, K.; Murakami, H.; Yeh, J.-W.; Jian, S.-R. The high temperature tensile and creep behaviors of high entropy superalloy. Sci. Rep. 2017, 7, 12658. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zhang, Y. Functional properties and promising applications of high entropy alloys. Scr. Mater. 2020, 187, 188–193. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J. Breakthrough applications of high-entropy materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- Romero, R.A.; Xu, S.; Jian, W.-R.; Beyerlein, I.J.; Ramana, C. Atomistic simulations of the local slip resistances in four refractory multi-principal element alloys. Int. J. Plast. 2022, 149, 103157. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Wadsworth, J.; Nieh, T.; Stephens, J. Recent advances in aerospace refractory metal alloys. Int. Mater. Rev. 1988, 33, 131–150. [Google Scholar] [CrossRef]
- Choudhary, S.; O’Brien, S.; Qiu, Y.; Thomas, S.; Gupta, R.; Birbilis, N. On the dynamic passivity and corrosion resistance of a low cost and low density multi-principal-element alloy produced via commodity metals. Electrochem. Commun. 2021, 125, 106989. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Tang, Y.-Z.; Zhang, Y.-Z.; Dong, A.-P.; Tu, J.; Chai, L.-J.; Zhou, Z.-M. Microstructure and dry sliding wear behavior of laser clad AlCrNiSiTi multi-principal element alloy coatings. Rare Met. 2017, 36, 562–568. [Google Scholar] [CrossRef]
- Goh, G.D.; Agarwala, S.; Goh, G.L.; Dikshit, V.; Sing, S.L.; Yeong, W.Y. Additive manufacturing in unmanned aerial vehicles (UAVs): Challenges and potential. Aerosp. Sci. Technol. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Diouf, S.; Molinari, A. Densification mechanisms in spark plasma sintering: Effect of particle size and pressure. Powder Technol. 2012, 221, 220–227. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef] [Green Version]
- Matizamhuka, W. Spark plasma sintering (SPS)-an advanced sintering technique for structural nanocomposite materials. J. South. Afr. Inst. Min. Metall. 2016, 116, 1171–1180. [Google Scholar] [CrossRef]
- Yousif, B. Design of newly fabricated tribological machine for wear and frictional experiments under dry/wet condition. Mater. Des. 2013, 48, 2–13. [Google Scholar] [CrossRef]
- Kato, K. Wear in relation to friction—A review. Wear 2000, 241, 151–157. [Google Scholar] [CrossRef]
- Alotaibi, J.; Yousif, B.; Yusaf, T. Wear behaviour and mechanism of different metals sliding against stainless steel counterface. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2014, 228, 692–704. [Google Scholar] [CrossRef]
- Česánek, Z.; Schubert, J. Potentiodynamic evaluation of corrosion resistant coatings. Metal 2014, 2014. [Google Scholar]
- Alaneme, K.K.; Olubambi, P.A. Corrosion and wear behaviour of rice husk ash—Alumina reinforced Al–Mg–Si alloy matrix hybrid composites. J. Mater. Res. Technol. 2013, 2, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Balasubramaniam, R.; Tiwari, S. Corrosion inhibition of 6061-SiC by rare earth chlorides. Anti-Corrosion Methods Mater. 2007, 54, 37–46. [Google Scholar] [CrossRef]



| Constituent Element | Weight Percent (wt.%) | Purity (%) | Particle Size | ||
|---|---|---|---|---|---|
| Copper, Cu | 40 | 99 | 44 µm | ||
| Niobium, Nb | 30 | 99.9 | 14 nm–44 µm | ||
| Titanium diboride, TiB2 | 20 | 99 | 75 µm | ||
| Graphite, C | 10 | 99.8 | 14.43 nm | ||
| Spark plasma sintering parameters | |||||
| Alloys | Sintering temperature (°C) | Pressure (MPa) | Heating rate (°C/min) | Holding time (min) | |
| Cu-Nb(nano)-TiB2-G | 650 | 700 | 60 | 50 | 5 |
| Cu-Nb(micron)-TiB2-G | 650 | 700 | 60 | 50 | 5 |
| Sintering dies | Diameter (mm) | Height (mm) | Volume (=πr2h) (mm3) | ||
| Graphite dies | 30 | 5 | 3534.29 | ||
| Multi-Principal Element Alloys | Micro-Hardness (HV0.2) | Density (g/cm3) | Relative Density (%) |
|---|---|---|---|
| CuNbTiB2C with nano-particles of Nb sintered at 650 °C | 786.03 | 7.309 | 90 |
| CuNbTiB2C with nano-particles of Nb sintered at 700 °C | 734.50 | 7.390 | 91 |
| CuNbTiB2C with micro-particles of Nb sintered at 650 °C | 754.16 | 7.806 | 96 |
| CuNbTiB2C with micro-particles of Nb sintered at 700 °C | 745.06 | 7.758 | 95 |
| Multi-Principal Element Alloys | Minimum Coefficient of Friction, µ | Maximum Coefficient of Friction, µ | Mean Value of µ | Standard Deviation Value of µ |
|---|---|---|---|---|
| CuNbTiB2C with nano-particle of Nb sintered at 650 °C | −0.024 | 0.719 | 0.484 | 0.084 |
| CuNbTiB2C with nano-particles of Nb sintered at 700 °C | −0.044 | 0.416 | 0.286 | 0.065 |
| CuNbTiB2C with micro-particles of Nb sintered at 650 °C | −0.020 | 0.201 | 0.107 | 0.043 |
| CuNbTiB2C with micro-particles of Nb sintered at 700 °C | −0.171 | 0.425 | 0.177 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eze, A.A.; Sadiku, E.R.; Kupolati, W.K.; Snyman, J.; Ndambuki, J.M.; Ibrahim, I.D. Spark Plasma Sintering (SPS) of Multi-Principal Element Alloys of Copper-Niobium-Titanium-Di-Boride-Graphite, Investigation of Microstructures, and Properties. Crystals 2022, 12, 1754. https://doi.org/10.3390/cryst12121754
Eze AA, Sadiku ER, Kupolati WK, Snyman J, Ndambuki JM, Ibrahim ID. Spark Plasma Sintering (SPS) of Multi-Principal Element Alloys of Copper-Niobium-Titanium-Di-Boride-Graphite, Investigation of Microstructures, and Properties. Crystals. 2022; 12(12):1754. https://doi.org/10.3390/cryst12121754
Chicago/Turabian StyleEze, Azunna Agwo, Emmanuel Rotimi Sadiku, Williams Kehinde Kupolati, Jacques Snyman, Julius Musyoka Ndambuki, and Idowu David Ibrahim. 2022. "Spark Plasma Sintering (SPS) of Multi-Principal Element Alloys of Copper-Niobium-Titanium-Di-Boride-Graphite, Investigation of Microstructures, and Properties" Crystals 12, no. 12: 1754. https://doi.org/10.3390/cryst12121754
APA StyleEze, A. A., Sadiku, E. R., Kupolati, W. K., Snyman, J., Ndambuki, J. M., & Ibrahim, I. D. (2022). Spark Plasma Sintering (SPS) of Multi-Principal Element Alloys of Copper-Niobium-Titanium-Di-Boride-Graphite, Investigation of Microstructures, and Properties. Crystals, 12(12), 1754. https://doi.org/10.3390/cryst12121754








