Effect of Pre-Oxidation on a Ti PVD Coated Ferritic Steel Substrate during High-Temperature Aging
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
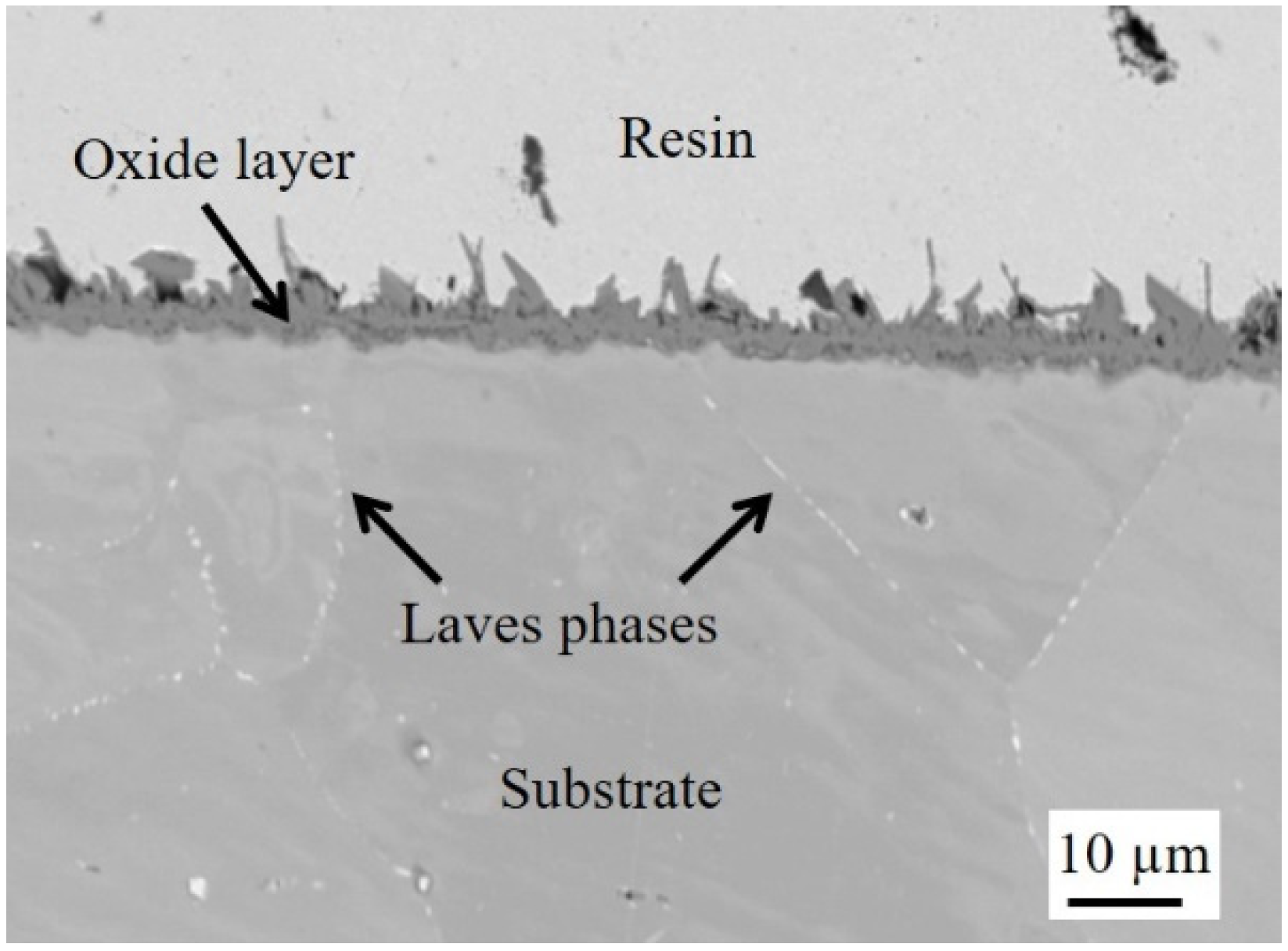
3.1. Oxidation of Polished Sample
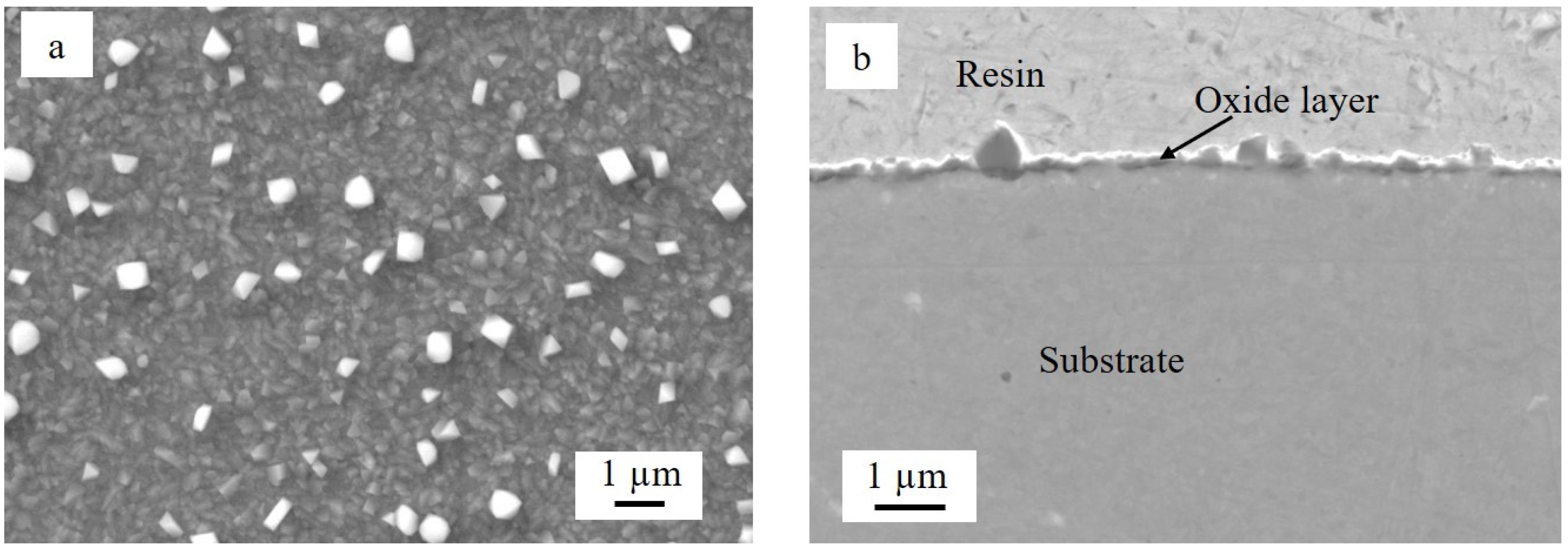
3.2. Oxidation of Ti-Coated Sample
3.3. Oxidation of Ti-Coated Sample after Pre-Oxidation
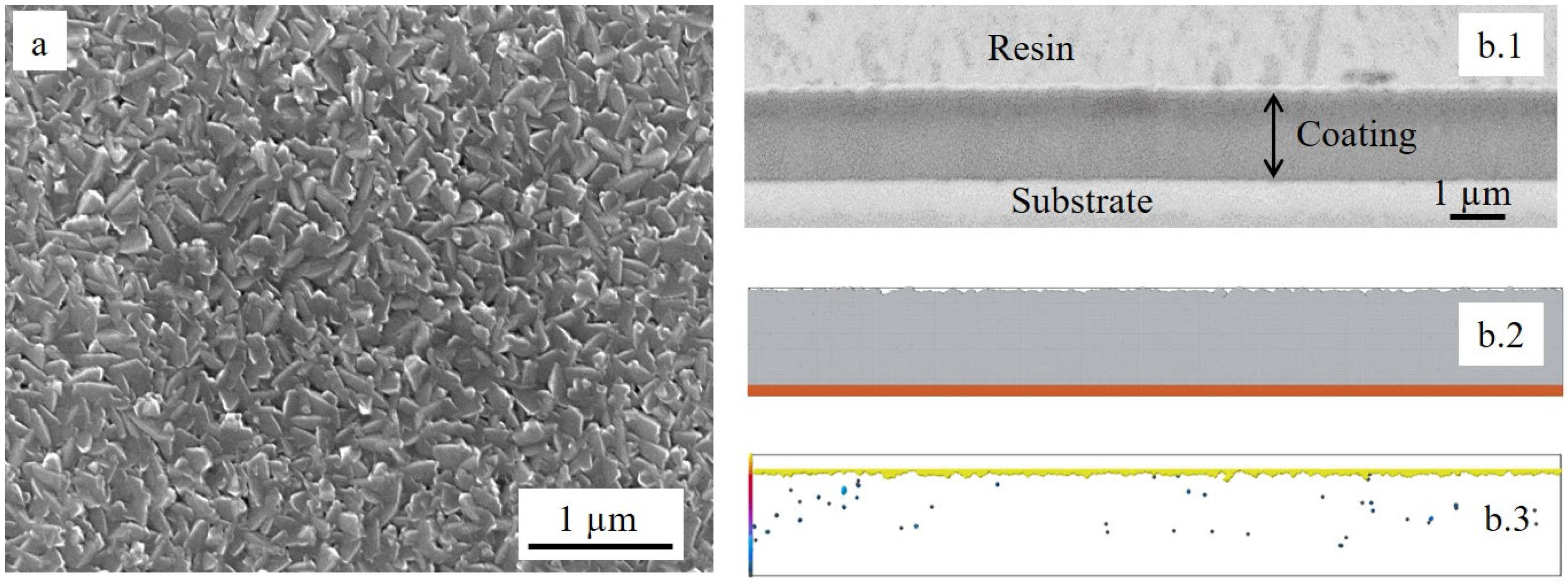
3.3.1. Ti Deposition after Pre-Oxidation
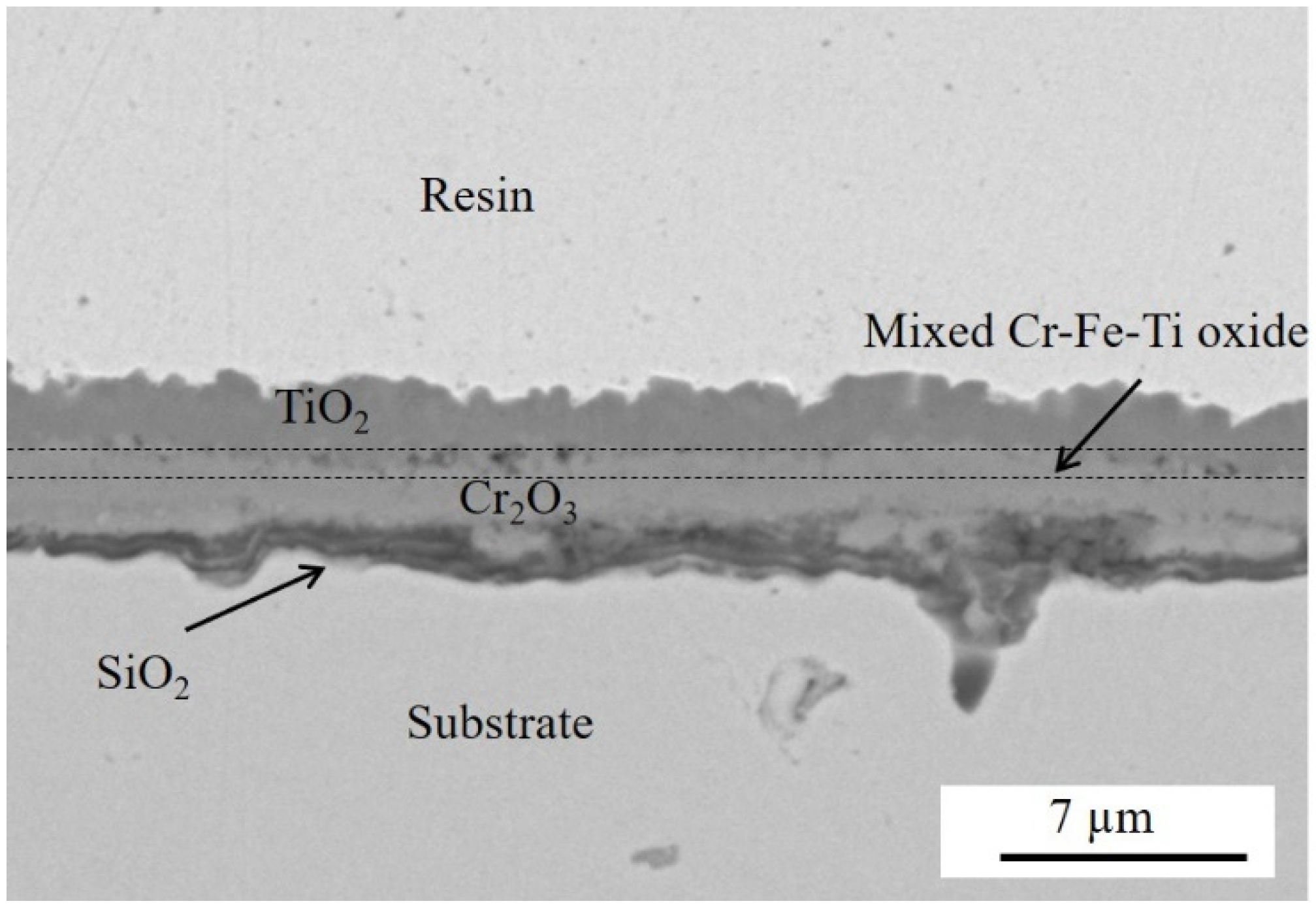
3.3.2. Oxidation of Coated Pre-Oxidized Sample
3.4. Discussion about Adhesion
4. Conclusions
- Metallic Ti converted into Ti oxide (TiO2) during high-temperature aging at 800 °C. TiO2 scale formed on the steel that was not pre-oxidized slowed down the oxidation of the substrate. Indeed, the thickness of the chromia layer ranged from about 4 to almost 8 µm in the case of the uncoated sample and between 2 and 4 µm for the sample that was not pre-oxidized. However, significant spallation of the TiO2 scale occurred during cooling down.
- The TiO2 scale grown on the pre-oxidized steel effectively protected the substrate against oxidation. The thickness of the duplex Cr2O3/(Cr,Mn)3O4 layer varied from about 500 nm to 1.2 µm and was more than four times lower than in the case of the steel that was not pre-oxidized.
- These different behaviors were the result of the combination of different kinds of stress, namely, residual, growing, and thermal stress.
- The film residual stress on the polished substrate before 100 h of oxidation was tensile. On the other hand, the coating on the pre-oxidized steel had about 3% of porosity. This value was 30 times higher than the one measured in the case of the substrate that was not pre-oxidized. The substrate residual stress determined for both steel samples (with and without pre-oxidation) before undergoing high-temperature oxidation was compressive, but it was five times higher in the case of the steel that was not pre-oxidized (−542.5 ± 55.7 and −108.6 ± 50.6 MPa, respectively). If stress is generated during oxidation, the sample without pre-oxidation will have less ability to resist than the pre-oxidized one.
- In the case of the substrate that was not pre-oxidized, the growing stress was due to the growth of the Cr2O3 scale underneath the TiO2 layer during isothermal oxidation, which led to the cracking of TiO2.
- The thermal stress occurred during cooling down and was due to differences between the thermal expansion coefficients (TEC) of the substrate and the different oxide scales. Cr2O3 has a TEC intermediate between TiO2 and the ferritic steel substrate. In the case of the pre-oxidized steel, the presence of a continuous, albeit thin, Cr2O3 layer covering the substrate before the application of the coating, i.e., from the first exposure instant of the Ti coating to the oxidant atmosphere, had a beneficial effect on the adhesion of the TiO2 scale forming during high-temperature exposure.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ardigo-Besnard, M.R.; Popa, I.; Chevalier, S. Spinel and perovskite coatings effect on long term oxidation of a ferritic stainless steel in H2/H2O atmosphere. Corros. Sci. 2019, 148, 251–263. [Google Scholar] [CrossRef]
- Ardigo, M.R.; Popa, I.; Chevalier, S.; Girardon, P.; Perry, F.; Laucournet, R.; Brevet, A.; Desgranges, C. Effect of coatings on long term behaviour of a commercial stainless steel for solid oxide electrolyser cell interconnect application in H2/H2O atmosphere. Int. J. Hydrogen Energy 2014, 39, 21673–21677. [Google Scholar] [CrossRef]
- Ardigo, M.R.; Popa, I.; Chevalier, S.; Parry, V.; Galerie, A.; Girardon, P.; Perry, F.; Laucournet, R.; Brevet, A.; Rigal, E. Coated interconnects development for high temperature water vapour electrolysis: Study in anode atmosphere. Int. J. Hydrogen Energy 2013, 38, 15910–15916. [Google Scholar] [CrossRef]
- Petric, A.; Ling, H. Electrical conductivity and thermal expansion of spinels at elevated temperatures. J. Am. Ceram. Soc. 2007, 90, 1515–1520. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Mardare, C.C.; Asteman, H.; Spiegel, M.; Savan, A.; Ludwig, A. Investigation of thermally oxidised Mn–Co thin films for application in SOFC metallic interconnects. Appl. Surf. Sci. 2008, 255, 1850–1859. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, J.; Hwang, H.J.; Whang, C.M.; Moon, J.M.; Kim, D.H. Lanthanum oxide-coated stainless steel for bipolar plates in solid oxide fuel cells (SOFCs). J. Power Sources 2008, 181, 281–286. [Google Scholar] [CrossRef]
- Cabouro, G.; Caboche, G.; Chevalier, S.; Piccardo, P. Opportunity of metallic interconnects for ITSOFC: Reactivity and electrical property. J. Power Sources 2006, 156, 39–44. [Google Scholar] [CrossRef]
- Lim, D.P.; Lim, D.S.; Oh, J.S.; Lyo, I.W. Influence of post-treatments on the contact resistance of plasma-sprayed La0.8Sr0.2MnO3 coating on SOFC metallic interconnector. Surf. Coat. Technol. 2005, 200, 1248–1251. [Google Scholar] [CrossRef]
- Wu, J.; Johnson, C.D.; Jiang, Y.; Gemmen, R.S.; Liu, X. Pulse plating of Mn–Co alloys for SOFC interconnect applications. Electrochim. Acta 2008, 54, 793–800. [Google Scholar] [CrossRef]
- Mikkelsen, L.; Chen, M.; Hendriksen, P.V.; Persson, A.; Pryds, N.; Rodrigo, K. Deposition of La0.8Sr0.2Cr0.97V0.03O3 and MnCr2O4 thin films on ferritic alloy for solid oxide fuel cell application. Surf. Coat. Technol. 2007, 202, 1262–1266. [Google Scholar] [CrossRef]
- Gorokhovsky, V.I.; Gannon, P.E.; Deibert, M.C.; Smith, R.J.; Kayani, A.; Kopczyk, M.; VanVorous, D.; Yang, Z.G.; Stevenson, J.W.; Visco, S.; et al. Deposition and Evaluation of Protective PVD Coatings on Ferritic Stainless Steel SOFC Interconnects. J. Electrochem. Soc. 2006, 153, A1886–A1893. [Google Scholar] [CrossRef]
- Hoyt, K.O.; Gannon, P.E.; White, P.; Tortop, R.; Ellingwood, B.J.; Khoshuei, H. Oxidation behavior of (Co,Mn)3O4 coatings on preoxidized stainless steel for solid oxide fuel cell interconnects. Int. J. Hydrogen Energy 2012, 37, 518–529. [Google Scholar] [CrossRef]
- Pan, Y.; Geng, S.; Chen, G.; Wang, F. Effect of pre-oxidation on surface scale microstructure and electrical property of Cu-Fe coated steel interconnect. Corros. Sci. 2020, 170, 108680. [Google Scholar] [CrossRef]
- Yang, P.; Liu, C.-K.; Wu, J.-Y.; Shong, W.-J.; Lee, R.-Y.; Sung, C.-C. Effects of pre-oxidation on the microstructural and electrical properties of La0.67Sr0.33MnO3-δ coated ferritic stainless steels. J. Power Sources 2012, 213, 63–68. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, S.; Chen, G.; Wang, F. Influence of preoxidation on high temperature behavior of NiFe2 coated SOFC interconnect steel. Int. J. Hydrogen Energy 2019, 44, 13744–13756. [Google Scholar] [CrossRef]
- Amendola, R.; Gannon, P.; Ellingwood, B.; Hoyt, K.; Piccardo, P.; Genocchio, P. Oxidation behavior of coated and preoxidized ferritic steel in single and dual atmosphere exposures at 800 °C. Surf. Coat. Technol. 2012, 206, 2173–2180. [Google Scholar] [CrossRef]
- Talic, B.; Molin, S.; Hendriksen, P.V.; Lein, H.L. Effect of pre-oxidation on the oxidation resistance of Crofer 22 APU. Corros. Sci. 2018, 138, 189–199. [Google Scholar]
- Goebel, C.; Alnegren, P.; Faust, R.; Svensson, J.-E.; Froitzheim, J. The effect of pre-oxidation parameters on the corrosion behavior of AISI 441 in dual atmosphere. Int. J. Hydrogen Energy 2018, 43, 14655–14674. [Google Scholar] [CrossRef]
- Evrard, M.; Besnard, A.; Lucas, S. Study of the influence of the pressure and rotational motion of 3D substrates processed by magnetron sputtering: A comparative study between Monte Carlo modelling and experiments. Surf. Coat. Tech. 2019, 378, 125070. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science: London, UK; Elsevier Applied Science: New York, NY, USA, 1988. [Google Scholar]
- Coddet, C.; Chaze, A.M.; Beranger, G. Measurements of the adhesion of thermal oxide film: Application to the oxidation of titanium. J. Mater. Sci. 1987, 22, 2969–2974. [Google Scholar] [CrossRef]
- Chaze, A.M.; Coddet, C. The role of nitrogen in the oxidation behavior of titanium and some binary alloys. J. Less Common Metals 1986, 124, 73–84. [Google Scholar] [CrossRef]
- Hasegawa, M. Chapter 3.3—Ellingham Diagram. In Treatise on Process Metallurgy Volume 1: Process Fundamentals; Seetharaman, S., McLean, A., Guthrie, R., Sridhar, S., Eds.; Elsevier Ldt.: Oxford, UK, 2014; pp. 507–516. [Google Scholar]
- Nakajima, H.; Koiv, M. Diffusion in Titanium. ISIJ Int. 1991, 31, 757–766. [Google Scholar] [CrossRef]
- Shapovalov, V.P.; Kurasov, A.N. Diffusion of titanium in iron. Metalloved. Termich. Obrab. Metall. 1975, 9, 71–73. [Google Scholar] [CrossRef]
- Noyan, I.C.; Cohen, J.B. Residual Stress, Measurement by Diffraction and Interpretation; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Besnard, A.; Ardigo, M.R.; Imhoff, L.; Jacquet, P. Curvature radius measurement by optical profiler and determination of the residual stress in thin films. Appl. Surf. Sci. 2019, 487, 356–361. [Google Scholar] [CrossRef]
- Depla, D.; Leroy, W.P. Magnetron sputter deposition as visualized by Monte Carlo modeling. Thin Solid Films 2012, 520, 6337–6354. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P. The stopping and range of ions in matter. In Treatise Heavy-Ion Science; Bromley, D.A., Ed.; Springer: Boston, MA, USA, 1985; Volume 6, Astrophys. Chem. Condens. Matter; pp. 93–129. [Google Scholar]
- Moskovkin, P.; Lucas, S. Computer simulations of the early stage growth of Ge clusters at elevated temperatures, on patterned Si substrate using the kinetic Monte Carlo method. Thin Solids Film 2013, 536, 313–317. [Google Scholar] [CrossRef]
- Niewolak, L.; Young, D.J.; Hattendorf, H.; Singheiser, L.; Quadakkers, W.J. Mechanisms of oxide scale formation on ferritic steel in simulated low and high pO2 service environments of solid oxide fuel cells. Oxid. Met. 2014, 82, 123–143. [Google Scholar] [CrossRef]
- Fontana, S.; Amendola, R.; Chevalier, S.; Piccardo, P.; Caboche, G.; Viviani, M.; Molins, R.; Sennour, M. Metallic interconnects for SOFC: Characterisation of corrosion resistance and conductivity evaluation at operating temperature of differently coated alloys. J. Power Sources 2007, 171, 652–662. [Google Scholar] [CrossRef]
- Bednarz, M.; Molin, S.; Bobruk, M.; Stygar, M.; Długoń, E.; Sitarz, M.; Brylewski, T. High-temperature oxidation of the Crofer 22 H ferritic steel with Mn1.45Co1.45Fe0.1O4 and Mn1.5Co1.5O4 spinel coatings under thermal cycling conditions and its properties. Mater. Chem. Phys. 2019, 225, 227–238. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly conductive nano-sized Magnéli phases titanium oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef] [PubMed]
- Waldner, P.; Eriksson, G. Thermodynamic modelling of the system titanium-oxygen. Calphad 1999, 23, 189–218. [Google Scholar] [CrossRef]
- Geng, S.; Zhao, Q.; Li, Y.; Mu, J.; Chen, G.; Wang, F.; Zhu, S. Sputtered MnCu metallic coating on ferritic stainless steel for solid oxide fuel cell interconnects application. Int. J. Hydrogen Energy 2017, 42, 10298–10307. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.G.; Singh, P.; Stevenson, J.W. Electrical contacts between cathodes and metallic interconnects in solid oxide fuel cells. J. Power Sources 2006, 155, 246–252. [Google Scholar] [CrossRef]
- Lobnig, R.E.; Schmidt, H.P.; Hennesen, K.; Grabke, H.G. Diffusion of cations in chromia layers grown on iron-base alloys. Oxid. Met. 1992, 37, 81–93. [Google Scholar] [CrossRef]
- Evans, H.E.; Donaldson, A.T.; Gilmour, T.C. Mechanisms of breakaway oxidation and application to a chromia-forming steel. Oxid. Met. 1999, 54, 379–402. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. 2003, A348, 227–243. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Liu, S.; Zhang, Y.; Xu, J.; Zhou, X.; Zhao, G.; Cai, Z.; Zhao, X. Ultra-high temperature oxidation resistance of a novel (Mo, Hf, W, Ti)Si2 ceramic coating with Nb interlayer on Ta substrate. J. Eur. Ceram. Soc. 2022, 42, 4866–4880. [Google Scholar] [CrossRef]
- Anton, R.; Hüning, S.; Laska, N.; Weber, M.; Schellert, S.; Gorr, B.; Christ, H.J.; Schulz, U. Graded PVD Mo-Si interlayer between Si coating and Mo-Si-B alloys: Investigation of oxidation behavior. Corros. Sci. 2021, 192, 109843. [Google Scholar] [CrossRef]
- Lima, C.R.C.; Cinca, N.; Guilemany, J.M. Study of the high temperature oxidation performance of Thermal Barrier Coatings with HVOF sprayed bond coat and incorporating a PVD ceramic interlayer. Ceram. Int. 2012, 38, 6423–6429. [Google Scholar] [CrossRef]




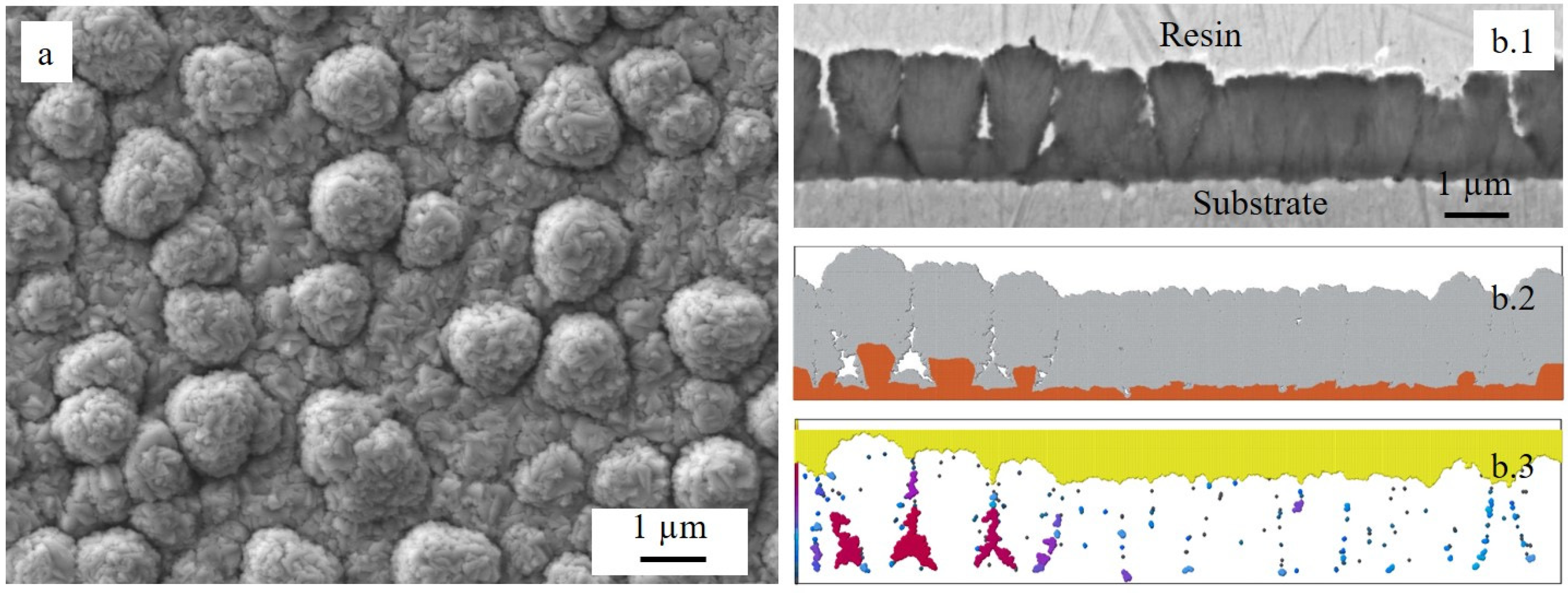
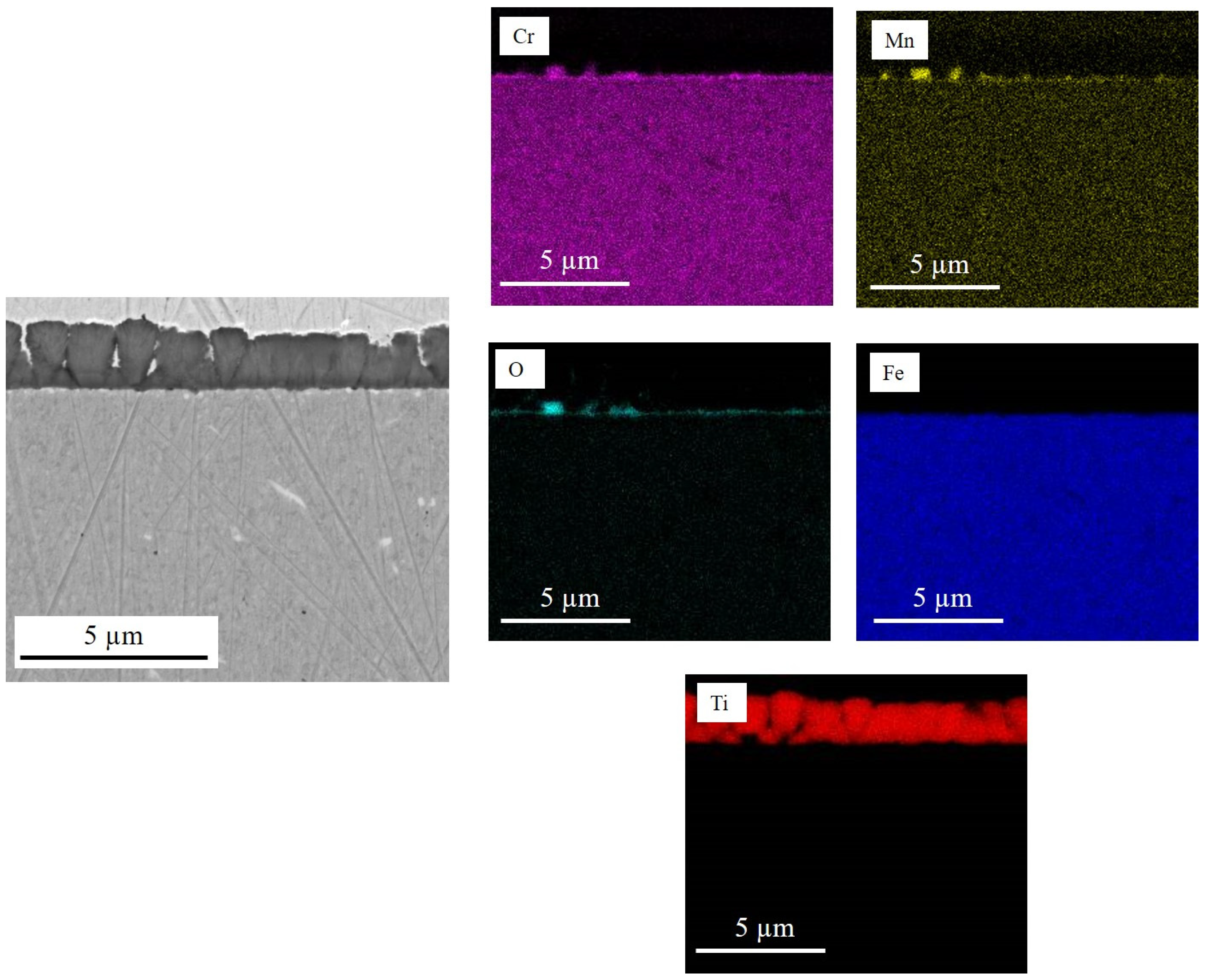
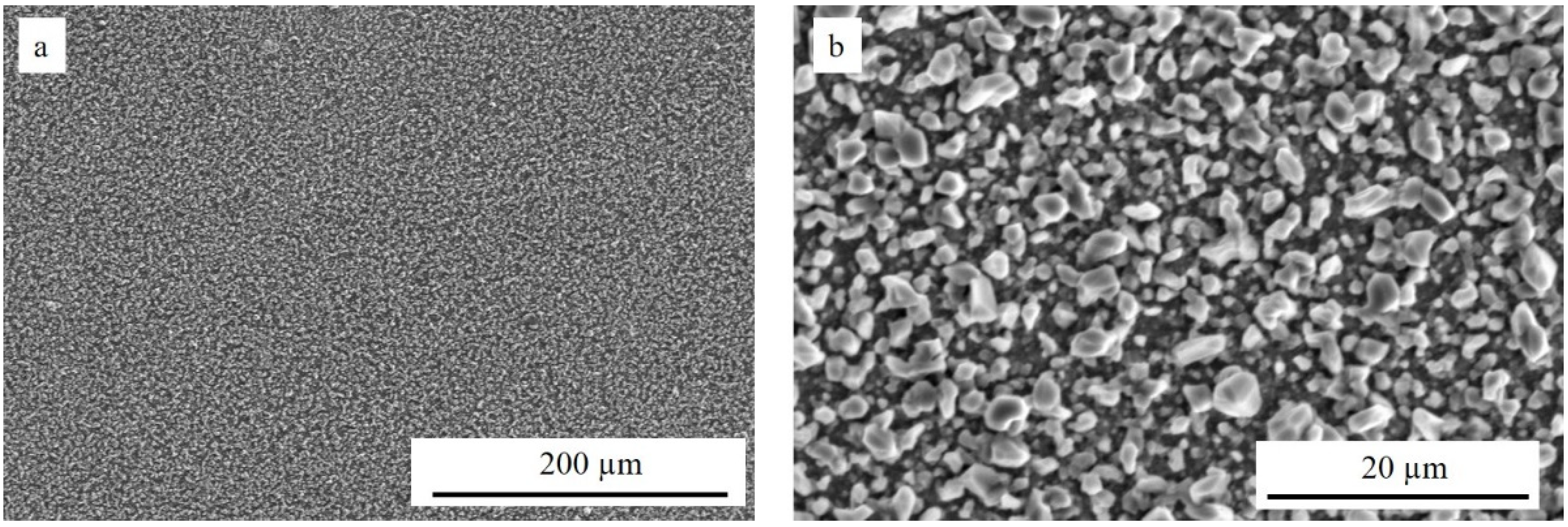

| Element | O | Si | Ti | Cr | Mn | Fe | Nb |
|---|---|---|---|---|---|---|---|
| Point 1 | 64.7 | 0.1 | 32.1 | 2.6 | 0.3 | 0.2 | - |
| Point 2 | 60.7 | - | 33.5 | 4.9 | 0.4 | 0.5 | - |
| Point 3 | 31.1 | 14.5 | 0.6 | 9.6 | - | 43.6 | 0.6 |
| Point 4 | 27.1 | 6.5 | 0.5 | 17.4 | 0.5 | 48.0 | - |
| Point 5 | 61.3 | - | 1.6 | 35.1 | - | 2.0 | - |
| Point 6 | 58.9 | 0.5 | 7.3 | 25.3 | - | 1.8 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardigo-Besnard, M.-R.; Besnard, A.; Nkou Bouala, G.; Boulet, P.; Pinot, Y.; Ostorero, Q. Effect of Pre-Oxidation on a Ti PVD Coated Ferritic Steel Substrate during High-Temperature Aging. Crystals 2022, 12, 1732. https://doi.org/10.3390/cryst12121732
Ardigo-Besnard M-R, Besnard A, Nkou Bouala G, Boulet P, Pinot Y, Ostorero Q. Effect of Pre-Oxidation on a Ti PVD Coated Ferritic Steel Substrate during High-Temperature Aging. Crystals. 2022; 12(12):1732. https://doi.org/10.3390/cryst12121732
Chicago/Turabian StyleArdigo-Besnard, Maria-Rosa, Aurélien Besnard, Galy Nkou Bouala, Pascal Boulet, Yoann Pinot, and Quentin Ostorero. 2022. "Effect of Pre-Oxidation on a Ti PVD Coated Ferritic Steel Substrate during High-Temperature Aging" Crystals 12, no. 12: 1732. https://doi.org/10.3390/cryst12121732
APA StyleArdigo-Besnard, M.-R., Besnard, A., Nkou Bouala, G., Boulet, P., Pinot, Y., & Ostorero, Q. (2022). Effect of Pre-Oxidation on a Ti PVD Coated Ferritic Steel Substrate during High-Temperature Aging. Crystals, 12(12), 1732. https://doi.org/10.3390/cryst12121732








