Photocatalytic Dye Decomposition over CaMnO3−δ and Pr0.5Ca0.5MnO3: A Combined XPS and DFT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Characterizations and Photocatalysis Experiments
2.3. Theoretical Method
3. Results and Discussion
3.1. Structural and Microstructural Properties
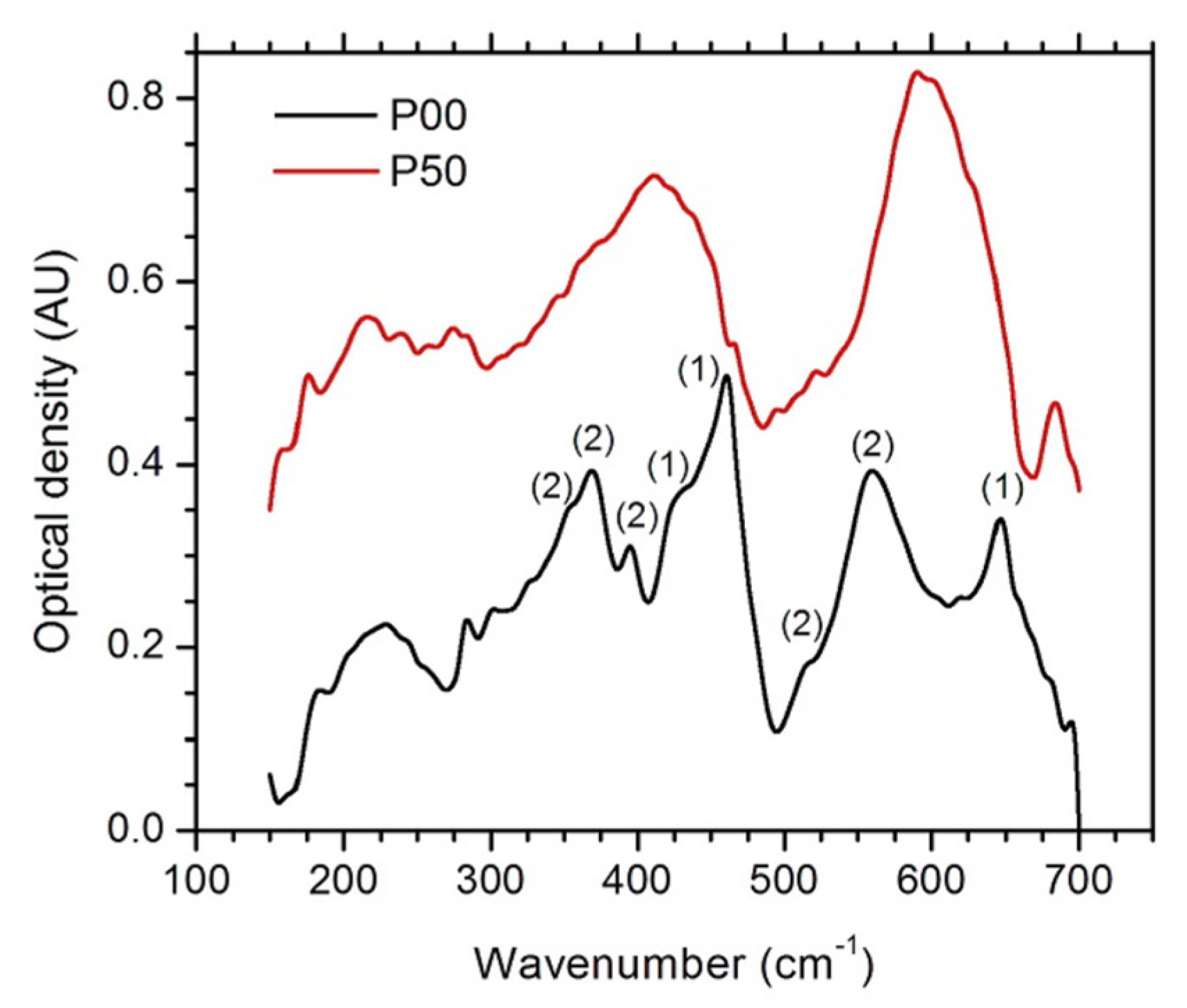
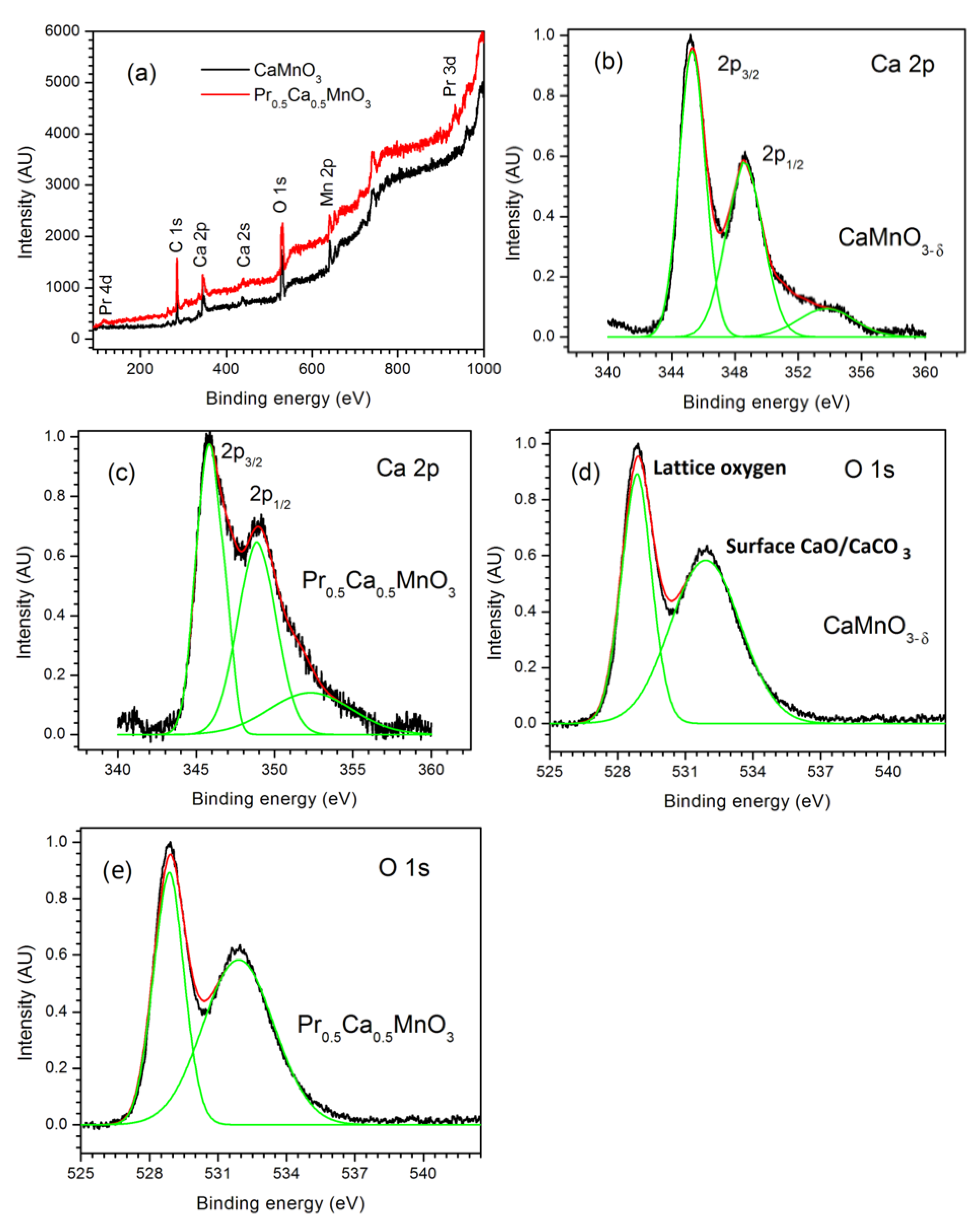
3.2. Spectroscopic Analysis
3.3. Photocatalytic Degradation Analysis
3.4. Electronic Structure of CaMnO3−δ and Pr1−xCaxMnO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Likodimos, V. Advanced Photocatalytic Materials. Materials 2020, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef] [PubMed]
- Dogan, F.; Lin, H.; Guilloux-Viry, M.; Peña, O. Focus on properties and applications of perovskites. Sci. Technol. Adv. Mater. 2015, 16, 020301. [Google Scholar] [CrossRef]
- Islam, M.S. Ionic transport in ABO3 perovskite oxides: A computer modelling tour. J. Mater. Chem. 2000, 10, 1027–1038. [Google Scholar] [CrossRef]
- Sotoudeh, M.; Rajpurohit, S.; Blöchl, P.; Mierwaldt, D.; Norpoth, J.; Roddatis, V.; Mildner, S.; Ifland, B.; Jooss, C. Electronic structure of Pr1−xCaxMnO3. Phys. Rev. B 2016, 95, 235150. [Google Scholar] [CrossRef]
- Benedetti, P.; Zeyher, R. Jahn-Teller distortion and electronic correlation effects in undoped manganese perovskites. Phys. Rev. B 1999, 59, 9923–9928. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.W.; Hayashi, N.; Saito, T.; Azuma, M.; Muranaka, S.; Shimakawa, Y. Temperature-induced A–B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature 2009, 458, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Angel, R.J. Octahedral tilts, symmetry-adapted displacive modes and polyhedral volume ratios in perovskite structures. Acta Crystallogr. Sect. B Struct. Sci. 2011, 67, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Levin, I.; Bendersky, L.A. Symmetry classification of the layered perovskite-derived AnBnX3n+2 structures. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Himanshu, A.; Sen, P.; Kumar, U.; Richter, M.; Sinha, T. Effects of octahedral tilting on the electronic structure and optical properties of d0 double perovskites A2ScSbO6 (A=Sr, Ca). J. Alloy. Compd. 2017, 705, 497–506. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Wu, L.; Bao, S.; Shen, Y.; Lin, Y.; Nan, C. Tunable magnetic and electrical behaviors in perovskite oxides by oxygen octahedral tilting. Sci. China Mater. 2015, 58, 302–312. [Google Scholar] [CrossRef]
- Teh, Y.W.; Chee, M.K.T.; Kong, X.Y.; Yong, S.-T.; Chai, S.-P. An insight into perovskite-based photocatalysts for artificial photosynthesis. Sustain. Energy Fuels 2019, 4, 973–984. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Lim, P.F.; Leong, K.H.; Sim, L.C.; Saravanan, P.; Aziz, A.A. Perovskite Oxide–Based Photocatalysts for Excellent Visible Light–Driven Photocatalysis and Energy Conversion. In Plant Nanobionics; Prasad, R., Ed.; Approaches in Nanoparticles, Biosynthesis, and Toxicity; Springer: Cham, Switzerland, 2019; Volume 2, pp. 35–54. [Google Scholar] [CrossRef]
- Husanu, M.-A.; Vistoli, L.; Verdi, C.; Sander, A.; Garcia, V.; Rault, J.; Bisti, F.; Lev, L.L.; Schmitt, T.; Giustino, F.; et al. Electron-polaron dichotomy of charge carriers in perovskite oxides. Commun. Phys. 2020, 3, 62. [Google Scholar] [CrossRef]
- Huang, C.; Fang, H.; Xu, Z.; Zheng, X.; Ruan, X. Evolutions of geometry and electronic state introduced by oxygen vacancy for CaMnO3 compound. Results Phys. 2019, 13, 102337. [Google Scholar] [CrossRef]
- Du, J.; Zhang, T.; Cheng, F.; Chu, W.; Wu, Z.; Chen, J. Nonstoichiometric Perovskite CaMnO3−δ for Oxygen Electrocatalysis with High Activity. Inorg. Chem. 2014, 53, 9106–9114. [Google Scholar] [CrossRef]
- Soleimani Varaki, M.; Jafari, A.; Ebrahimizadeh Abrishami, M. Laser-induced photocatalytic reduction of CO2 into methanol over perovskite LaMnO3. J. Laser Appl. 2020, 32, 042005. [Google Scholar] [CrossRef]
- Wang, T.; Qian, X.; Yue, D.; Yan, X.; Yamashita, H.; Zhao, Y. CaMnO3 perovskite nanocrystals for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 398, 125638. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, W.; Huang, X.; Zou, J. Oxygen vacancy induced enhancement of photochemical water oxidation on calcium manganese oxide catalyst. Catal. Commun. 2017, 89, 117–120. [Google Scholar] [CrossRef]
- Gagrani, A.; Sousa, S.; Monteiro, O.; Tsuzuki, T. Solid state synthesis and photocatalytic activity of bio-inspired calcium manganese oxide catalysts. J. Solid State Chem. 2020, 288, 121390. [Google Scholar] [CrossRef]
- Klarbring, J.; Simak, S. Nature of the octahedral tilting phase transitions in perovskites: A case study of CaMnO3. Phys. Rev. B 2018, 97, 024108. [Google Scholar] [CrossRef]
- Yu, X.; Li, F.; Huang, C.; Fang, H.; Xu, Z. Anisotropic electronic structure and geometry of CaMnO3 perovskite with oxygen nonstoichiometry. J. Mater. Res. Technol. 2020, 9, 6595–6601. [Google Scholar] [CrossRef]
- Abrishami, M.E.; Risch, M.; Scholz, J.; Roddatis, V.; Osterthun, N.; Jooss, C. Oxygen Evolution at Manganite Perovskite Ruddlesden-Popper Type Particles: Trends of Activity on Structure, Valence and Covalence. Materials 2016, 9, 921. [Google Scholar] [CrossRef]
- Mierwaldt, D.; Roddatis, V.; Risch, M.; Scholz, J.; Geppert, J.; Abrishami, M.E.; Jooss, C. Environmental TEM Investigation of Electrochemical Stability of Perovskite and Ruddlesden-Popper Type Manganite Oxygen Evolution Catalysts. Adv. Sustain. Syst. 2017, 1, 1700109. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Fedorov, I.; Lorenzana, J.; Dore, P.; De Marzi, G.; Maselli, P.; Calvani, P.; Cheong, S.W.; Koval, S.; Migoni, R. Infrared-active phonons of LaMnO3 and CaMnO3. Phys. Rev. B 1999, 60, 11875–11878. [Google Scholar] [CrossRef]
- Sopracase, R.; Gruener, G.; Olive, E.; Soret, J.-C. Infrared study of the phonon modes in PrMnO3 and CaMnO3. Phys. B: Condens. Matter 2010, 405, 45–52. [Google Scholar] [CrossRef]
- Celorrio, V.; Calvillo, L.; Dann, E.; Granozzi, G.; Aguadero, A.; Kramer, D.; Russell, A.E.; Fermín, D.J. Oxygen reduction reaction at LaxCa1−xMnO3 nanostructures: Interplay between A-site segregation and B-site valency. Catal. Sci. Technol. 2016, 6, 7231–7238. [Google Scholar] [CrossRef]
- Joy, L.K.; Shanmukharao Samatham, S.; Thomas, S.; Ganesan, V.; Al-Harthi, S.; Liebig, A.; Albrecht, M.; Anantharaman, M.R. Colossal thermoelectric power in charge ordered lanthanum calcium manganites (La0.5Ca0.5MnO3). J. Appl. Phys. 2014, 116, 213701. [Google Scholar] [CrossRef]
- Machkova, M.; Brashkova, N.; Ivanov, P.; Carda, J.; Kozhukharov, V. Surface behavior of Sr-doped lanthanide perovskites. Appl. Surf. Sci. 1997, 119, 127–136. [Google Scholar] [CrossRef]
- Ivanov-Emin, B.N.; Nevskaya, N.A.; Zaitsev, B.E.; Ivanova, T. Synthesis and properties of calcium and strontium hydroxomanganates(III). Zh. Neorg. Khim. 1982, 27, 3101–3104. [Google Scholar]
- Nelson, A.J.; Reynolds, J.G.; Roos, J.W. Core-level satellites and outer core-level multiplet splitting in Mn model compounds. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 1072–1076. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of Photocatalyzed Reactions: Five Lessons Learned. Front. Chem. 2018, 6, 378. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Lachgar, A. Effect of particle size on the photocatalytic activity of BiNbO4 under visible light irradiation. J. Physics: Conf. Ser. 2016, 758, 012017. [Google Scholar] [CrossRef]
- Liu, L.; Luo, C.; Xiong, J.; Yang, Z.; Zhang, Y.; Cai, Y.; Gu, H. Reduced graphene oxide (rGO) decorated TiO2 microspheres for visible-light photocatalytic reduction of Cr(VI). J. Alloy. Compd. 2017, 690, 771–776. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Nolan, P.D.; Wheeler, M.C.; Davis, J.E.; Mullins, C.B. Mechanisms of Initial Dissociative Chemisorption of Oxygen on Transition-Metal Surfaces. Accounts Chem. Res. 1998, 31, 798–804. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Lu, C. Detection of Oxygen Vacancies in Oxides by Defect-Dependent Cataluminescence. Anal. Chem. 2015, 87, 7313–7320. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, Y.; Lu, C.; Ma, Y.; Yuan, S.; Qian, G. Soot Oxidation over CeO2 or Ag/CeO2: Influences of Bulk Oxygen Vacancies and Surface Oxygen Vacancies on Activity and Stability of the Catalyst. Eur. J. Inorg. Chem. 2018, 2018, 2944–2951. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Mastrikov, Y.A.; Heifets, E.; Maier, J. Adsorption of atomic and molecular oxygen on the LaMnO3(001) surface: Ab initio supercell calculations and thermodynamics. Phys. Chem. Chem. Phys. 2008, 10, 4644–4649. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to Near-Infrared Light: Towards Full Solar-Light Spectrum Activity. ChemCatChem 2014, 7, 559–573. [Google Scholar] [CrossRef]
- Chabri, S.; Dhara, A.; Show, B.; Adak, D.; Sinha, A.; Mukherjee, N. Mesoporous CuO–ZnO p–n heterojunction based nanocomposites with high specific surface area for enhanced photocatalysis and electrochemical sensing. Catal. Sci. Technol. 2015, 6, 3238–3252. [Google Scholar] [CrossRef]
- Shafaee, M.; Goharshadi, E.K.; Mashreghi, M.; Sadeghinia, M. TiO2 nanoparticles and TiO2@graphene quantum dots nanocomposites as effective visible/solar light photocatalysts. J. Photochem. Photobiol. Chem. 2018, 357, 90–102. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rezaee Roknabadi, M.; Behdani, M.; Kompany, A. Enhancement of visible and UV light photocatalytic activity of rGO-TiO2 nanocomposites: The effect of TiO2/Graphene oxide weight ratio. Ceram. Int. 2019, 45, 12625–12634. [Google Scholar] [CrossRef]
- Weng, B.; Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Toward the enhanced photoactivity and photostability of ZnO nanospheres via intimate surface coating with reduced graphene oxide. J. Mater. Chem. A 2014, 2, 9380–9389. [Google Scholar] [CrossRef]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef]
- Raiser, D.; Mildner, S.; Ifland, B.; Sotoudeh, M.; Blöchl, P.; Techert, S.; Jooss, C. Evolution of Hot Polaron States with a Nanosecond Lifetime in a Manganite Perovskite. Adv. Energy Mater. 2017, 7, 1602174. [Google Scholar] [CrossRef]
- Sotoudeh, M. First-principles calculations of polaronic correlations and reactivity of oxides: Manganites, water oxidation and Pd/rutile interface. Ph.D. Thesis, Fakultät für Physik (inkl. GAUSS), Georg-August-University Göttingen, Göttingen, Germany, 2019. [Google Scholar] [CrossRef]



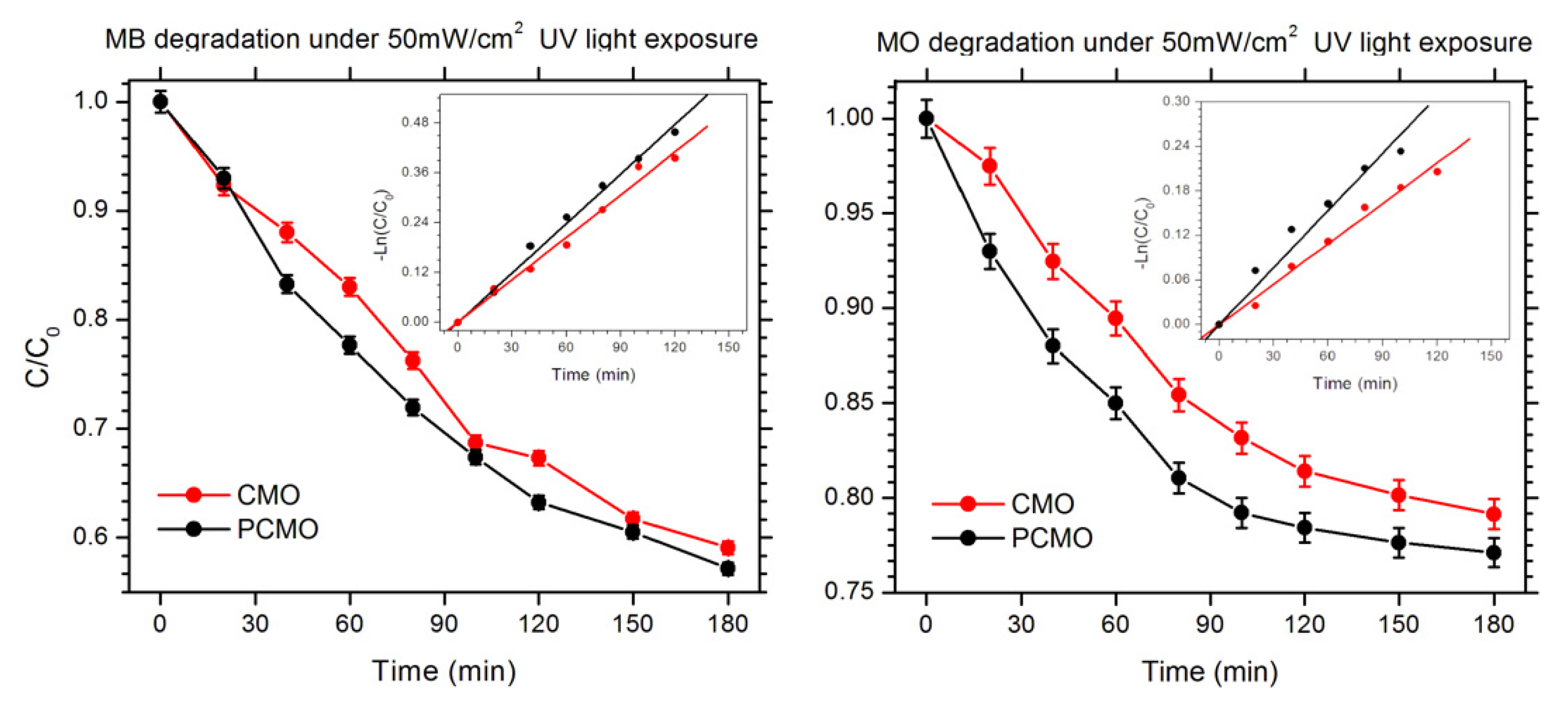
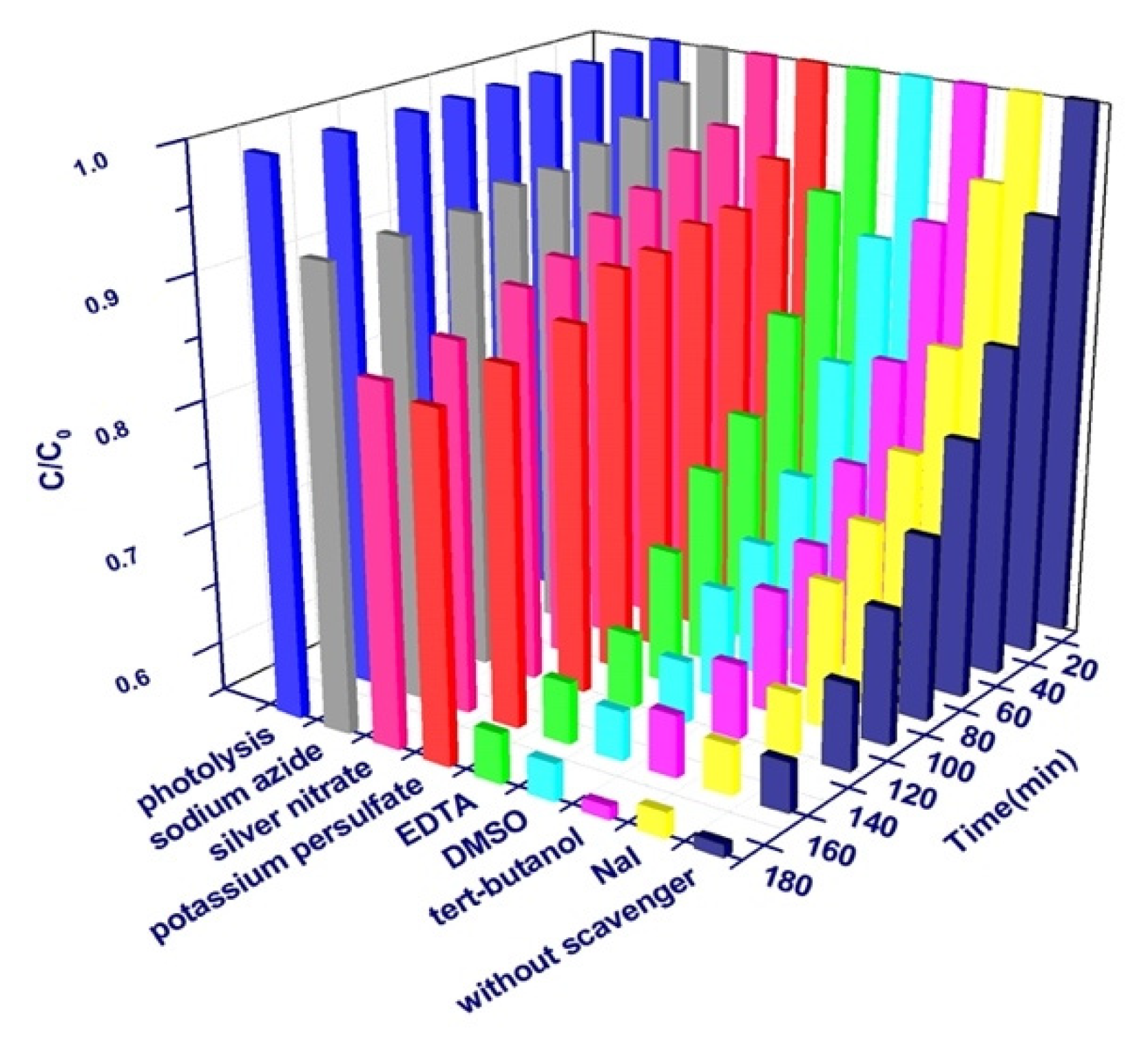

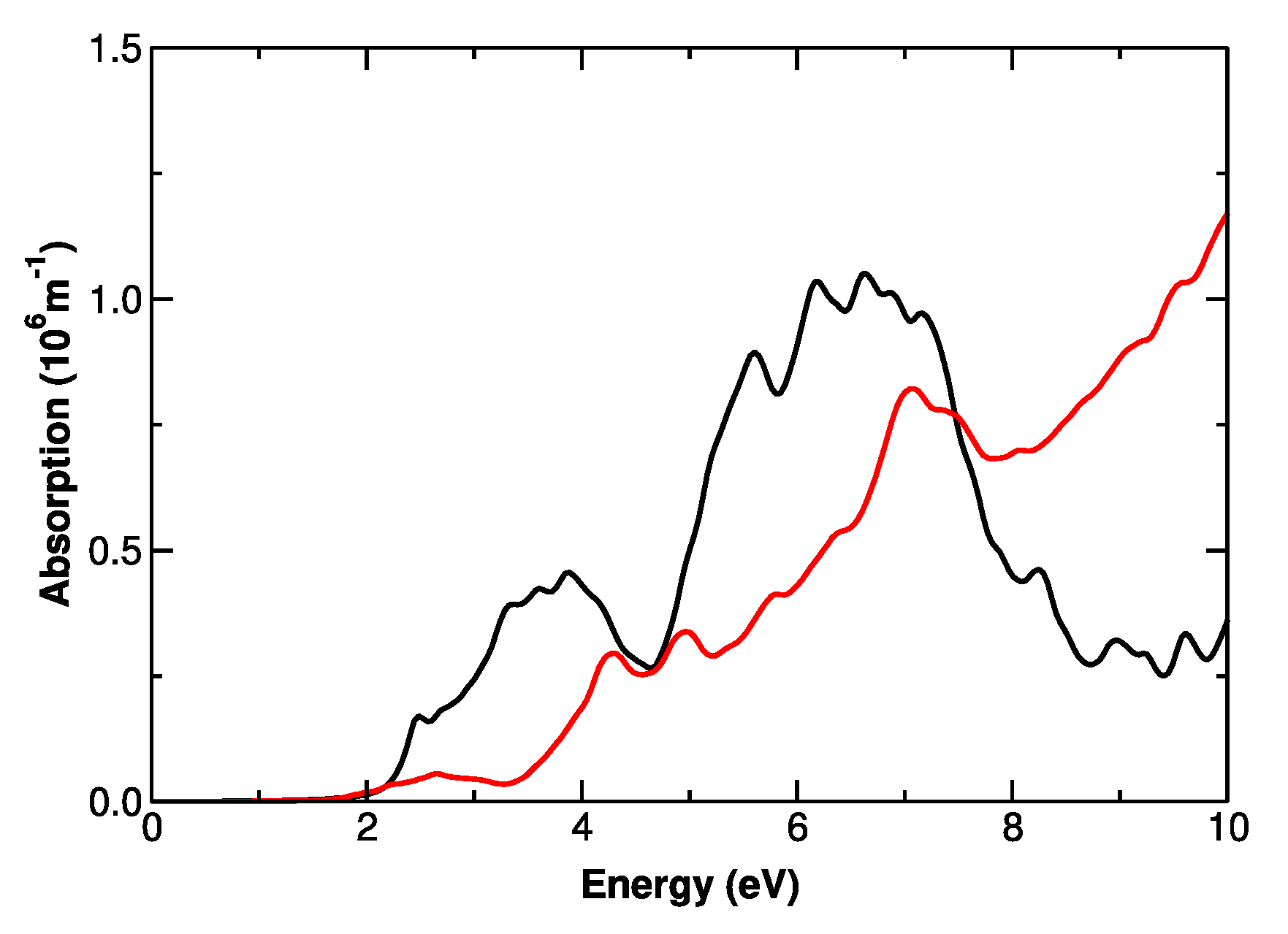
| Material | Particle Size (nm) [27] | Specific Surface Area (cm2/mg) | kapp for MB (10−3 min−1) | kapp for MO (10−3 min−1) |
|---|---|---|---|---|
| CMO | 70 | 81.63 | 3.98 | 2.56 |
| PCMO | 64 | 49.96 | 3.42 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimizadeh Abrishami, M.; Mohammadi, M.; Sotoudeh, M. Photocatalytic Dye Decomposition over CaMnO3−δ and Pr0.5Ca0.5MnO3: A Combined XPS and DFT Study. Crystals 2022, 12, 1728. https://doi.org/10.3390/cryst12121728
Ebrahimizadeh Abrishami M, Mohammadi M, Sotoudeh M. Photocatalytic Dye Decomposition over CaMnO3−δ and Pr0.5Ca0.5MnO3: A Combined XPS and DFT Study. Crystals. 2022; 12(12):1728. https://doi.org/10.3390/cryst12121728
Chicago/Turabian StyleEbrahimizadeh Abrishami, Majid, Mojtaba Mohammadi, and Mohsen Sotoudeh. 2022. "Photocatalytic Dye Decomposition over CaMnO3−δ and Pr0.5Ca0.5MnO3: A Combined XPS and DFT Study" Crystals 12, no. 12: 1728. https://doi.org/10.3390/cryst12121728
APA StyleEbrahimizadeh Abrishami, M., Mohammadi, M., & Sotoudeh, M. (2022). Photocatalytic Dye Decomposition over CaMnO3−δ and Pr0.5Ca0.5MnO3: A Combined XPS and DFT Study. Crystals, 12(12), 1728. https://doi.org/10.3390/cryst12121728