Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development
Abstract
1. Zeolites
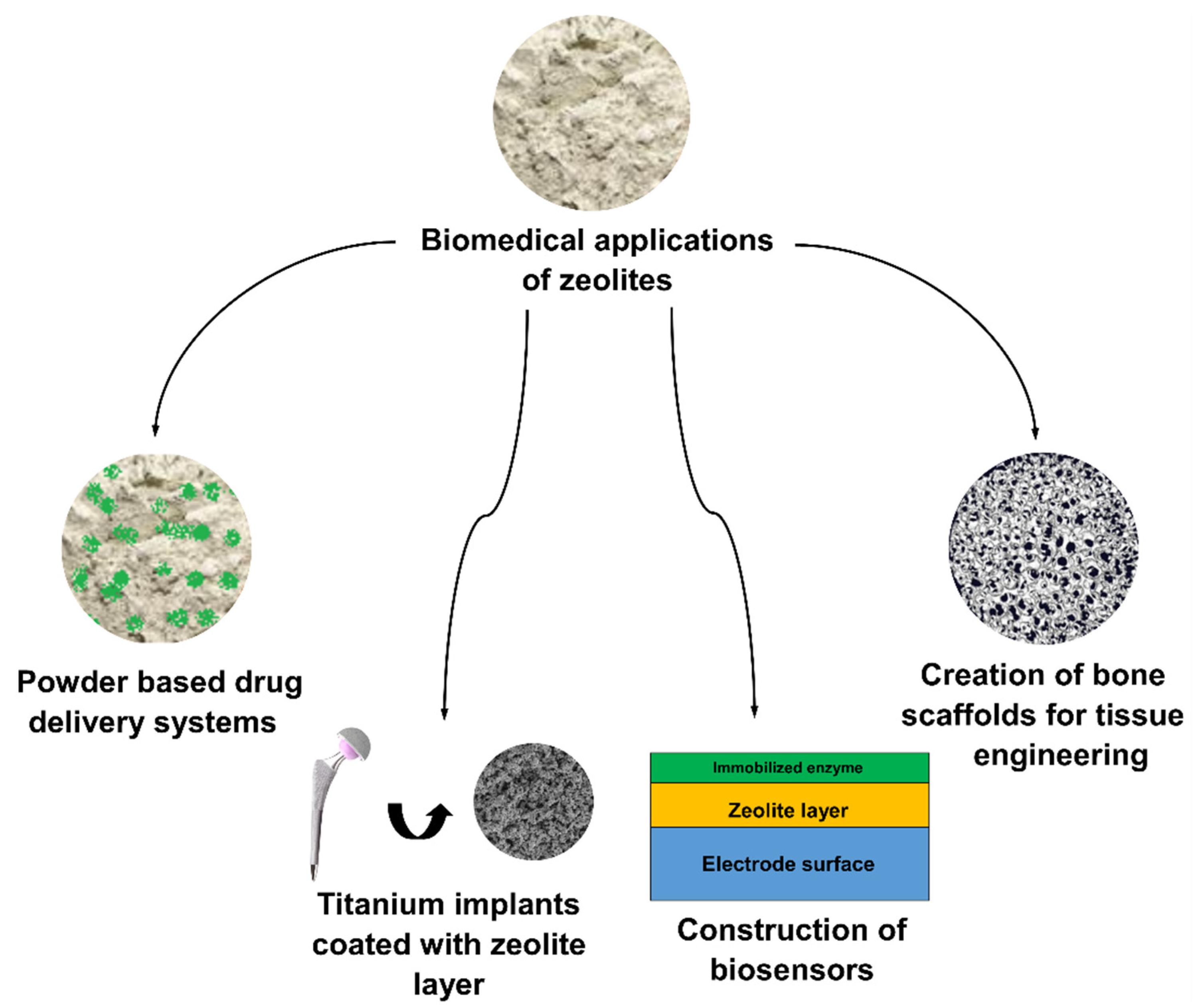
2. Zeolites in Biomaterials
3. Modification of Zeolite Properties
4. Zeolites in Modification of Titanium Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purnomo; Setyarini, P.H.; Sulistyaningsih, D. Zeolite-Based Biomaterials for Biomedical Application: A Review. AIP Conf. Proc. 2018, 1977, 030013. [Google Scholar] [CrossRef]
- Pan, M.; Omar, H.M.; Rohani, S. Application of Nanosize Zeolite Molecular Sieves for Medical Oxygen Concentration. Nanomaterials 2017, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-H.; Fang, F.; Lu, C.-H.; Zheng, L. Removal of Cs+, Sr2+, and Co2+ Ions from the Mixture of Organics and Suspended Solids Aqueous Solutions by Zeolites. Nucl. Eng. Technol. 2017, 49, 556–561. [Google Scholar] [CrossRef]
- Kianfar, E.; Hajimirzaee, S.; Mousavian, S.; Mehr, A.S. Zeolite-Based Catalysts for Methanol to Gasoline Process: A Review. Microchem. J. 2020, 156, 104822. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Voelkel, A. Calcium Zeolites as Intelligent Carriers in Controlled Release of Bisphosphonates. Int. J. Pharm. 2020, 578, 119117. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Espinoza-Santos, N. Cation Exchange of Natural Zeolites: Worldwide Research. Sustainability 2021, 13, 7751. [Google Scholar] [CrossRef]
- Abou-Mesalam, M.M.; Abass, M.R.; Zakaria, E.S.; Hassan, A.M. Metal Doping Silicates as Inorganic Ion Exchange Materials for Environmental Remediation. Silicon 2022, 14, 7961–7969. [Google Scholar] [CrossRef]
- Chen, P.; Xie, M.; Zhai, Y.; Wang, Y.; Huang, Z.; Yang, T.; Sun, W.; Wang, Y.; Sun, J. Stabilization of Extra-Large-Pore Zeolite by Boron Substitution for the Production of Commercially Applicable Catalysts. Chem. Eur. J. 2022, e202202170. [Google Scholar] [CrossRef]
- Kots, P.A.; Zabilska, A.V.; Khramov, E.V.; Grigoriev, Y.V.; Zubavichus, Y.V.; Ivanova, I.I. Mechanism of Zr Incorporation in the Course of Hydrothermal Synthesis of Zeolite BEA. Inorg. Chem. 2018, 57, 11978–11985. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 27 September 2022).
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. Towards a Better Understanding of Lewis Acidic Aluminium in Zeolites. Nat. Mater. 2020, 19, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of Zeolites in Biotechnology and Medicine—A Review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Leng, S.; Guo, H.; Yu, J.; Li, W.; Cao, L.; Huang, J. Quantitative Arrangement of Si/Al Ratio of Natural Zeolite Using Acid Treatment. Appl. Surf. Sci. 2019, 498, 143874. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.-L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.-P. Facile and Fast Determination of Si/Al Ratio of Zeolites Using FTIR Spectroscopy Technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Ramezani Shabolaghi, K.; Irani, M. Ethanol Adsorption in Cation-Exchanged Linde Type L Zeolite, Studied by Molecular Simulations. Comput. Theor. Chem. 2022, 1207, 113498. [Google Scholar] [CrossRef]
- Hernandez-Tamargo, C.; Kwakye-Awuah, B.; O’Malley, A.J.; de Leeuw, N.H. Mercury Exchange in Zeolites Na-A and Na-Y Studied by Classical Molecular Dynamics Simulations and Ion Exchange Experiments. Microporous Mesoporous Mater. 2021, 315, 110903. [Google Scholar] [CrossRef]
- Hasan, F.; Singh, R.; Li, G.; Zhao, D.; Webley, P.A. Direct Synthesis of Hierarchical LTA Zeolite via a Low Crystallization and Growth Rate Technique in Presence of Cetyltrimethylammonium Bromide. J. Colloid Interface Sci. 2012, 382, 1–12. [Google Scholar] [CrossRef]
- Petushkov, A.; Freeman, J.; Larsen, S.C. Framework Stability of Nanocrystalline NaY in Aqueous Solution at Varying PH. Langmuir 2010, 26, 6695–6701. [Google Scholar] [CrossRef]
- Karimi, M.; Habibizadeh, M.; Rostamizadeh, K.; Khatamian, M.; Divband, B. Preparation and Characterization of Nanocomposites Based on Different Zeolite Frameworks as Carriers for Anticancer Drug: Zeolite Y versus ZSM-5. Polym. Bull. 2019, 76, 2233–2252. [Google Scholar] [CrossRef]
- Zakeri, N.; Rezaie, H.R.; Javadpour, J.; Kharaziha, M. Effect of PH on Cisplatin Encapsulated Zeolite Nanoparticles: Release Mechanism and Cytotoxicity. Mater. Chem. Phys. 2021, 273, 124964. [Google Scholar] [CrossRef]
- de Gennaro, B.; Catalanotti, L.; Cappelletti, P.; Langella, A.; Mercurio, M.; Serri, C.; Biondi, M.; Mayol, L. Surface Modified Natural Zeolite as a Carrier for Sustained Diclofenac Release: A Preliminary Feasibility Study. Colloids Surf. B Biointerfaces 2015, 130, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical Applications of Zeolite-Based Materials: A Review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef] [PubMed]
- Sağir, T.; Huysal, M.; Durmus, Z.; Kurt, B.Z.; Senel, M.; Isık, S. Preparation and in Vitro Evaluation of 5-Flourouracil Loaded Magnetite–Zeolite Nanocomposite (5-FU-MZNC) for Cancer Drug Delivery Applications. Biomed. Pharmacother. 2016, 77, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M.; Kucinska, M.; Ratajczak, M.; Pokora, M.; Murias, M.; Voelkel, A.; Sandomierski, M. Zinc Forms of Faujasite Zeolites as a Drug Delivery System for 6-Mercaptopurine. Microporous Mesoporous Mater. 2022, 343, 112194. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in Nanomaterial Application in Enzyme-Based Electrochemical Biosensors: A Review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Kucherenko, I.S.; Soldatkin, O.O.; Pyeshkova, V.M.; Dudchenko, O.Y.; Akata Kurç, B.; Dzyadevych, S.V. Development of Electrochemical Biosensors with Various Types of Zeolites. Appl. Nanosci. 2019, 9, 737–747. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R. A Polyaniline–Zeolite Nanocomposite Material Based Acetylcholinesterase Biosensor for the Sensitive Detection of Acetylcholine and Organophosphates. New J. Chem. 2015, 39, 6899–6906. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Z.; Xia, Q.; Zhou, D. Progress and Perspective of Enzyme Immobilization on Zeolite Crystal Materials. Biochem. Eng. J. 2021, 172, 108033. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Holmes, S.M. Immobilization of Cobalt Ions Using Hierarchically Porous 4A Zeolite-Based Carbon Composites: Ion-Exchange and Solidification. J. Water Process Eng. 2020, 33, 101059. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Yahaya, N.A.B.; Park, I.-K.; Elain, A.; Wong, T.W.; Thomas, S.; Grohens, Y. Antibacterial and Wound Healing Analysis of Gelatin/Zeolite Scaffolds. Colloids Surf. B Biointerfaces 2014, 115, 244–252. [Google Scholar] [CrossRef]
- Akmammedov, R.; Huysal, M.; Isik, S.; Senel, M. Preparation and Characterization of Novel Chitosan/Zeolite Scaffolds for Bone Tissue Engineering Applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 110–118. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Qing, Y.; Wei, Y.; Wang, Q.; Zhang, T.; Sun, C.; Qin, Y.; Li, D.; Yu, J. Antibacterial Activity of Ag-Incorporated Zincosilicate Zeolite Scaffolds Fabricated by Additive Manufacturing. Inorg. Chem. Commun. 2019, 105, 31–35. [Google Scholar] [CrossRef]
- Qing, Y.; Li, K.; Li, D.; Qin, Y. Antibacterial Effects of Silver Incorporated Zeolite Coatings on 3D Printed Porous Stainless Steels. Mater. Sci. Eng. C 2020, 108, 110430. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Ustaoğlu, Z.; Yılmazer, G.A.; Sahin, F.; Baç, N. Antimicrobial Properties of Zeolite-X and Zeolite-A Ion-Exchanged with Silver, Copper, and Zinc Against a Broad Range of Microorganisms. Appl. Biochem. Biotechnol. 2014, 172, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, X.; Tong, S.; Zhang, T.; Song, Y.; Wang, Y.; Hao, Z.; Huang, C.; Xia, D. Modification of Zeolite by Metal and Adsorption Desulfurization of Organic Sulfide in Natural Gas. J. Nat. Gas Sci. Eng. 2019, 69, 102941. [Google Scholar] [CrossRef]
- Mousavi, M.; Fini, E. Silanization Mechanism of Silica Nanoparticles in Bitumen Using 3-Aminopropyl Triethoxysilane (APTES) and 3-Glycidyloxypropyl Trimethoxysilane (GPTMS). ACS Sustain. Chem. Eng. 2020, 8, 3231–3240. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Zeolite Nanoparticle as a Superior Adsorbent with High Capacity: Synthesis, Surface Modification and Pollutant Adsorption Ability from Wastewater. Microchem. J. 2019, 145, 74–83. [Google Scholar] [CrossRef]
- Sandomierski, M.; Voelkel, A. Diazonium Modification of Inorganic and Organic Fillers for the Design of Robust Composites: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1–21. [Google Scholar] [CrossRef]
- Sandomierski, M.; Okulus, Z.; Voelkel, A. Active Diazonium-Modified Zeolite Fillers for Methacrylate-Based Composites. Compos. Interfaces 2019, 26, 643–657. [Google Scholar] [CrossRef]
- Sandomierski, M.; Strzemiecka, B.; Grams, J.; Chehimi, M.M.; Voelkel, A. Diazonium-Modified Zeolite Fillers. Effect of Diazonium Substituent Position on the Filler Surface Modification and the Mechanical Properties of Phenolic/Zeolite Composites. Int. J. Adhes. Adhes. 2018, 85, 157–164. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk Factors for Chronic Biofilm-Related Infection Associated with Implanted Medical Devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, J.; Han, Y.; Li, D.; Cui, K. Microstructure and Bioactivity of Ca, P and Sr Doped TiO2 Coating Formed on Porous Titanium by Micro-Arc Oxidation. Surf. Coat. Technol. 2010, 205, 1702–1713. [Google Scholar] [CrossRef]
- Guo, S.; Yu, D.; Xiao, X.; Liu, W.; Wu, Z.; Shi, L.; Zhao, Q.; Yang, D.; Lu, Y.; Wei, X.; et al. A Vessel Subtype Beneficial for Osteogenesis Enhanced by Strontium-Doped Sodium Titanate Nanorods by Modulating Macrophage Polarization. J. Mater. Chem. B 2020, 8, 6048–6058. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Drug Distribution Evaluation Using FT-IR Imaging on the Surface of a Titanium Alloy Coated with Zinc Titanate with Potential Application in the Release of Drugs for Osteoporosis. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 281, 121575. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Zeolitic Imidazolate Framework-8 (ZIF-8) Modified Titanium Alloy for Controlled Release of Drugs for Osteoporosis. Sci. Rep. 2022, 12, 9103. [Google Scholar] [CrossRef] [PubMed]
- Sandomierski, M.; Zielińska, M.; Buchwald, T.; Patalas, A.; Voelkel, A. Controlled Release of the Drug for Osteoporosis from the Surface of Titanium Implants Coated with Calcium Titanate. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, Y.; Li, X.; Guo, Z. Improving the Osteointegration of Ti6Al4V by Zeolite MFI Coating. Biochem. Biophys. Res. Commun. 2015, 460, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of Corrosion-Resistant Zeolite Coatings for Titanium Alloy Biomedical Implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Guo, S.; Zhang, J.; Song, Y.; Dong, X.; Wang, X.; Yu, J. Antibacterial and Anti-Adhesive Zeolite Coatings on Titanium Alloy Surface. Microporous Mesoporous Mater. 2011, 146, 216–222. [Google Scholar] [CrossRef]
- Guimarães, M.B.; Antes, T.H.; Dolacio, M.B.; Pereira, D.D.; Marquezan, M. Does Local Delivery of Bisphosphonates Influence the Osseointegration of Titanium Implants? A Systematic Review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1429–1436. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, T.; Li, D.; Li, Z.; Leng, Y.; Ji, X.; Liu, H.; Wu, D.; Ding, J. Bisphosphonate-Functionalized Scaffolds for Enhanced Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1901073. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Voelkel, A. A Long-Term Controlled Release of the Drug for Osteoporosis from the Surface of Titanium Implants Coated with Calcium Zeolite. Mater. Chem. Front. 2021, 5, 5718–5725. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Shan, H. Improving Biocompatibility of Titanium Alloy Scaffolds by Calcium Incorporated Silicalite-1 Coatings. Inorg. Chem. Commun. 2019, 102, 61–65. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Li, D.; Zhang, Z.-Y.; Liu, G.; Liang, H.; Qin, Y.; Yu, J.; Li, Y. Fabrication of Bioactive 3D Printed Porous Titanium Implants with Sr Ion-Incorporated Zeolite Coatings for Bone Ingrowth. J. Mater. Chem. B 2018, 6, 3254–3261. [Google Scholar] [CrossRef]
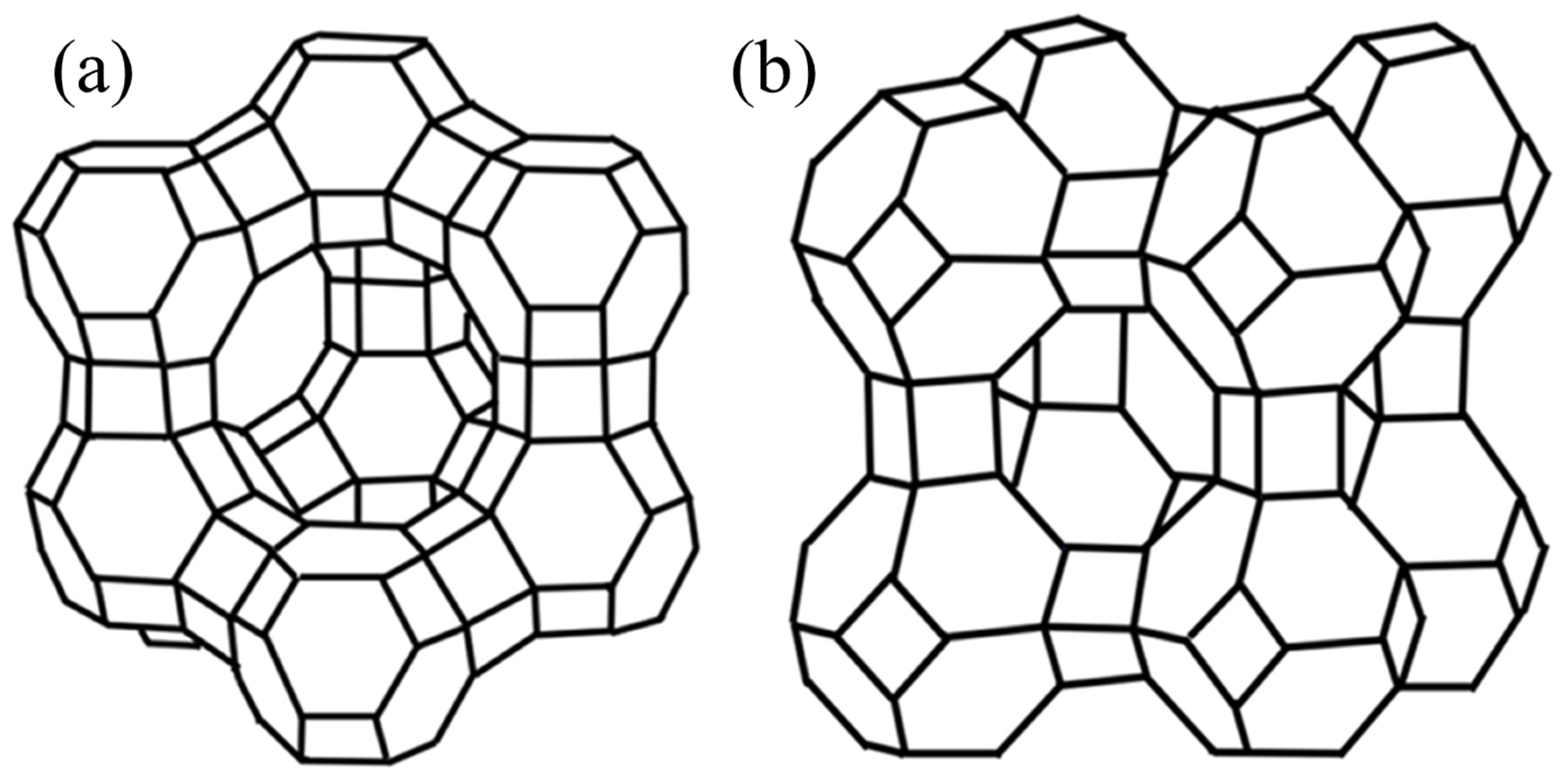

| Titanium Alloy Type | Zeolite Type | Ion in Zeolite Structure | Influence of Modification on Material Properties | Ref. |
|---|---|---|---|---|
| Ti6Al4V | MFI | - | improvement of the growth of hydroxyapatite, higher biocompatibility, promote cell migration, increase in the expression of bone-related genes | [54] |
| Ti6Al4V | MFI | - | lower corrosion potential, enhanced cell development, higher biocompatibility, improvement of cell adhesion | [55] |
| Ti6Al4V | A | Ag+ | antibacterial properties, low cytotoxicity, biocompatibility | [56] |
| Ti6Al4V | Sodalite | Ca2+ | controlled long drug release (risedronate release), | [59] |
| Ti6Al4V | Silicalite-1 | Ca2+ | releasing less toxic ions, very good adhesion properties to the cells, significant acceleration of cell proliferation | [60] |
| Ti6Al4V | A | Sr2+ | improvement of the growth of hydroxyapatite, excellent | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowski, M.; Voelkel, A.; Sandomierski, M. Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development. Crystals 2022, 12, 1520. https://doi.org/10.3390/cryst12111520
Jakubowski M, Voelkel A, Sandomierski M. Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development. Crystals. 2022; 12(11):1520. https://doi.org/10.3390/cryst12111520
Chicago/Turabian StyleJakubowski, Marcel, Adam Voelkel, and Mariusz Sandomierski. 2022. "Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development" Crystals 12, no. 11: 1520. https://doi.org/10.3390/cryst12111520
APA StyleJakubowski, M., Voelkel, A., & Sandomierski, M. (2022). Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development. Crystals, 12(11), 1520. https://doi.org/10.3390/cryst12111520







