Abstract
The electrochemical energy storage that based on earth-abundant materials is essential because of the future demands. Because of carbon-based architecture supercapacitors, rapid charging/discharging, and long life cycle, they considered attractive compared to chemical to batteries. Therefore, copper oxide (CuO) as positive electrode and reduced grapheme oxide (rGO) as negative electrode materials were used for a high-performance supercapacitor in a low cost, simple, and ecofriendly method. During the present work, synthesized reduced graphene oxide/copper oxide (rGO/CuO) nanocomposite using a simple chemical method is carried and investigated. The crystallinity index (Ic) of CuO, 1.0 M rGO/CuO and rGO was 90.61%, 88.42%, and 86.25%, respectively, at 500 °C and one h, while it was 76.30%, 73.51%, and 67.77respectively, at 500 °C and 30 h. As the test temperature increases, Ic% of both rGO and 1.0 M rGO/CuO increases, and that of CuO decreases. As the test period increases, Ic% for rGO, CuO, and 1.0 M rGO/CuO decreases. As the molarity concentration increased, the crystallinity index of rGO/CuO composites increased. The specimens characteristics are carried and investigated using; EDX, SEM, GC/MS, and XRD analysis. The appearance of the peaks at 2θ = 22.20° and 43.58° were related to GO, and peaks at 22°, 20°, 43.58°, 50.70°, and 74.37° indicated the synthesis of the nanocomposite.
1. Introduction
The need to reduce fossil fuel consumption and environmental pollution caused the supercapacitors development, [1]. Supercapacitors are energy storage devices that have high power density, excellent stability, and environmentally friendly characteristics. They are therefore commonly used for many applications, such as portable electronic devices, hybrid vehicles, and renewable energy systems [2,3,4,5,6,7,8,9]. To solve the solar energy systems intermittency in time and space problems, Latent heat thermal energy storage is required.
Using metal fins, both thermal conductivity of phase change materials (PCMs) and energy storage efficiency increases and improved. The metal fins positive on heat transfer has not been fully utilized. A novel design of angled fins used to improve PCMs thermal transport in a shell-and-tube thermal storage unit proposed by Guo et al. [10]. Also, non-uniform angled fin type with small angles coupled with designed and built visual experimental platform with the reliability of numerical model based on experimental results verified by Guo et al. [11]. The effect of adding longitudinal fins in a horizontal shell-and-tube heat storage unit on heat transfer is discussed by Yang et al. [12]. Both the optimal number of fins and the longitudinal fins effect on improving melting speed which is significant for storage energy application quantified. Nagarajarao et al. [13] and Majumdar et al. [14] showed that the electrochemical characteristics of supercapacitors are strongly dependent on electrode materials. Bhavyasree and Xavier [15] focused on copper and copper oxide (Cu/CuO)- based nanomaterial current state green synthesis utilizing plant extracts as reducing and capping agents. Jay et al. [16] used Hibiscus sabdariffa L. aqueous extract which is non-toxic, environmentally friendly, and cost-effective for chemically reduction of graphene oxide (GO). Perumal et al. [17] used extracts from different plant parts that act as reductants in rGO green synthesis and have potential applications in industries. El-Kady et al. [18] deduced that grapheme is the best material for making superconductors due to its exceptionally high surface area and fast electrical connection. Supercapacitors can subsequently store as much energy as conventional batteries and can be charged up to 1000 times faster.
The effectiveness of synthesized reduced graphene oxide (rGO) in dyes depends on the electrostatic interaction between them [19,20,21,22,23,24,25]. Chen et al. [2] used piranha acid treatment at different time intervals with graphene oxide-silica particle nanocomposites. Graphene oxide (GO) and silicon oxide template methods were also used to produce excellent supercapacitor performance [26,27,28,29,30,31,32,33]. Kotsyubynsky et al. [34] synthesized colloidal GO using the modified Tour method. Rosli et al. [35] used microwave-assisted reaction of sodium cholate at temperatures between 120 and 180 °C for 1 h for producing rGO. Wu et al. [36] synthesized Co@CNR/rGO with a one-pot hydrothermal process. Makal et al. [37] fabricated rGO composites using a high-pressure and high-temperature hydrothermal method. Kuzmany et al. [28] synthesized reduced graphene oxide with thermal treatment. Fulari et al. [38] used the modified Hummers method for synthesizing graphene oxide. Patil et al. [39] used the liquid–air interface for growing highly uniform graphene nanosheet thin films. Zhang et al. [40] used a homogeneous precipitation method for preparing Ni(OH)2/graphite nanosheets. Tamilselvi et al. [41] synthesized series of composites with NiO, Co3O4, and NiCo2O4 and rGO useing single-step hydrothermal approach with subsequent heat treatment.
Chandel et al. [9] enhanced the super-capacitive performance of the (AgxNi1 − x)y rGO (100 − y) nanocomposite using a combination of Ni, Ag, and rGO nanoparticles. Makal et al. [42] manufactured a promising electrode material for high-performance supercapacitors using (CoNiD)60r-GO40 composites. Xu et al. [43] manufactured a high-capacity electrode based on PPy-coated GC-SC products. For symmetric supercapacitor applications, Sahoo et al. [44] fabricated nano-gold-decorated rGO wrapped polymethyl methacrylate nanohybrids (PMMA/r-GO/Au). Vivas and Singh [45] used green synthesis in the design of an rGO-Au supercapacitor active electrode. Chandel et al. [46] utilized reduced graphene oxide (rGO)in their study. Umeshbabu et al. [47] fabricated nanostructured NiCo2O4/graphene composites for supercapacitor applications. Bo et al. [48] produced high performance supercapacitors using rGO paper with unimpeded liquid permeation. Lui et al. [49] fabricated dye sensitized solar cell (DSSC) photoanodes using synthesized rGO@TiO2 nanotube hybrids. Makkarand Ghosh [50] introduced an inorganic supercapacitor with high-performance electrochemical energy storage from earth-abundant materials.
Chen et al. [51] used simple two-step method for synthesizing a highly efficient CoSx–reduced graphene oxide (rGO)/TiO2 composite photocatalyst. Deng et al. [52] used one-step hydrothermal method for synthesizing S and N codoped graphene supported cobalt–nickel sulfide composite catalyst (rGO@SN-CoNi2S4). They concluded that rGO@ SNCoNi2S4 has high great potential for excellent cathode and anode electrolyzer usage during the water splitting process. Bruno and Shangc [53], Diab [54], and Barnes and Levine [55] found that with the increase in temperature, the capacitance increases, and the thermal capacitor resistance decreases. For supercapacitors, the temperature change does not affect their ability to store energy, and their resistance increases with the decrease in temperature, causing a decrease in the unloaded value of the energy, which means that the energy yield decreases relative to temperature. Therefore, low temperatures are used to identify the lowest energy yield and supercapacitor efficiency.
She et al. [56] demonstrated mixed metal oxide nanomaterials for energy storage electrode applications because of their synergistic enhancement effects. Due to their unique advantages as catalysts, Copper oxides (CuO and Cu2O)facile fabrication and noticeable electrochemical response make them efficient electrochemical energy storage systems [57,58]. Keihan et al. [59] concluded that special features such as non-toxicity, abundance, low cost, and ease of fabrication in the form of nano-dimensions causes cupric oxide (CuO) is a well-known metal oxide [60]. Because of its low toxicity and low cost, copper oxide p-type semiconductor material has been used in catalyst, solar energy storage, and lithium-ion battery anode materials. They found that CuO electrode has stable charge and discharge platforms, and a discharge-specific capacity up to 19 mA h g−1 at 0.3 A g−1. While Senthilkumar et al. [61] found that CuO electrode has high energy and power density of 29.4 Wh/kg and 12.7 W/kg, respectively and Lu et al. [62] observed that etched rGO electrode has 47 W hkg−1 and 100 kW kg−1 maximum energy and high power density respectively.
Raghavan and Thangavel [63] hypothesized that simple sonication in incorporating elemental sulfur (S) into graphene-oxide (GO) is suitable for photocatalytic and waste-water treatment/remedial applications. Sampaio and Viana [64] green synthesized of Cu2O/Cu nanoparticles using artichoke extract with ascorbic acid (AA) in a one-bath process. RGO-CuO nanocomposite electrochemical impedance spectroscopy is important for future energy storage devices [65,66,67,68,69,70]. Majumdar et al. [65] emphasized the facility of the one-pot chemical technique to fabricate rGO-CuO(II) (rGO-CuO) nanocomposite with superior electrochemical signature. For supercapacitors and H2O2 sensors, Lohar et al. [71] indicated that nanopellet structured CuO/rGO composite could be a promising electrode material. Vijayakumar et al. [72] evaluated a CuCo2O4battery type electrode for hybrid supercapacitor applications. Using a copper anchored boron doped grapheme nanosheet (CuBG) as a negative electrode and reduced grapheme nanoplate as a positive electrode with H2SO4/PVA as the quasi-solid electrolyte, Pandian and Pandurangan [73] designed and fabricated a novel asymmetric solid-state supercapacitor (ASSC). Ansari et al. [70] and El-Abeid et al. [74] coated silver (Ag) decorated reduced graphene oxide (rGO) with ultrafine CuO nanosheets for combination of a microwave-assisted hydrothermal route and a chemical methodology. Because of copper oxide (CuO)good reactivity and stability, Li et al. [75] used in semiconductor gas sensors. They also prepared graphene-CuO and metal oxide-CuO composites using a microwave-assisted hydrothermal synthesis (MWHS)process. However, Purushothaman et al. [76] showed that the electrochemical performance of CuO is hampered due to the low electrical conductivity and structural destruction during ion intercalation/deintercalation.
Environmental warming and pollution create challenges requiring lithium batteries, green chemistry, advanced fuel cells, electrocatalytic water splitting devices, and supercapacitor [77,78,79].
Shwetha et al. [80] sensitized copper oxide nanoparticles (CuO NPs) using Areca catechu leaf extract as a bio-reducing and capping. Taera et al. [7] produced activated carbon nanosheets from the Syzygiumoleana leaf biomass using a one-stage integrated pyrolysis method for manufacturing a supercapacitor electrode for energy storage applications. Qian et al. [81] concluded that the high loading of the pseudo-capacitive components may cause the blockage of the pores and the destruction of the surface functional groups.
Taera et al. [7] found that the XRD pattern for Syzygiumoleana leaf ACS monoliths based on different carbonization temperatures of 500, 600, and 700 °C has two clear characteristic peaks around 24–26° related to the (002) diffraction plane and 43–46° related to the (100) plane that represents hexagonal graphite small amounts in agreement with [82]. The same results obtained from previous studies with durian shell and mangosteen [83]. Two broad strong peaks at 22–24° and 42–44° indicatedthatdue to the existence of both micropores, random combination of graphitic and chaotic stacking, the interlayer spacing of ACS700 was greater than that of the ACS500 and ACS600 (the number on the label indicates carbonization temperature) that leads to higher specific surface and the electronic conductivity increment [84,85]. The diffraction peaks at 35.5° and 38.7° are related to the (−111) and (111) planes of CuO (JCPDS No. 80-1917) [86]. Fulari et al. [38] concluded that rGO has a peak at 2θ= 11.40° related to the graphite and successfully synthesized graphene oxide. the peak at 2θ = 11.40° related to the orientation along the (002) plane [87,88]. Also, at about 25°, there is a small peak related to the start of graphene oxide reduction [89,90].
Ansari et al. [70] found that pure CuO nanosheet and rGO@CuO nanocomposite patterns have high-intensity peaks located at the same diffraction angles of 32.67°, 35.44°, 38.66°, 48.81°, 53.63°, 58.22°, 61.35°, 66.17°, 67.80°, and 75.05°corresponded to the planes (110), (002), (111), (20-2), (020), (202), (11-3) (20-2), (020), (202), (11-3), (022) and (113). El-Abeid [74] illustrated that the of rGO-CuO NPs diffraction peaks at 16.20°, 31.21°, 39.63°, 50.44°, and 52.49° related to planes (101), (113), (024), (033) and (220), respectively. Ju et al. [90] calculated the crystallinity index (Ic) using the XRD data at 2θ = 18° as follows [91,92]:
where I002 is related to the maximum intensity and Iam to the minimum intensity of diffraction in the same units at θ = 18°.
Ic = 100 × [(I002 − Iam)/I002]
The aim of the present work was to fabricate rGO/CuO nanocomposites with six reaction temperatures of 20, 300, 350, 400, 450, and 500 °C; six test periods of 1, 6, 12, 18, 24, and 30 h; and four molar values of 0.125, 0.25, 0.5, and 1.0 M rGO. The Ic values were calculated at different test variables. The effect of the test variables on the various rGO/CuO nanocomposite values at various rGO molarity concentration was assessed. XRD, SEM, GC/MS, and EDX devices were used.
2. Experiment
2.1. Materials
Both graphite and copper sulphate hexahydrate (Cu(SO4)2·6H2O) analytical reagent (AR) were purchased from Sigma-Aldrich (St. Louis, MO, USA) with 99% purity. Grass plant wastes were collected from Taif Stadiums, Saudi Arabia. H2SO4; 99%), NaNO3, KMnO4, H2O2; 30%, HCL; 12%, and NaOH were used.
2.2. Synthesis of Graphene Oxide Nanoparticles
With continuous agitation, 3 g of graphite powder reacted with 69 mL of H2SO4, 1.8 g NaNO3, and 9 g KmnO4 in a water bath at 36 °C for 60 min. A total of 200 mL of double-distilled water was gradually added to the reacted mixture for 50 min at 100 °C to increase the product oxidation degree. The resulting suspension volume mixture with 420 mL was mixed with double-distilled water and 9 mL of H2O2 until the suspension color turned from brown to bright yellow. The oxidized graphite was isolated from the suspension by vacuum filtration. To remove H2SO4 and salt impurities, a mixture of HCl was added to the triple volume of distilled water using vacuum filtration followed by vacuum drying of the wet product under 55 °C in agreement with [93,94,95,96,97].
2.3. Grass Waste Extraction
Grass plant wastes collected from Taif Stadiums Saudi Arabia were washed with distilled water to release impurities, dried, ground, and stirred mechanically at 5000 rpm/min for 45 min into powder. 25 g of the powder dispersed into 200 mL of distilled water and boiled at 100 °C until the color of the aqueous solution turned dark brown and pH was 8. The extract cooled at room temperature, filtered using Whatman no. 1 filter paper (Maidstone, UK) and stored in the glass bottle and kept in the refrigerator to avoid fungal growth until it was used as reducing agent for the synthesis of CuO NPs. The GC/MS System of a TRACE 1312 Gas Chromatograph, Thermo Fisher Scientific S.p.A. Milan, Italy, attached to a mass spectrometer was used to analyze the grass compounds.
2.4. Preparation of CuO Nanoparticles
A total of 10 mL of the grass waste extract was added drop by drop to copper sulphate hexahydrate (5 g) until the solution pH value was adjusted to 7. The mixture dissolved in 100 mL distilled water, sonicated for 40 min at room temperature, heated to 100 °C for 1 h, and finally the deposited light green nanoparticles were centrifuged (4000 rpm, 15 min), washed with the distilled water of 20 mL three times, and washed with 20 mL ethanol three times. Finally, the obtained CuO nanoparticles were dried in an oven for 6 h at 60 °C in according to [59].
2.5. Synthesis of rGO-CuO Nanoparticles
Four different rGO molarity concentrations of 0.125, 0.250, 0.500, and 1.0 M used for preparating of CuO/reduced graphene oxide composites. Graphite oxide exfoliated in a mixed solvent of distilled water (6 mL) and DMF (54 mL) using ultrasonic treatment to form a stable graphene oxide (GO) nanosheet suspension. 5.0 g CuSO4·6H2O dissolved in 10 mL extracted grass plant with flow of N2 in a round-bottomed flask. The above graphene oxide (GO)nanosheet suspension quickly mixed with the solution containing cuprous chloride and ammonia solution. Under reflux at 90 °C for 2 h with vigorous stirring, the reduction reaction carried out in one step. The (CuO/rGO) solid product separated by centrifugation (4000 rpm) and washed using deionized water and anhydrous ethanol several times to remove other ions, with pH = 7. The final product collected and dried in a vacuum at 60 °C for 6 h. The CuO solution sample concentration were 97% (for 0.125 M rGO), 94% (for 0.5 M rGO), 91% (for 0.75 M rGO), and 88% (for 1.0 M rGO).
2.6. Electrochemical Measurements
The corresponding EDS spectra, JOEL, Santa Clara, CA, USA was used to illustrate the primary elements exhibited in the appropriate rGO-CuO composition. Furthermore, the three elements; carbon, copper, and oxygen formation and presence investigated, confirming the occurrence of rGO in the rGO-CuO sample. EIS measurements at the range from 0.1 Hz to 100 kHz frequency with ±2 V voltage used.
2.7. Characterization Techniques
The synthesized materials were analyzed using the X-ray diffraction (XRD) PANalytical (Malvern Instruments, Malvern, UK) X’Pert-PRO MPD high resolution (PW 3064/60) device with 2° range from 5 to 90° of Cu Ka radiation, 1.5406 A, 30 mA and 40 kV, used to investigate structural conformation. Scanning electron microscopy (JEOL, JSM, ARM-200F, HRTEM, Tokyo, Japan) was used for analyzing the sample morphology at a voltage of 15 kV. Energy dispersive spectroscopy (EDS, JEOLJSM-6510 LA, CA, USA) of r 0 to 20 KeV range and 15 kV used to record the element contents.
3. Results and Discussion
3.1. Analysis of Grass Plant Wastes
Many bioactive compounds and various pharmacologically compounds that contain common compounds belonged to hydrocarbon classes such as; carbonochloridic acid, 1,6-hexanediyl ester, o-methyl o-butyl isopropylphosphnate,benzoicac-id3-(3-hydroxy-3-methyl-1-butynyl)-,methanol,3-(hydroxy methyl) bicycle [2.2.1]hept-2-yl], butanimidamide, 1-butanamine, N-methyl-N-nitro-, hexahydrocyclopenta[1,3]cyclopropa-pa[1,2]benzen-3-one,ethanone, and 1-(2-pyridinyl)- obtained from the GC-MS analysis of grass plant wastes using water extract as listed in Figure 1.
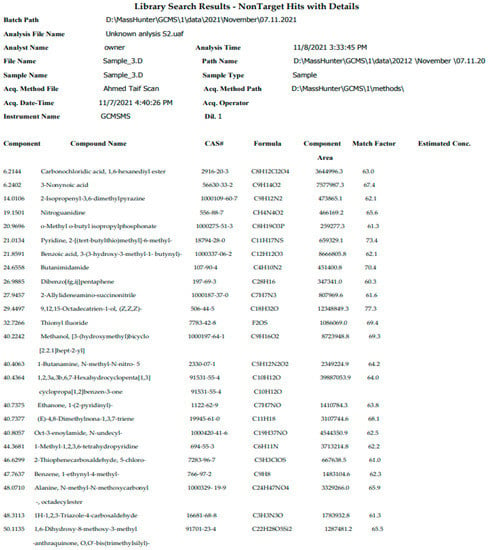
Figure 1.
GC-MS analysis and chemical composition of grass plant wastes.
These compounds contained hydroxyl groups and/or double bonds that belonged to the free radical scavenging activity of the grass extract in agreement with [98,99,100]. Richard in [101] showed that there is unsaturated fatty acids act as antioxidants based on their instauration. Abed et al. [102] revealed the grass protective effect against genotoxicity and oxidative stress. Several antioxidant vitamins, Eantioxidant-containing chlorophyll, antioxidant enzymes such as SOD, cytochrome oxidase, and other enzymes are found in the grass plant wastes. Banerjee et al. [103] considered grass plant wastes as plentiful source of numerous flavonoids; apigenin, antioxidant and anticarcinogenic potential.
3.2. Temperature Effect on the Crystallinity Index (Ic)
Figure 2 shows the effect of temperature variations of 20, 300, 350, 400, 450and 500 °C on rGO, CuO, and 1.0 M rGO/CuO. It is clear that as the test temperature increases, the trends of both rGO and rGO/CuO increase gradually, while the CuO trend decreases with the increase in temperature as listed in Table 1 in agreement with Pisarkiewicz et al. [104], who concluded that with increasing test temperature, both CuO and rGO thickness layer trends decrease due to the decrease in the change of response and that the CuO trend is higher than the rGO trend at various temperature levels. They also proved that the rGO trend is lower than rGO/CuO at the same temperature due to its lower sensitivity response.
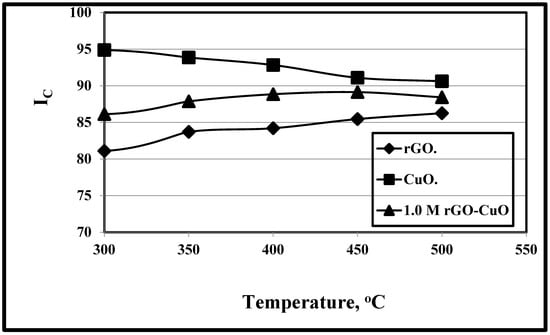
Figure 2.
Variation of Ic/temperature at rGO, CuO, and 1.0 M rGO/CuO and 1 h.

Table 1.
Effect of temperature and test period on crystallinity index (Ic) of rGO, CuO and 1.0 M rGO/CuO.
Isah et al. [105] found that the CuO trends decrease with the increase in temperature due to the thin Cu film. Azmi et al. [106] found that the optimum conditions of the developed rGO-modified screen-printed carbon electrode (mSPCE)layer was achieved by incubating the rGO on the electrode surface for 24 h at 25 °C, due to its layer stability decrease. Folorunso et al. [27] related the decrease in rGO trend with the increase in temperature to the reduced graphene oxide decomposition, its good thermal stability and higher mixed homogeneity. CuO was subjected to three decomposition steps, and its trend position was higher than the rGO/CuO trend due to its higher stability compared to rGO/CuO. Li et al. [107] concluded that all the trends of rGO/CuO have the same decreasing trend at different temperature values because of the carbon skeleton decomposition from rGO during the oxidation process.
3.3. The Effect of the Test Peroid on the Crystallinity Index
Figure 3 illustrates the variation of the Ic/test period at rGO, CuO, and 1 M rGO/CuO and 500 °C. It is clear that with the increase in test duration, as shown by Kumari et al. [108], the adsorption of CuO, rGO/CuO and rGO increases, respectively, which results in the decrease in crystallinity index. Li et al. [109] and Gijare et al. [110] related the decrease in rGO, CuO, and rGO/CuO with the increase in test duration to the residual degradation rate of rGO/CuO which is lower than that of CuO and rGO, in agreement with our work.
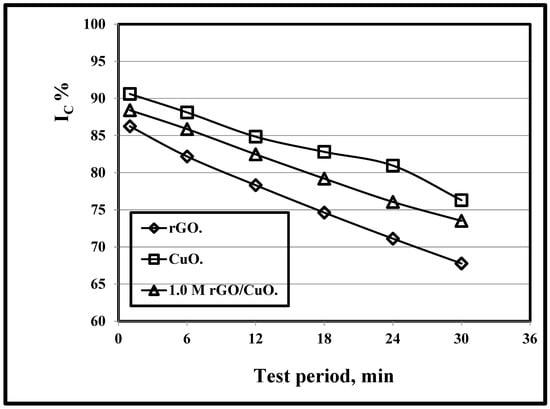
Figure 3.
Variation of Ic/test period at rGO, CuO, and 1.0 M rGO/CuO and 500 °C.
3.4. Effect of rGO Molar Concentrations on the Crystallinity Index
Figure 4 shows the effect of rGO molarity concentrations on both the crystallinity index and rGO/CuO composites for various test periods and 500 °C. It is clear that as the rGO molarity concentrations increase, the crystallinity index increases as listed in Table 2 in agreement with Pisarkiewicz et al. [104] due to the enhancement of the catalytic property that is attributed to the CuO and the of reduced graphene oxide molarity concentrations interaction. CuO can provide more active sites for CO oxidation.
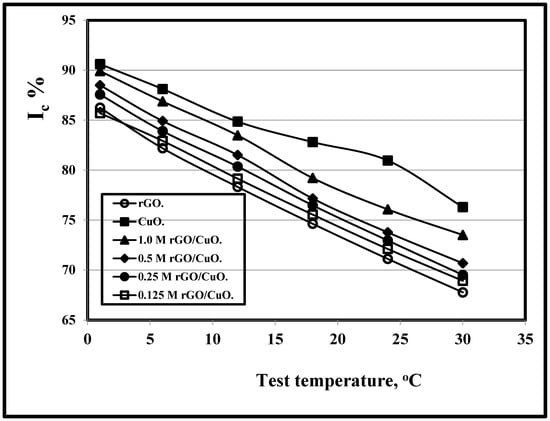
Figure 4.
Variation of Ic/test period at 500 °C and different rGO molarity concentrations in rGO/CuO.

Table 2.
Effect of rGO molarity concentration on crystallinity index (Ic) of rGO, CuO, and rGO/CuO at different test periods and 500 °C.
Xu et al. [111] related the crystallinity index increment to the Rgo good consistency that preventing CuO nanoparticles agglomeration and resulting in smaller CuO particles with an apparent specific surface area increment. Dutta et al. [112] related this to the charge transfer from the rGO lowest unoccupied molecular orbital to the conduction band of CuO.
3.5. Structural Analysis
Figure 5 shows the synthesized rGO-CuO XRD analysis at different rGO molar concentrations. The four diffraction patterns have slight changes due to the rGO molar concentration variations. Five diffraction peaks observed at; 22°, 20°, 43.58°, 50.70°, and 74.37° were related to planes (111), (002), (202), (220), and (102) of pure CuO. The other two diffraction peaks of 22.20° and 43.58° were related to planes (002) and (100) of reduced graphene oxide. It is clear that as the rGO molar concentration increases, the intensity peaks increase in agreement with [113,114,115,116]. However, Wang et al. [117] found that rGO-CuO has a diffraction peak at 25.1° related to plane (002) of CuO with no peaks of rGO; they related thisto the CuO’s high degree of crystallinity and the lower rGO content used that makes the strong CuO peaks cover rGO peaks. Sagadevan et al. [118] concluded that rGO has peaks at 10.9°, 42.6°, 35.6°, and 38.8° of planes (002) due inclusion of their hexagonal structure, and the other two peaks corresponded to the (111) planes due to the rGO impregnating copper precursors. In addition, rGO-CuO has diffraction peaks closely aligned with the CuO monoclinic phase corresponding to planes (110), (111), (111), (202), (020), (202), and (311). Sudhakar et al. [119] illustrated that rGO has a peak at 21° related to plane(111) and CuO peaks observed at; 30.1°, 37.4°, 44.3°, 62.5°, and 74.2° related to (110), (111), (200), (220), and (311) planes, respectively, which confirmed the presence of the CuO phase. The rGO–CuO composite has all the diffraction peaks related to CuO with a small peak 2θ = 26.4° related to rGO due to the small stacking of rGO sheets.
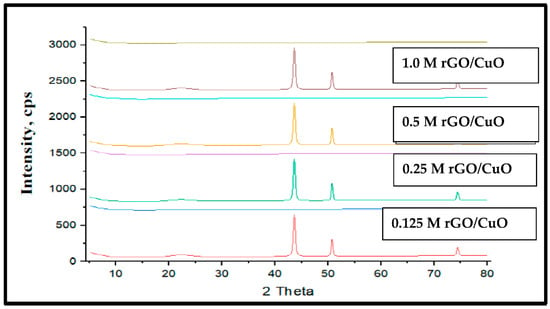
Figure 5.
XRD patterns of pure CuO and rGO/CuO composites at different rGO molarity concentrations.
Sagadevan et al. [116] depicts that GO has XRD diffraction peaks at; 42.6° and 10.9° because of the inclusion of various oxygen functional groups (hydroxyl, epoxy, carbonyl groups, etc.) on the graphene layerseither sides. The peak at 42.6° corresponds to the (100) plane of the hexagonal structure. The additional diffraction peaks at 2θ = 35.6° and 38.8° related to copper precursor that impregnates GO with the (111) and (111) planes of the monoclinic structured CuO.
CuO/rGO has diffraction peaks closely aligned with the monoclinic phase of CuO corresponding to the planes (110), (111), (111), (202), (020), (202), and (311) (JCPDS 48-1548). Siburian et al. [113] produced an rGO peak structure at 21°, while CuO peaks at 37.4°, along with 30.1°, 44.3°, 62.5°, and 74.2° corresponded to (111), (110), (200), (220), and (311) planes respectively confirming the presence of CuO phase. RGO–CuO nanocomposite diffraction peaks were perfectly related to the monoclinic CuO, except the small peak at 2θ = 26.4° which related to small stacking of rGO sheets in agreement with Sagadevan et al. [114] conclusion. They found that rGO/CuO peaks of planes (110), (−111), (111), (−202), (020), (202), (−113), (−311), and (220) were consistent with the CuO monoclinic phase extra peaks around (002) and (100) that attributed to the rGO. The GO peak at (001) disappeared from the composite material. Figure 6 illustrates the rGO-CuO composite XRD pattern at 1.0% rGO molar concentration in intensity counts. The figure has the same peaks.
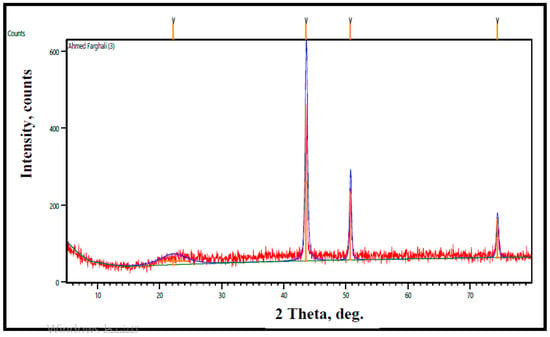
Figure 6.
XRD patterns of pure CuO and rGO/CuO composites at room temperature.
3.6. Morphological Analyses
Room temperature, 300 and 500 °C rGO SEM micrographs are shown in Figure 7a–c, respectively. Figure 7a illustrates the existence of fewer O stacks with coupled graphite structure exfoliation in agreement with [116]. With the increase in temperature to 300 °C (Figure 7b), the graphene layers become creased, damaged, twisted, and crumbled as detailed in [113] and [114] with the decomposition of GO that is followed by the abrupt eruption of gaseous products (CO, CO2). As a consequence, a partial separation of graphene layers followed by significant GO weight loss and volume expansion is observed in agreement with [115]. With the increase in temperature to 500 °C (Figure 7c), O volume increases, and GO is decomposed followed by abrupt eruption of gaseous products (CO, CO2) with less crumpled, relatively large volume, layered, and stacked configurations [110,111,112,113,114,115,116,117,118,119,120,121]. Figure 7c indicates expanded gaps between the graphene layers that generated unfolded rGO in agreement with [122,123] due to the existence of oxygen-containing groups that makes the GO structure display typical rippled and crumpled surfaces indicative of graphene.

Figure 7.
RGOSEM image sat (a) room temperature, (b) 300 °C, and (c) 500 °C.
Figure 8 illustrate CuO SEM images at different temperatures. As function temperature change, the morphology changes significantly in agreement with [124,125,126,127]. At room temperature, the morphology of Cu and O is fine, round shaped, uniform, dense, and very smooth, and the aggregates are almost spherical. Tiny nanoparticles tightly stuck together can also be seen in agreement with [106]. With the increase in temperature (Figure 8b), Cu particles agglomerated and well-dispersed, with regularly spherical shapes, which means that temperature has a significant effect on structure morphology in agreement with Anwar et al. [128] with smaller vacancies. O nanoparticles have a fibrous structure, with a mixture of rods and needle-like rods coupled with random orientation like sheaf feather mixture with non-agglomerated morphologies in agreement with [129,130,131,132]. At 500 °C (Figure 8c), the SEM micrograph indicates well-dispersed and regularly spherical shapes of highly crystalline Cu nanoparticles. The O nanoparticles showed incremental needle-shaped expanded volumes due to increased temperature, in agreement with [125].

Figure 8.
CuO SEM images at (a) room temperature, (b) 300 °C, and (c) 500 °C.
SEM images of rGO/CuO composites are shown in Figure 9a–c at various temperatures. The images revealed clearly that rGO and CuO stick together, forming stacked bundles and crumpled flakes in agreement with [122,123,124,125,126,127,128]. Figure 9a shows that rGO found as wrinkled and scaled sheets porous fluffy network resemblance with spongy-like structure. The CuO nanoparticles randomly distributed on the rGO basal plane in agreement with [129,130]. With a temperature of 300 °C, the scanning electron images of CuO/rGO illustrate a cluster leaf type ofCuO/rGO on which CuO is randomly distributed over the rGO skeleton [131,132,133,134,135,136]. With the increase in temperature to 500 °C (Figure 9c), the cluster leaf type increased in agglomeration and concentration with CuO that was entrapped and embedded in the rGO, producing identical flake morphology and evenly distributed particles with well-defined morphology [137,138]. CuO monoclinic phase.
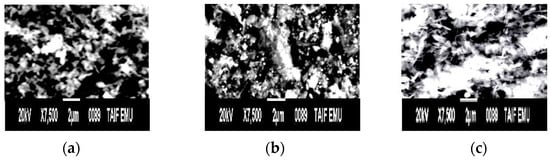
Figure 9.
1 M rGO/CuO SEM images at (a) room temperature, (b) 300 °C, and (c) 500 °C.
3.7. Energy Dispersive Analysis (EDS)
Figure 10a,b illustrate the energy dispersive (EDS) analysis of 0.125 M rGO/CuO and 1 M rGO/CuO, respectively. Cu, C, K, and O primary elements found in the rGO/CuO composites confirmed the rGO in the rGO/CuO composite. CuO particles are distributed within the rGO layers in agreement with the SEM morphological images. The amount of C is not observed because rGO-CuO and CuO monoclinic phase has closely aligned diffraction peaks and is coupled with some extra peaks of GO according to Sagadevan et al. [134] and Sudhakar et al. [119]. Sagadevan et al. [116] and Talande et al. [139] observed that the amount of C was not detected through their XRD analysis; they related this to the lower C distribution in the rGO/CuO composite and the GO partially quenching into graphene. Thus, both of EDAXand EDS analyses proved that the rGO-CuO nanocomposites were successfully synthesized.
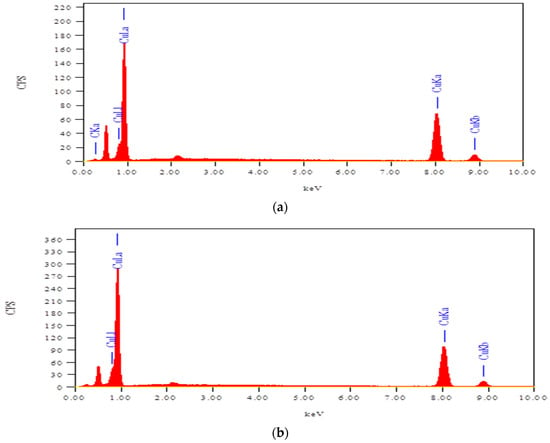
Figure 10.
EDAX analysis at (a) 0.125 M rGO/CuO and (b) 1.0 M rGO/CuO.
4. Conclusions
- The rGO/CuO nanocomposites were successfully prepared at various rGO molarity concentrations, test temperatures, and periods using plant wastes.
- SEM images of rGO/CuO composites at 500 °C showed that the cluster leaf type increased in agglomeration and concentration with entrapped and embedded CuO in the rGO producing identical flake morphology. The reaction temperature of 300and 500 °C produce nanosheet morphology emblazoned with nanofibers. SEM images are in good agreement with the energy dispersive analysis (EDS).
- As the rGO molarity increases, the XRD intensity and the crystallinity index of the rGO/CuO increases, illustrating that grafting rGO on CuO exhibits the highest catalytic activity for CuO and photocatalytic activity.
- As the test temperature increases, the crystallinity index of both rGO and 1.0 M rGO/CuO increase, and that of CuO decreases. However, with the increase in test duration, the crystallinity index of CuO, 1.0 M rGO/CuO, and rGO decreases.
Author Contributions
Formal analysis, A.K.A.; Funding acquisition, A.K.A.; Investigation, A.T.M.; Methodology, A.T.M., A.K.A., H.M.A.-D. and A.M.F.; Software, H.M.A.-D., A.M.F. and A.K.A.; Supervision, T.P.; Visualization, Z.A.A., T.P., J.T.A. and A.K.A.; Writing—original draft, H.M.A.-D. and A.K.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to extend their sincere thanks to the High Altitude Research Center, Taif University for its funding of this research through the Research Group of Project number: 1-442-47.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the study’s findings are included in the article.
Acknowledgments
All the authors would like to extend their sincere thanks to High Altitude Research Center, Taif University for its funding of this research through the Research Group; Project number: 1-442-47.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ansarinejad, H.; Nooshabadi, M.S.; Ghoreishi, S.M. Enhanced supercapacitor performance using a Co3O4@Co3S4 nanocomposite on reduced graphene oxide/Ni foam electrodes. Chem. Asian J. 2021, 16, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, P.S. Electrochemical Supercapacitors: From Mechanism Understanding to Multifunctional Applications. Adv. Energy Mater. 2020, 11, 2003311. [Google Scholar] [CrossRef]
- Ramlee, F.; Farhana, N.; Bashir, S.; Saidi, N.M.; Omar, F.S.; Ramesh, S.; Ramesh, K. Electrical property enhancement of poly (vinyl alcohol-co-ethylene)–based gel polymer electrolyte incorporated with triglyme for electric double-layer capacitors. Univ. Malaya Res. Repos. 2020, 27, 361–373. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, Y.; Liu, X.; Lic, X.; Gao, S. A treasure map fornonmetallic catalysts: Optimal nitrogen and fluorine distribution of biomass-derived carbon materials for high-performance oxygen reduction catalysts. J. Mater. Chem. A 2021, 9, 18251–18259. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.; Liu, T.; Chen, H.; Chen, S.; Jiang, Z.-J.; Liu, J.; Huang, J.; Liu, M. Wood-Derived Hierarchically Porous Electrodes for High-Performance All-Solid-State Supercapacitors. Adv. Funct. Mater. 2018, 28, 1806207. [Google Scholar] [CrossRef]
- Adán-Más, A.; Silva, M.T.; Demourgues, L.G.; Bourgeois, L.; Sarroste, C.L.; Montemor, M.F. Nickel-cobalt oxide modified with re-duced grapheme oxide: Performance and degradation for energy storage Applications. J. Power Source 2019, 419, 12–26. [Google Scholar] [CrossRef]
- Taera, E.; Apriwandia, A.; Taslimb, R.; Agutinoa, A.; Yusraa, D.A. Conversion syzygiumoleana leaves biomass waste to porous activated carbon nanosheet for boosting supercapacitor performances. J. Mater. Res. Technol. 2020, 9, 13332–13340. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, X.; Miao, J.; You, M.; Zhu, Y.; Pan, J.; Wang, L.; Cheng, X. Super-conductive silver nanoparticles functioned three-dimensional CuxO foams as a high-pseudocapacitive electrode for flexible asymmetric supercapacitors. J. Mater. 2020, 7, 156–165. [Google Scholar] [CrossRef]
- Chandel, M.; Makkar, P.; Ghosh, N.N. Ag–Ni nanoparticle anchored reduced graphene oxide nanocomposite as advanced electrode material for supercapacitor application. ACS Appl. Electron. Mater. 2019, 1, 1215–1224. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Yang, B.; Yang, X.; Yan, J. Melting assessment on the angled fin design for a novel latent heat thermal energy storage tube. Renew. Energy 2022, 183, 406–422. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Yang, B.; Yang, X.; Li, M.J. Thermal assessment on solid-liquid energy storage tube packed with non-uniform angled fins. Sol. Energy Mater. Sol. Cells 2022, 236, 111526. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, Z.; Luo, X.; Yan, J. Effect of fin number on the melting phase change in a horizontal finned shell-and-tube thermal energy storage unit. Sol. Energy Mater. Sol. Cells 2021, 236, 111527. [Google Scholar] [CrossRef]
- Nagarajarao, S.H.; Nandagudi, A.; Viswanatha, R.; Basavaraja, B.M.; Santosh, M.S.; Praveen, B.M.; Pandith, A. Recent developments in supercapacitor electrodes: A mini review. ChemEngineering 2022, 6, 5. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent progress in ruthenium oxide-based composites for supercapacitorapplications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Bhavyasree, P.; Xavier, T. Green synthesised copper and copper oxide based nanomaterials using plant extracts and their application in antimicrobial activity: Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100249. [Google Scholar] [CrossRef]
- Jay, H.; Chi, C.; Nyan, Y.L.; Tai, H. Green reduction of graphene oxide by Hibiscus sabdariffa L. to fabricate flexible graphene electrode. Carbon 2014, 80, 725–733. [Google Scholar]
- Perumal, D.; Albert, E.L.; Abdullah, C.A.C. Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups. J. Compos. Sci. 2022, 6, 58. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Bo Long, B.; Zhou, X. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 2016, 768, 18–26. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Szmidt, M.; Stankiewicz, A.; Urbańska, K.; Jaworski, S.; Kutwin, M.; Wierzbicki, M.; Grodzik, M.; Burzyńska, B.; Góra, M.; Chwalibog, A.; et al. Graphene oxide down-regulates genes of the oxidative phosphorylation complexes in a glioblastoma. BMC Mol. Biol. 2019, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405, 127018–127027. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cheng, C.; Qin, Z.; Zhong, D.; Cheng, Z.; Zhang, Q. Improvement of thermoplastic polyurethane’s flame retardancy and thermal conductivity by leaf-shaped cobalt-zeolitic imidazolate framework–modified graphene and intumescent flame retardant. Polym. Adv. Technol. 2021, 32, 228–240. [Google Scholar] [CrossRef]
- Sala, A.; Zou, Z.; Carnevali, V.; Panighel, M.; Genuzio, F.; Menteş, T.O.; Locatelli, A.; Cepek, C.; Peressi, M.; Comelli, G.; et al. Quantum confinement in aligned zigzag “pseudo-ribbons” embedded in graphene on Ni (100). Adv. Funct. Mater. 2021, 32, 2105844. [Google Scholar] [CrossRef]
- Folorunso, O.; Sadiku, R.; Hamam, Y.; Ray, S.S. An investigation of copper oxide-loaded reduced graphene oxide nanocomposite for energy storage applications. Appl. Phys. A 2021, 128, 54. [Google Scholar] [CrossRef]
- Kuzmany, H.; Shi, L.; Martinati, M.; Cambré, S.; Wenseleers, W.; Kürti, J.; Koltai, J.; Kukucska, G.; Cao, K.; Kaiser, U.; et al. Well-defined subnanometer graphene ribbons synthesized inside carbon nanotubes. Carbon 2020, 171, 221–229. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Hassanzadeh, N.; Eslami-Farsani, R. A review study on the recent advances in developing the heteroatom-doped graphene and porous graphene as superior anode materials for Li-ion batteries. Ceram. Int. 2021, 47, 22269–22301. [Google Scholar] [CrossRef]
- Ding, D.D.; Maeyoshi, Y.; Kubota, M.; Wakasugi, J.; Takemoto, K.; Kanamura, K.; Abe, H. Li-ion conducting glass ceramic (LICGC)/reduced graphene oxide sandwich-like structure composite for high-performance lithium-ion batteries. J. Power Source 2021, 500, 229976–229986. [Google Scholar]
- Garakani, M.A.; Sebastiano Bellani, S.; Pellegrini, V.; Nuñez, R.O.; Del Rio Castilloa, A.E.; Abouali, S.; Najafia, L.; Garcíaa, B.M.; Ansaldoa, A.; Bondavalli, P.; et al. Scalable spray-coated graphene-based electrodes for high-power electrochemical double-layer capacitors operating over a wide range of temperature. Energy Storage Mater. 2021, 34, 1–11. [Google Scholar] [CrossRef]
- Shi, X.; Yin, Z.Z.; Xu, J.; Li, S.; Wang, C.; Wang, B.; Qin, Y.; Kong, Y. Preparation, characterization and the supercapacitive behaviors of electrochemically reduced graphene quantum dots/polypyrrole hybrids. Electrochim. Acta 2021, 385, 138435–138445. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, J.; Xu, C.; Gao, T.; Wang, X. Electric Double Layer Capacitors Based on Porous Three-Dimensional Graphene Materials for Energy Storage. J. Electron. Mater. 2021, 50, 3043–3063. [Google Scholar] [CrossRef]
- Kotsyubynsky, V.O.; Boychuk, V.M.; Budzulyak, I.M.; Rachiy, B.I.; Hodlevska, M.A.; Kachmar, A.I.; Hodlevsky, M.A. Graphene oxide synthesis using modified Tour method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035006. [Google Scholar] [CrossRef]
- Rosli, N.H.A.; Lau, K.S.; Winie, T.; Chin, S.; Chia, C.H. Microwave-assisted reduction of graphene oxide for an electrochemical supercapacitor: Structural and capacitance behavior. Mater. Chem. Phys. 2021, 262, 124274. [Google Scholar] [CrossRef]
- Wu, H.; He, D.; Wang, Y. Electrode materials of Cobalt@Nitrogen doped carbon nano rod/reduced graphene oxide on Nickel foam by electrophoretic deposition and 3D rGO aerogel for a high-performance asymmetrical supercapacitor. Electrochim. Acta 2020, 343, 136117. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Superior photocatalytic dye degradation undervisible light by reduced graphene oxide laminated TiO2-B nanowire composite. J. Environ. Chem. Eng. 2019, 7, 103358. [Google Scholar] [CrossRef]
- Fulari, A.V.; Reddy, M.V.R.; Jadhav, S.T.; Ghodake, G.S.; Kim, D.Y.; Lohar, G.M. TiO2/reduced graphene oxide composite based nano-petals for supercapacitor application: Effect of substrate. J. Mater. Sci. Mater. Electron. 2018, 29, 10814–10824. [Google Scholar] [CrossRef]
- Patil, S.; Patil, V.; Shivaram Sathaye, S.; Patil, K. Facile room temperature methods for growing ultra thin films of graphene nanosheets, nanoparticulate tin oxide and preliminary assessment of graphene–tin oxide stacked layered composite structure for supercapacitor application. RSC Adv. 2014, 4, 4094–4104. [Google Scholar] [CrossRef]
- Zhang, J.T.; Liu, S.; Pan, G.L.; Li, G.R.; Gao, X.P. A 3D hierarchical porous a-Ni(OH)2/graphite nanosheet composite as an elec-trode material for supercapacitors. J. Mater. Chem. A 2014, 2, 1524. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Padmanathan, N.; Rahulan, K.M.; Mohana Priya, P.; Sasikumar, R.; Mandhakini, M. Reduced graphene oxide (rGO): Supported NiO, Co3O4 and NiCO2O4 hybrid composite on carbon cloth (CC)—Bi-functional electrode/catalyst for energy storage and conversion devices. J. Mater. Sci. Mater. Electron. 2018, 29, 4869–4880. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Reduced Graphene Oxide-Laminated One-Dimensional TiO2–Bronze Nanowire Composite: An Efficient Photoanode Material for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 4362–4373. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, H.; Wang, K.; Wang, B.; Xu, Q. Effect of supercritical CO2 on fabrication of free-standing hierarchical graphene ox-ide/carbon nanofiber/polypyrrole film and its electrochemical property. Phys. Chem. Chem. Phys. 2014, 16, 7350. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.; Sarkar, N.; Sahu, D.; Swain, S.K. Nano gold decorated reduced graphene oxide wrapped polymethylmethacrylate for supercapacitor applications. RSC Adv. 2017, 7, 2137. [Google Scholar] [CrossRef]
- Vivas, L.; Singh, D.P. A Highly Efficient Graphene Gold Based Green Supercapacitor Coin Cell Device for Energy Storage. Front. Energy Res. 2022, 9, 794604. [Google Scholar] [CrossRef]
- Chandel, M.; Makkar, P.; Ghosh, B.K.; Moitra, D.; Ghosh, N.N. A facile synthesis methodology for preparation of Ag–Ni-reduced graphene oxide: A magnetically separable versatile nanocatalyst for multiple organic reactions and density functional study of its electronic structures. RSC Adv. 2018, 8, 37774–37788. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Rajeshkhanna, G.; Rao, G.R. Effect of solvents on the morphology of NiCO2O4/grapheme nanostructures for electrochemical pseudocapacitor application. J. Solid State Electrochem. 2016, 20, 1837–1844. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, W.; Tu, X.; Yang, Y.; Mao, S.; He, Y.; Chen, J.; Yan, J.; Cen, K. Instantaneous Reduction of Graphene Oxide Paper for Supercapacitor Electrodes with Unimpeded Liquid Permeation. J. Phys. Chem. C 2014, 118, 13493–13502. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, Y.; Song, Y.; Song, K.; Liu, C. Enhanced efficiency of dye-sensitized solar cells using rGO@TiO2 nanotube hybrids. Chem. Res. Chin. Univ. 2018, 34, 269–273. [Google Scholar] [CrossRef]
- Makkar, P.; Ghosh, N.N. Facile synthesis of MnFe2O4 hollow sphere-reduced graphene oxide nanocomposites as electrode materials for all-solid-state flexible high-performance asymmetric supercapacitors. ACS Appl. Energy Mater. 2020, 3, 2653–2664. [Google Scholar] [CrossRef]
- Chen, F.; Feng, H.-F.; Luo, W.; Wang, P.; Yu, H.-G.; Fan, J.-J. Simultaneous realization of direct photo deposition and high H2-production activity of amorphous cobalt sulfide nanodot-modified rGO/TiO2 photocatalyst. Rare Met. 2021, 40, 3125–3134. [Google Scholar] [CrossRef]
- Deng, B.-L.; Guo, L.-P.; Lu, Y.; Rong, H.-B.; Cheng, D.-C. Sulfur–nitrogen co-doped graphene supported cobalt–nickel sulfide rGO@SN-CoNi2S4 as highly efficient bifunctional catalysts for hydrogen/oxygen evolution reactions. Rare Met. 2021, 41, 911–920. [Google Scholar] [CrossRef]
- Bruno, G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar]
- Diab, Y. Using energy storage systems for improving therenewable energies performance. In Proceedings of the First Conference Franco-Syrian on the Renewable Energies, Damascus, Syria, 2010; pp. 1–10. [Google Scholar]
- Barnes, F.S.; Levine, J.G. (Eds.) Large Energy Storage Systems Hand Book; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–260. [Google Scholar]
- She, Y.; Tang, B.; Li, D.; Tang, X.; Qiu, J.; Shang, Z.; Hu, W. Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications. Coatings 2018, 8, 340. [Google Scholar] [CrossRef]
- Majumdar, D.; Ghosh, S. Recent advancements of copper oxide based nanomaterials for supercapacitor. J. Energy Storage 2021, 34, 101995. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maitic, S.; Liu, D. Efficiancy of shape-monitored reduced grapheme oxide–copper nanohybrids: Anti-bacterial attributes for food safety and dye degradation. New J. Chem. 2019, 43, 662. [Google Scholar] [CrossRef]
- Keihan, R.E.; Ledari, R.T.; Mehrabad, M.S.; Dalvand, S.; Sohrabi, H.; Maleki, A.; Khoshdel, S.M.M.; Shalan, A.E. Effective Combination of rGO and CuO Nanomaterials through Poly (pphenylenediamine) Texture: Utilizing It as an Excellent Supercapacitor. Energy Fuels 2021, 35, 10869–10877. [Google Scholar] [CrossRef]
- Meng, J.; Yang, Z.; Chen, L.; Qin, H.; Cui, F.; Jiang, Y.; Zeng, X. Energy storage performance of CuO as a cathode material for aqueous zinc ion battery. Mater. Energy Today 2020, 15, 100370. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Kim, Y.S.; Chandrasekaran, S.; Rajagopalan, B.; Kim, E.J., II; Chung, J.S. Comparative supercapacitance performance of CuO nanostructures for energy storage device applications. RSC Adv. 2015, 5, 20545–20553. [Google Scholar] [CrossRef]
- Lu, L.; Peng, L.; Zhan, C.; You, W.; Xiao, S. Enhanced electrochemical energy storage performance of reduced graphene oxide by incorporating oxygen-rich in-plane pores. J. Mater. Chem. A 2014, 2, 1802–1808. [Google Scholar] [CrossRef]
- Raghavan, N.; Thangavel, S. Investigation of photocatalytic performances of sulfur based reduced graphene oxide-TiO2 nanohybrids. Appl. Surf. Sci. 2018, 449, 712–718. [Google Scholar] [CrossRef]
- Sampaio, S.; Viana, J.C. Green synthesis of Cu2O/Cu nanoparticles and conversion to Cu microparticles in one-bath reaction method for improved electrical conductivity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 025009. [Google Scholar] [CrossRef]
- Majumdar, D.; Baugh, N.; Bhattacharya, S.K. Ultrasound assisted formation of reduced graphene oxide-copper (II) oxide nanocomposite for energy storage applications. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 512, 158–170. [Google Scholar] [CrossRef]
- Saini, H.; Srinivasan, N.; Šedajová, V.; Majumder, M.; Dubal, D.P.; Otyepka, M.; Zbořil, R.; Kurra, N.; Fischer, R.A.; Jayaramulu, K. Emerging MXene@Metal–Organic Framework Hybrids: Design Strategies toward Versatile Applications. ACS Nano 2021, 15, 18742–18776. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, B.; Gao, Z.; Lan, D.; Zhao, Z.; Wei, F.; Zhu, Q.; Lu, X.; Wu, G. Two-dimensional nanomaterials for high-efficiency electromagnetic wave absorption: An overview of recent advances and prospects. J. Alloys Compd. 2021, 893, 162343. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Kospa, D.A.; Ahmed, A.I.; Samra, S.E.; Ibrahim, A.A. High efficiency solar desalination and dye retention of plasmonic/reduced graphene oxide based copper oxide nanocomposites. RSC Adv. 2021, 11, 15184–15194. [Google Scholar] [CrossRef]
- Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications. Materials 2021, 14, 5032. [Google Scholar] [CrossRef]
- Lohar, G.M.; Pore, O.C.; Fulari, A.V. Electrochemical behavior of CuO/rGO nanopellets for flexible supercapacitor, non-enzymatic glucose, and H2O2 sensing application. Ceram. Int. 2021, 47, 16674–16687. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Ryu, K.S. CuCo2O4 flowers/Ni-foam architecture as a battery type positive electrode for high performance hybrid supercapacitor applications. Electrochim. Acta 2017, 238, 99–106. [Google Scholar] [CrossRef]
- Pandian, P.M.; Pandurangan, A. Copper nanoparticles anchored onto boron-doped graphene nanosheets. RSC Adv. 2019, 9, 3443. [Google Scholar] [CrossRef] [PubMed]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.-A.; Mohamed, M.A. Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide Nanoparticles: A Potent Antifungal Nanocomposite against Fusarium Root Rot and Wilt Diseases of Tomato and Pepper Plants. Nanomaterials 2022, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.-L.; Wu, K.-D.; Zhang, D.-Z.; Debliquy, M.; Zhang, C. Microwave-assisted hydrothermal synthesis of copper oxide-based gas-sensitive nanostructures. Rare Met. 2021, 40, 1477–1493. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Saravanakumar, B.; Babu, I.M.; Sethuraman, B.; Muralidharan, G. Nanostructured CuO/reduced graphene oxide composite for hybrid supercapacitors. RSC Adv. 2014, 4, 23485–23491. [Google Scholar] [CrossRef]
- Vicentini, R.; Nunes, W.G.; da Costa, L.H.; Da Silva, L.M.; Freitas, B.; Pascon, A.M.; Vilas-Boas, O.; Zanin, H. Multi-walled carbon nanotubes and activated carbon composite material as electrodes for electrochemical capacitors. J. Energy Storage 2021, 33, 100738. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose nanocrystals/graphene hybrids—A promising new class of materials for ad-vanced applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Shwetha, U.R.; Latha, M.S.; Kumar, C.R.R.; Kiran, M.S.; Onkarappa, H.S.; Betageri, V.S. Potential antidiabetic and anticancer activity of copper oxide nanoparticles synthesised using Areca catechu leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 025008. [Google Scholar] [CrossRef]
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent development in the synthesis of agricultural and for-estrybiomass-derived porous carbons for supercapacitor applications: A review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Muliastri, D.; Susanti, D.; Widyastuti. Influence of composition grafit oxide, irradiation-time variation analyzes on reduced graphene oxide—Copper oxide (rGO/CuO) composite toward photocatalytic conversion of CO2 to methanol. AIP Conf. Proc. 2018, 2014, 020102-1–020102-6. [Google Scholar]
- Samir, M.A.S.A.; Alloin, F.; Paillet, M.; Dufresne, A. Tangling Effect in Fibrillated Cellulose Reinforced Nanocomposites. Macromolecules 2004, 37, 4313–4316. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation and characterization of nanocrystalline cellulose from roselle-derived microcrystalline cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaimi, A.A.; Wanrosli, W.D. Isolation and characterization of nanocrystalline cellulose from totally chlorine free oil palm empty fruit bunch pulp. J. Polym. Environ. 2017, 25, 192–202. [Google Scholar] [CrossRef]
- Yang, V.; Senthil, R.A.; Pan, J.; Khan, A.; Osman, S.; Wang, L.; Jiang, W.; Sun, Y. Highly ordered hierarchical porous carbon derived from biomass waste mangosteen peel as superior cathodematerial for high performance supercapacitor. J. Electroanal. Chem. 2019, 855, 113616. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghosh, A.; Achak, O.; Chafik, T.; Ghamouss, F. A high energy storage supercapacitor based on nanoporous activated carbon electrode made from Argan shells with excellent ion transport in aqueous and non-aqueous electrolytes. J. Energy Storage 2019, 26, 100958. [Google Scholar] [CrossRef]
- Hussain, N.; Das, M.R. Magnetically recoverable graphene-based nanocomposite material as an efficient catalyst for the synthesis of propargylamines via A3 coupling reaction. New J. Chem. 2017, 41, 12756–12766. [Google Scholar] [CrossRef]
- Frindy, S.; El Kadib, A.; Lahcini, M.; Primo, A.; García, H. Copper nanoparticles supported on graphene as an efficient catalyst for A3 coupling of benzaldehydes. Catal. Sci. Technol. 2019, 6, 4306–4317. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Reiner, R.S.; Baez, C. Probing crystallinity of never-dried wood cellulose with Raman spectroscopy. Cellulose 2016, 23, 125–144. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kotsyubynsky, V.O.; Boychuk, V.M.; Budzulyak, I.M.; Rachiy, B.I.; Zapukhlyak, R.I.; Hodlevska, M.A.; Kachmar, A.I.; Bi-logubka, O.R.; Malakhov, A.A. Structural Properties of Graphene Oxide Materials Synthesized Accordingly to Hummers, Tour and Modified Methods: XRD and Raman Study. Phys. Chem. Solid State 2021, 22, 31–38. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Khatoon, F.; Siddiq, M.; Khan, G.S. Synthesis of graphene oxide–metal nanoparticle nanocomposites for catalytic reduction of nitrocompounds in aqueous medium. J. Taibah Univ. Sci. 2021, 15, 493–506. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rajaura, R.S.; Sharma, K. Synthesis and characterization of graphene oxide nanoparticles and their anti-bacterial activity. Suresh GyanVihar Univ. Int. J. Environ. Sci. Technol. 2015, 1, 16–24. [Google Scholar]
- Kashinath, L.; Kumar, R.S.; Hayakawa, Y.; Ravi, G. Synthesis & Structural Study on Graphene Nano Particles. Int. J. Sci. Eng. Appl. 2013. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Wu, S.; Wang, H.; Yang, W. Green synthesis of capacitive carbon derived from Platanus catkins with high energy density. J. Mater. Sci. Mater. Electron. 2019, 30, 4184–4195. [Google Scholar] [CrossRef]
- Eldamaty, H.S.E.; Elbasiouny, H.; Elmoslemany, A.M.; Abd El-Maoula, L.M.; El-Desoky, O.I.; Rehan, M.; Abd El Moneim, D.; Zedan, A. Protective effect of wheat and barley grass against the acute toxicological effects of the concurrent administration of excessive heavy metals in drinking water on the rats liver and brain. Appl. Sci. 2021, 11, 5059. [Google Scholar] [CrossRef]
- Iordache, A.; Culea, M.; Gherman, C.; Cozar, O. Characterization of some plant extracts by GC–MS. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 338–342. [Google Scholar] [CrossRef]
- Shakya, G.; Pajaniradje, S.; Hoda, M.; Durairaj, V.; Rajagopalan, R. GC-MS analysis, in vitro antioxidant and cytotoxic studies of wheatgrass extract. Am. J. Phytomed. Clin. Ther. 2014, 2, 877–893. [Google Scholar]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Abed, K.A.K.; Yaqoob, K.; Abdoh, A.O.O.; Mohammed, S.M.; Pankaj, T.; Hakeem, S.M.A.; Mamoon, H.S. Investigation of Anti-genotoxic Potential of Wheatgrass (Triticumaestivum) Powder on Cyclophosphamide Induced Genotoxicity and Oxidative Stress in Mice. Austin J. Pharmacol. Ther. 2017, 5, 1098. [Google Scholar]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Waghmode, B.; Nathani, S.; Krishnan, V.; Sircar, D.; Roy, P. Wheatgrass inhibits the lipo-polysaccharide-stimulated inflammatory effect in RAW 264.7 macrophages. Curr. Res. Toxicol. 2021, 2, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.; Rydosz, A. Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor. Coatings 2020, 10, 1015. [Google Scholar] [CrossRef]
- Isah, K.U.; Bakeko, M.M.; Ahmadu, U.; Uno, U.E.; Kimpa, M.I.; Yabagi, J.A. Effect of oxidation temperature on the properties of copper oxide thin films prepared from thermally oxidised evaporated copper thin films. IOSR J. Appl. Phys. 2013, 3, 61–66. [Google Scholar]
- Azmi, A.F.M.; Kannan, V.; Yasin, N.S.; Abdul Rashi, J.I.; Omar, A.; Salleh, E.M. Effect of time and tem-perature on reduced graphene oxide (rGO) layer stability and cyclic voltametric behaviour of modified screen-printed carbon electrode (mSPCE) for biosensing purposes. Malays. J. Anal. Sci. 2020, 24, 800–809. [Google Scholar]
- Li, Y.; Duan, C.N.; Jiang, Z.; bin Zhou, X.; Wang, Y. CuO/rGO nanocomposite as an anode material for high-performance lithium-ion batteries. Mater. Res. Express 2021, 8, 055505. [Google Scholar] [CrossRef]
- Kumari, V.; Kaushal, S.; Singh, P.P. Green synthesis of a CuO/rGO nanocomposite using a Terminalia arjuna bark extract and its catalytic activity for the purification of water. Mater. Adv. 2022, 3, 2170–2184. [Google Scholar] [CrossRef]
- Li, D.; Liang, Z.; Zhang, W.; Dai, S.; Zhang, C. Preparation and photocatalytic performance of TiO2-RGO-CuO/Fe2O3 ternary composite photocatalyst by solvothermal method. Mater. Res. Express 2021, 8, 015025. [Google Scholar] [CrossRef]
- Gijare, M.; Chaudhari, S.; Ekar, S.; Anil Garje, A. A facile synthesis of GO/CuO-blended nanofiber sensor electrode for efficient enzyme-free amperometric determination of glucose. J. Anal. Sci. Technol. 2021, 12, 40. [Google Scholar] [CrossRef]
- Xu, X.; Shen, J.; Qin, J.; Duan, H.; He, G.; Chen, H. Cytotoxicity of Bacteriostatic Reduced Graphene Oxide-Based Copper Oxide Nanocomposites. JOM 2018, 71, 294–301. [Google Scholar] [CrossRef]
- Dutta, K.; Das, K.; Chakrabarti, K.; Jana, D.; De, S.K. Highly efficient photocatalytic activity of CuO quantum dot decorated rGO nanocomposites. J. Phys. D Appl. Phys. 2016, 49, 315107. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Raja, S.L.; Supeno, M.; Simanjuntak, C. New Route to Synthesize of Graphene Nano Sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef]
- Drewniak, S.E.; Pustelny, T.P.; Muzyka, R.; Plis, A. Studies of physicochemical properties of graphite oxide and thermally exfoliated/reduced graphene oxide. Pol. J. Chem. Technol. 2015, 17, 109–114. [Google Scholar] [CrossRef]
- Gurzęda, B.; Buchwald, T.; Nocuń, M.; Bąkowicz, A.; Krawczyk, P. Graphene material preparation through thermal treatment of graphite oxide electrochemically synthesized in aqueous sulfuric acid. RSC Adv. 2017, 7, 19904. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Abdul Aziz, F.; Salleh, E.M.; Hawa, A.; Rafique, R.F. A one-step facile route syn-thesis of copper oxide/reduced graphene oxide nanocomposite for supercapacitor applications. J. Exp. Nano-Sci. 2018, 13, 284–295. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Z.; Zhang, H.; Cao, G.; Sun, Q.; Cao, J. CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO. Catalysts 2016, 6, 214. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Won-Chun Oh, W.C.; Hamizi, N.A.; Johan, M.R. Enhanced photocatalytic activity of rGO-CuO nanocomposites for the degradation of organic pollutants. Catalysts 2021, 11, 1008. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Hemant, H.; Nitinkumar, S.S.; Poornesh, P.; Selvakumar, M. Green synthesis and electrochemical characterization of rGO–CuO nanocomposites for supercapacitor applications. Ionics 2016, 23, 1267–1276. [Google Scholar] [CrossRef]
- Park, H.; Lim, S.; Nguyen, D.D.; Suk, J.W. Electrical measurements of thermally reduced graphene oxide powders under pressure. Nanomaterials 2019, 9, 1387. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Y.; Dong, H.; Zhao, X.; Xiao, Y.; Liang, Y.; Hu, Y.; Liu, H.; Zheng, Y.M. Hierarchically porous carbon nanosheets derived from Moringa oleifera stems as electrode material for high-performance electric double-layer capacitors. J. Power Source 2017, 53, 260–269. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Rekos, K.; Kampouraki, Z.-C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene Oxide Based Magnetic Nanocomposites with Polymers as Effective Bisphenol—A Nanoadsorbents. Materials 2019, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Lai, C.W.; Bin Johan, M.R. Recent developments in biomass-derived carbon as a potential sustainable material for super-capacitor-based energy storage and environmental applications. J. Anal. Appl. Pyrolysis 2019, 140, 54–85. [Google Scholar] [CrossRef]
- Cruz, D.S.; Hernández, S.A.M.; Flores, F.d.M.; Álvarez, J.C.; Mou Pal, M.; Cruz, J.S. CuOX thin films by direct oxidation of Cu films deposited by physical vapor deposition. Results Phys. 2017, 7, 4140–4144. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Yang, W.; Zhu, Z.; Wu, Y. Positron Annihilation Spectroscopy Study on Annealing Effect of CuO Nanoparticles. Mater. Res. 2015, 19, 316–321. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M.R.; Motamedi, E. Synthesis of green and pure copper oxide nanoparticles using two plant resources via solid-state route and their phytotoxicity assessment. RSC Adv. 2021, 11, 3346–3353. [Google Scholar] [CrossRef]
- Anwar, H.; Naqvi, S.M.B.; Abbas, B.; Shahid, M.; Iqbal, M.; Shaharyar, M.; Islam, A.; Batool, F.; Khalid, M.; Jamil, A. Investigation of the effect of annealing temperature on structural, optical and antibacterial properties of copper oxide nanoparticles pre-pared by facile co-precipitation route. J. Optoelectron. Biomed. Mater. 2020, 12, 43–50. [Google Scholar]
- Gonçalves, A.M.B.; Campos, L.C.; Ferlauto, A.S.; Lacerda, R.G. On the growth and electrical characterization of CuO nanowires by thermal oxidation. J. Appl. Phys. 2009, 106, 034303. [Google Scholar] [CrossRef]
- Jnaktiyok, J.; Özer, A.K. Synthesis of copper oxide (CuO) from thermal decomposition of copper acetate monohydrate (Cu(CH3COO)2 H2O). Omer Halisdemir Univ. J. Eng. Sci. 2019, 8, 1292–1298. [Google Scholar]
- Khen, A.; Hadjersi, T.; Brihi, N.; Moulai, F.; Fellahi, O.; Naama, S.; Ifires, M.; Manseri, A. Preparation of SiNWs/rGO/CuO nanocomposites as effective photocatalyst for degradation of ciprofloxacin assisted with peroxymonosulfate. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1078–1091. [Google Scholar] [CrossRef]
- Siddique, S.; Abdin, Z.U.; Waseem, M.; Naseem, T.; Bibi, A.; Hafeez, M.; Din, S.U.; Haq, S.; Qureshi, S. Photo-Catalytic and Anti-microbial Activities of rGO/CuO Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1359–1372. [Google Scholar] [CrossRef]
- Morales, J.; Sanchez, L.; Martin, F.; Ramos-Barrado, J.R.; Sanchez, M. Use of low-temperature nanostructured CuO thin films de-posited by spray-pyrolysis in lithium cells. Thin Solid Film 2005, 474, 133–140. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, Z.; Song, P.; Wang, Q. Facile synthesis of CuO nanoribbons/rGO nanocomposites for high-performance formaldehyde gas sensor at low temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 19297–19308. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, Q.; Yaseen, M.; Ahmad, I.; Hussain, F.; Jan, B.M.; Ikram, R.; Stylianakis, M.M.; Kenanakis, G. Development of waste polystyrene-based copper oxide/reduced graphene oxide composites and their mechanical, electrical and thermal properties. Nanomaterials 2021, 11, 2372. [Google Scholar] [CrossRef]
- Gupta, M.; Hawari, H.F.; Kumar, P.; Burhanudin, Z.A. Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications. Crystals 2022, 12, 264. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Arya, S. Highly selective and efficient electrochemical sensing of ascorbic acid via CuO/rGO nanocomposites deposited on conductive fabric. Appl. Phys. A 2022, 128, 262. [Google Scholar] [CrossRef]
- Pratheepa, M.L.; Lawrence, M. Synthesis of CuO—Reduced graphene oxide nanocomposite for high performance electrochemical capacitors. Int. J. Res. 2021, 5, 4519–4524. [Google Scholar]
- Talande, S.V.; Bakandritsos, A.; Zdrăzil, L.; Jakubec, P.; Mohammadi, E.; Tomanec, O.; Otyepka, M.; Presser, V.; Zbŏril, R.; Tŭcek, J. Pinning ultrasmallgreigite nanoparticles ongraphene for effective transition-metal-sulfide supercapacitors in an ionic liquid electrolyte. J. Mater. Chem. A 2020, 8, 25716–25726. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).