Abstract
Microalgae and other microorganisms often play a significant role in the transportation of heavy metal ions in the environment, while at the same time they are closely related to the formation of minerals in aquatic systems, especially with the formation of calcium carbonate (CaCO3). The biomineralization of calcite was investigated in aqueous solutions, supersaturated with respect to calcium carbonate (7.94 < SRcalcite < 31.36) in the presence of heavy metals (Zn, Cd, Ni, Cu) and of colonies of Acutodesmus obliquus (A. obliquus). The presence of metals tested in the supersaturated solutions, at concentration levels below the threshold of precipitation of the respective hydroxides, reduced the rate of calcium carbonate precipitation by 40 to 90% depending on the solution supersaturation. The presence of A. obliquus culture increased the rates of calcium carbonate precipitation by 80%. The presence of the test metals inhibited the growth of A. obliquus, especially the presence of Cd. The uptake of the test metals on calcite fitted Langmuir adsorption isotherm. Cadmium uptake onto calcite reached 85% of the total amount in the solutions. Charged ion pairs of test metals play an important role in their activity with respect to calcium carbonate precipitation and algal growth.
1. Introduction
In general, the term heavy metal (HM) refers to any metallic chemical element with relatively high density that is toxic at low concentrations. However, any metal or metalloid causing environmental pollution, or which is not biodegraded in nature, could be considered as HM [1]. Some of these metals, such as Zn, Cu, Mn, Ni and Co, are micronutrients necessary for plant growth, while others, including Cd, Pb and Hg, are toxic with still rather unknown biological functions [2]. Metal speciation in the environment depends on a broad range of biochemical processes (oxidation, reduction, chelation) influenced by biotic (interactions with living organisms) and abiotic factors (temperature, pH, organic matter and ionic strength) [3].
Population growth, industrialization and agricultural practices have resulted in higher concentrations of toxic HM in the environment, causing global concern. The presence of HM in the environment is often associated with human, industrial and agricultural activities [1,4,5,6]. The metal industry adds 0.39 million tons/year of heavy metals to the environment, while agriculture contributes 1.4 million tons/year [6]. Specifically, water pollution by HM, which is associated with industrial processes, needs urgent attention [7,8,9,10]. The most encountered HM in the environment include Cd, Cr, Cu, Hg, Pb and Zn. The global contamination from Cd, Cu, Ni and Zn is about 5.6–38 ktons/year, 541–1367 ktons/year, 106–544 ktons/year and 689–2054 ktons/year for Cd, Cu, Ni and Zn, respectively [11].
The processes applied for the removal of HM from wastewater include chemical precipitation, ion exchange, membrane filtration, flotation, coagulation flocculation and electrochemical methods [12]. These processes are generally applied to reduce HM to very low concentration levels, but they suffer from high operation and maintenance costs and produce secondary pollution due to toxic sludge formation [13]. Clearly, there is a need for new and improved technologies for the removal of HM from aquatic systems [14]. The environmentally friendly and cost-efficient bioremediation is limited to rather low concentration levels of the pollutants. Alternative methods for the removal of HM from aquatic media, involving bacteria, microalgae and fungi, have drawn considerable attention [15]. Of all microorganisms, microalgae are of particular interest, not only because of their outstanding biological characteristics, such as their high photosynthetic efficiency and simple structure (i.e., they are unicellular and simple multicellular photosynthetic micro-organisms, be they prokaryotes (cyanobacteria) or eukaryotes), but also because of their ability to grow under extreme environmental conditions, including the presence of heavy metals, high salinity, lack of nutrients and extreme temperatures. It should be noted that both living cells and non-living biomass of microalgae may be used as biosorbents for the uptake of HMs from polluted aquatic systems.
Photosynthetic microalgae have been associated with minerals formation—in particular, the formation of calcium carbonate [16,17,18,19]. It seems that only calcium carbonate polymorphism is affected by both the presence of microalgae and the heavy metals present in the aqueous medium. The formation of precipitates in the presence of metal ions is an effective method for transferring HM from the aqueous to the solid phase. In aquatic systems, when oxygenic photosynthesis takes place, the following equilibria are established:
CO2 is used by photosynthetic microorganisms in their metabolic process, while at the same time there is a dynamic equilibrium between HCO3– and CO32–. The use of CO2 or HCO3—results in the release of OH−, and the subsequent increase in the solution pH [20]. In the presence of enough calcium in the aqueous medium, the supersaturation with respect to calcium carbonate may be sufficient to cause the precipitate of this mineral salt [21]. The precipitation of CaCO3(s), as may be seen in Equation (4), is accompanied with proton release and to some extent counterbalances the equilibrium shown in Equation (3).
Metal ion species take part in the equilibria, affecting the rate of calcium carbonate precipitation, but at the same time their effective concentration in the aqueous medium may be reduced due to the presence of biomass, which may also interact with the aqueous species.
The present work studies the precipitation of calcium carbonate in both the presence of ions resulting from the dissolution in water of soluble salts of zinc, cadmium, nickel and copper, and in the presence of Acutodesmus obliquus (A. obliquus), a photosynthetic microalga. The effect of the test HM and of A. obliquus in the kinetics of calcium carbonate precipitation is expected to provide insight into the mechanism of interaction of HM aqueous species at the interface of calcium carbonate polymorphs forming and with A. obliquus.
2. Materials and Methods
A. obliquus, a freshwater green photosynthetic microalga, was selected for the investigation and was obtained from SAG (Strain number 276-1). A. obliquus was grown in Bolds Basal Medium (BBM) [22] in 250 mL conical flasks (batch cultures) under continuous air flow through an oil-free air pump at a flow rate of ca. 6 L per minute. The illumination for the growth of the A. obliquus culture was applied by cold white 3500 lx lamps (TL-D Super 80, Philips, Pila, Poland). Cultures were grown at 25 ± 2 °C and the pH was adjusted to 6.5.
2.1. Mineralization Experiments in the Presence of Heavy Metals (HM) and A. obliquus
The precipitation of calcium carbonate was carried out in solutions supersaturated with respect to all calcium carbonate anhydrous polymorphs. The supersaturated solutions were prepared in a double-walled Pyrex® glass reactor. Water from a thermostat circulated between the reactor walls with the help of a pump so that the temperature in the reactor was maintained at 25.0 ± 0.2 °C. All stock solutions were prepared from the respective crystalline solids (ACS reagent, ≥99.8%, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) by dissolution in triply distilled water. The stock solutions of sodium bicarbonate, sodium carbonate and calcium chloride were prepared by weighing exactly the quantities needed and were next filtered through 0.2 μm membrane filters (Sartorius, Goettingen, Germany). The calcium chloride solution was standardized by titration with standard EDTA solution using murexide indicator, and by atomic absorption spectrometry (AAS, AAnalyst 300, Perkin Elmer, Norwalk, CT, USA). NaHCO3 and Na2CO3 solutions were prepared fresh for each experiment and were used without any further standardization. Stock solutions of the studied metals, zinc, cadmium, nickel and copper, were prepared by weighing crystalline solids Zn(NO3)2∙4H2O, Cd(NO3)2∙4H2O, Ni(NO3)2 and CuSO4∙5H2O (ACS reagent, ≥99.8%, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), respectively. Following filtration, these solutions were standardized by AAS using the appropriate standard solutions (AA standard solutions, Merck KGaA, Darmstadt, Germany). The supersaturated calcium carbonate solutions were prepared by directly mixing equal volumes of calcium and carbonate solutions, which were prepared by diluting the needed quantities from the stock solutions. The solutions’ pH was adjusted to 8.5 with 0.1 M of NaOH standard solution (Merck titrisol). The effect of the test metals on the precipitation of calcium carbonate was investigated by adding the appropriate amounts from the respective stock solutions in the solutions supersaturated with respect to calcium carbonate. Next, the supersaturated solutions were inoculated with 10 mL of slurry of the active A. Obliquus culture, which was withdrawn directly from the respective batch cultures. In all experiments, the only parameter kept constant was temperature, while the activities of all species were not controlled (free drift method).
The onset of precipitation was signaled by the pH decrease in the supersaturated solutions because of proton release (Equation (4)). The precipitation of calcium carbonate was accompanied by the decrease in calcium and carbonate ions, the of which the change in concentration was monitored. The samples were withdrawn from the suspension in the reactor during precipitation and filtered. For the analysis of metals with AAS, the filtrates were acidified by the addition of concentrated HCl. Due to the continuous growth of A. obliquus, the total chlorophyll in the reactor was measured regularly to assess the status of A. obliquus culture during precipitation. The samples were withdrawn, filtered, and the absorbance of the filtrates was measured at 650 nm with a UV-VIS spectrophotometer (Perkin Elmer UV/VIS Spectrometer, Perkin Elmer, Model Lambda 35, Norwalk, CT, USA) [23].
The formation of calcium carbonate in the supersaturated solutions started, as already mentioned, after inoculation with A. obliquus culture and the nutrient medium in which the appropriate amount of zinc (or any other of the test metals) was added to the corresponding calcium carbonate in the supersaturated solution. The culture was illuminated to ensure growth. The composition of the modified nutrient medium is shown in Table 1.

Table 1.
Composition of modified A. obliquus culture medium added to the supersaturated calcium carbonate solutions upon inoculation with the culture.
The total volume of the supersaturated solutions was 250 mL, and the tested total calcium (Cat) concentrations (equal to the total carbonate, Ct) were 1.7 m M and 3.4 mM. The final concentration of total zinc in the supersaturated solutions was between 5.6 and 92 μΜ. A total of 10 mL of active microalgae culture (108 cells) was introduced into these solutions and left under illumination for 2 to 3 days, until the solution pH increased due to photosynthesis (Equations (1)–(3)) and reached a value of ca. 9. Next, the appropriate concentration of Na2CO3 solution was added so that the ratio of total calcium to total carbonate was Cat:Ct = 1:1.
At the end of the precipitation process, which was indicated by the levelling off of pH and metals concentration as a function of time profiles, the solution in the reactor was filtered through membrane filters (0.2 um, Sartorius). The solid on the filters was examined by powder X ray diffraction (XRD, Siemens D-500, Frankfurt, Germany) and scanning electron microscopy (SEM, Leo Supra, Zeiss Oberkochen, Germany) with a Bruker XS (Billerica, MA, USA) EDX microanalysis unit.
2.2. Measurements of the Metals Uptake
The uptake of metals on the calcite crystals was studied over a range of concentrations for each metal. The calcite crystals were prepared by precipitation and were aged suspended in the mother liquor for one week under stirring at room temperature (specific surface area using nitrogen absorption, SSA, and calculated according to the Brunauer–Emmett–Teller equation, was 0.33 m−2·g−1). Saturated solutions with respect to calcite were prepared by suspending 100 mg of calcite crystals in sodium chloride aqueous solutions of 0.15 M, and the pH was adjusted to 8.5. After equilibration, accurately measured amounts of stock solutions of the corresponding metals (Zn, Cd, Ni and Cu) were added in the equilibrated calcite suspensions, which were sufficient to reach the desired initial concentrations. The solutions reached equilibrium under continuous stirring at 25 °C. After three or five days, the samples were withdrawn and analyzed for calcium and total metals concentration. The same procedure was used for the investigation of the adsorption of heavy metals on A. obliquus cultures and on calcite crystals grown on air-dried A. obliquus cultures.
The study of zinc sorption in calcite in the presence of A. obliquus culture was carried out in solutions saturated with respect to calcite with an ionic strength of 0.15 M of NaCl, in the presence of 5 mL of microalgae culture, at its exponential growth phase. 108 cells corresponded to 5 mL of microalgae culture. A total of 100 mg of calcite, SSA 0.33 m−2·g−1, was suspended in the calcite-saturated solutions (50 mL) and was equilibrated under stirring. Next, zinc from the respective stock solution, 0.1 M of Zn(NO3)2·4H2O, was added to achieve a corresponding initial concentration of (total) zinc. During a time period of seven days, the samples were withdrawn and filtered through membrane filters. The residual (total) zinc was measured in the filtrates with AAS. The solid on the filters was identified by powder X-ray diffraction (XRD).
Zinc uptake was measured on calcite grown on air-dried A. obliquus (SSA = 2.64 m2·g−1) and of air-dried A. obliquus culture (SSA 0.38 m2·g−1). The ionic strength of the solutions used was adjusted to 0.15 M with NaCl, which was saturated in the presence of calcite and had a pH of 8.5. The suspensions were stirred for 1 h. Next, the zinc concentration was adjusted to 70 μM of Zn(NO3)2 with the appropriate volume of the corresponding stock solution. The suspensions were equilibrated under stirring at 25 °C for 48 h. At regular intervals, the samples were withdrawn from the suspensions and the zinc concentration in the suspensions was measured relative to the initial concentration. The experimental setup used for the investigation of mineralization of A. Obliquus and for the adsorption of metal ions is shown in Figure 1.
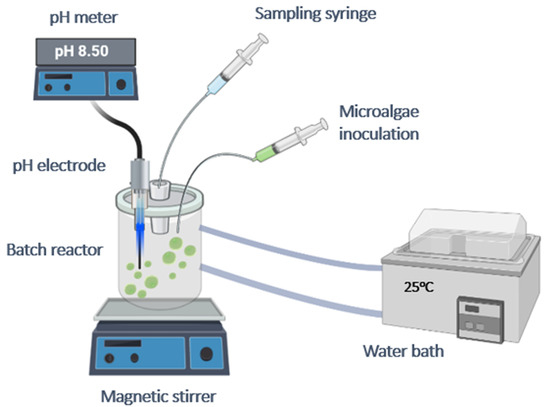
Figure 1.
Experimental setup for the study of mineralization and adsorption of HM on solids suspensions.
3. Results and Discussion
3.1. Mineralization Experiments in the Presence of Heavy Metals and of A. obliquus Culture
3.1.1. Precipitation of Calcium Carbonate in the Presence of A. obliquus Including the Corresponding Nutrient Growth Medium in the Presence of Zn
The driving force for the precipitation of calcium carbonate from supersaturated solutions with respect to calcite is the change in Gibbs free energy, ΔG, for going from the supersaturated solution to equilibrium.
where α are the activities of the subscripted ions and is the thermodynamic solubility product of calcite, R is the gas constant and T the absolute temperature. The supersaturation ratio, SRcalcite, with respect to calcite is defined as:
The relative supersaturation with respect to calcite, σcalcite, is defined as:
As may be seen from Equations (5)–(7), the driving force for the precipitation depends on the activities of the ions of the precipitating solid. It is therefore higher, as is the total concentration of the respective components (total calcium, Cat, and total carbonate, Ct, concentrations). The activities of the free ions in the supersaturated solutions were computed from the total concentrations of the elements present (Cat and Ct), pH and NaCl concentration, using the equilibrium calculations software PHREEQC® [24].
The experimental conditions for calcium carbonate precipitation in supersaturated solutions in the presence of zinc and A. obliquus culture are summarized in Table 2. The A. obliquus culture was at the beginning of the exponential growth phase.

Table 2.
Experimental conditions for calcium carbonate precipitation in the presence of zinc under illumination in the absence and presence of A. obliquus culture and nutrient medium; Expt. #, total calcium, Cat = total carbonate, Ct, concentration, zinc (total) concentration, CZn, and volume of A. obliquus culture; 25 °C, pHinitial 8.50, 0.15 Μ of NaCl.
The measurements of total chlorophyll concentration over a time period of three days are shown in Figure 2. The increasing concentration of chlorophyll during the increasing precipitation of calcium carbonate was attributed to the growth of the microalgal culture in the supersaturated solutions.
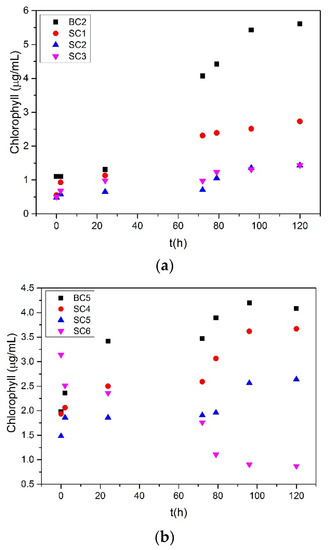
Figure 2.
Total chlorophyll concentration time profile during the precipitation of calcium carbonate from supersaturated solutions, in the presence of zinc, under illumination in the presence of 10 mL of A. obliquus culture and nutrient medium, (a) Cat = Ct = 1.7 mM; (b) Cat = Ct = 3.4 mM; 25 °C, pHinitial 8.50, 0.15 Μ of NaCl.
In the presence of 92 μM of zinc, as shown in Figure 2b, the chlorophyll concentration decreased with time. It is obvious that the relatively high concentration of Zn in comparison with the rest of the solutions inhibited the growth of A. obliquus. Logarithmic plots of the chlorophyll concentration as a function of time are shown in Figure 3. From the slopes obtained from the linear fitting of the data, the growth rate of the microalgae culture was calculated on a case-by-case basis. The results of the calculations are summarized in Table 3.
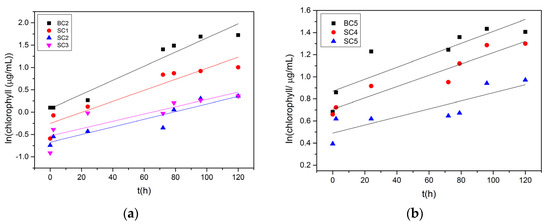
Figure 3.
Logarithmic plots of the variation of chlorophyll concentrations as a function of time during precipitation of calcium carbonate in the presence of zinc under illumination and in the presence of A. obliquus culture and nutrient medium. (a) Cat = Ct = 1.7 mM, σcalcite = 0.68; (b) Cat = Ct = 3.4 mM, σcalcite = 2.28; 25 °C, pHinitial 8.50, 0.15 Μ of NaCl.

Table 3.
Calculated slopes from the linear fit (Figure 3) of the calculated A. obliquus growth during precipitation of calcium carbonate in supersaturated solutions in the presence of A. obliquus culture and nutrient medium, under illumination, in the presence of zinc; 25 °C, pHinitial 8.50, 0.15 Μ of NaCl, Expt. #, total calcium.
The growth rate of the microalgae culture was affected by the presence of zinc. A. obliquus culture growth rates decreased with the increasing total zinc concentration in the solutions. As suggested for Cu [25], zinc aqueous species (Zn2+, ZnOH+ and ZnCl+) at a first step were electrostatically associated (surface ion pairs or complexes) with negatively charged A. obliquus cell wall molecules. At longer residence times, the charged metal species were transported across the cell membrane and entered the cell via ion pores, channels or transporters. Both steps could be responsible for the reduction in the growth rate of the A. obliquus culture. The exact mechanisms responsible for metal uptake by microalgae have not always been clearly elucidated. It should be noted, however, that studies on marine microalgae showed that zinc levels as high as 4 mM did not fully suppress their growth [26]. Similar toxicity results have been reported for Cd and Zn on two green microalga species, Scenedesmus obliquus and Desmodesmus pleiomorphus [27].
The change in (total) calcium concentration during the precipitation of calcium carbonate, as a function of time, is shown in Figure 4.
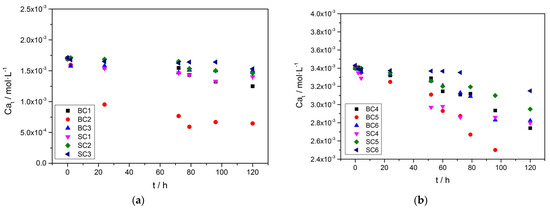
Figure 4.
Calcium concentration as a function of time during the precipitation of calcium carbonate in the presence of A. obliquus culture and nutrient medium under illumination, in the presence of Zn; (a) Cat = Ct = 1.7 mM; (b) Cat = Ct = 3.4 mM; 25 °C, pHinitia 8.50, 0.15 Μ of NaCl.
In all cases, the decrease in calcium concentration in the supersaturated solutions was due to the precipitation of calcium carbonate. The rates (initial) of calcium carbonate precipitation were calculated from the calcium concentration time profiles at time 0 (initial rates). The differences between the precipitation rates of calcium carbonate on the inoculating materials tested are presented in the plots of Figure 5 (the corresponding inoculating materials may be found from the experiment code symbol in Table 2).
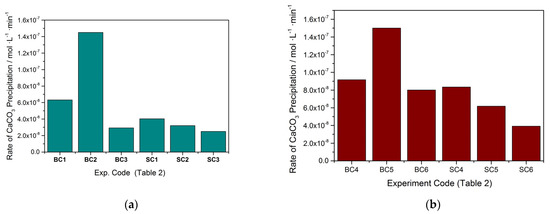
Figure 5.
Calcium carbonate precipitation rates in the presence of A. obliquus culture and nutrient medium under illumination in the presence of zinc. (a) Cat = Ct = 1.7 mM; (b) Cat = Ct = 3.4 mM; 25 °C, pHinitial 8.50, 0.15 Μ of NaCl; BC1: No solid (reference); BC2: 10 mL of metabolically active culture A. obliquus; BC3: 5.6 μM of Zn, No solid; SC1: 5.6 μM of Zn and 10 mL of metabolically active culture A. obliquus; 11.0 μM of Zn and 10 mL of metabolically active culture A. obliquus; 15.0 μM of Zn and 10 mL of metabolically active culture A. obliquus.
As may be seen, the rate of calcium carbonate precipitation in the presence of A. obliquus culture alone in the supersaturated solutions was higher in both supersaturations tested (experiment codes BC2 and BC5). In the presence of zinc, in addition to A. obliquus culture, the rate of calcium carbonate precipitation decreased, as may be seen in Figure 5. In the presence of higher Zn concentrations (15 and 92 μΜ), the reduction in the precipitation rate reached 83% and 74%, respectively, for the two values of relative supersaturation investigated.
The inhibition of calcium carbonate precipitation was calculated by a comparison of the rate in each case with the corresponding value in the absence of zinc. Specifically, the precipitation rate of SC1, SC2 and SC3 was compared with that of BC2, and the rate for experiments SC4, SC5 and SC6 was compared with experiment BC5.
In Equation (8), subscript x refers to experiments # SC1, SC2, SC3, and in Equation (9) the subscript y corresponds to experiments # SC4, SC5 and SC6. The % inhibition of calcium carbonate precipitation at the conditions of the experiments summarized in Table 2, is shown in the diagram of Figure 6.
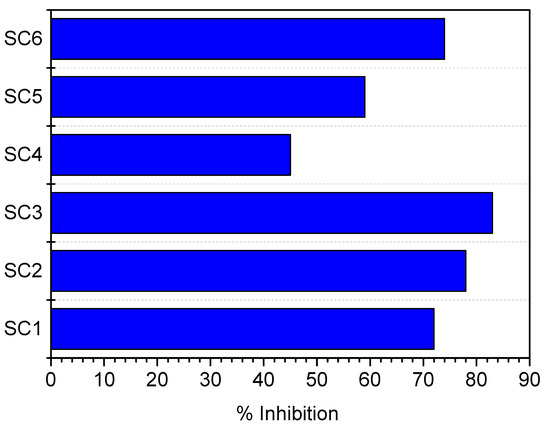
Figure 6.
% Inhibition of calcium carbonate precipitation rate in the presence of zinc and A. obliquus culture, at 25 °C, pHinitial 8.50, 0.15 Μ of NaCl.
Higher zinc concentrations in the supersaturated solutions increased the inhibition of calcium carbonate precipitation. The inhibition was higher with a decreasing supersaturation ratio. It should be noted that the concentration levels of zinc and of the A. obliquus culture in the supersaturated solutions did not affect the calculated value of the supersaturation ratio, taking all involved equilibria into consideration. The effect of the presence of Zn in the supersaturations solutions, together with active A. obliquus culture, resulted in a spectacular decrease in the rate of calcium carbonate mineralization. It should be kept in mind that the presence of A. obliquus culture alone accelerated the precipitation of calcium carbonate. Therefore, the reduction of the rate of calcium carbonate mineralization should, on the one hand, be attributed to the reduction in growth of the A. obliquus culture, which is equivalent to “less acceleration”, i.e., a relative reduction in the rate of calcium carbonate precipitation. The predominant zinc species at the experimental conditions of the present work were Zn2+, ZnOH+ and ZnCl+. These species mainly block the active sites on the growing crystals by adsorption onto the surface CO32− sites of the calcium carbonate crystal lattice. A significant reduction in the rate of calcium carbonate crystallization during electrocrystallization in the presence of just 10 μM of zinc concentrations was reported and was attributed to the adsorption of zinc on the growing calcium carbonate crystals [28]. This suggestion is corroborated by the fact that the zinc concentration in the solutions did not change significantly enough to be detected by AAS.
3.1.2. Precipitation of Calcium Carbonate in the Presence of A. obliquus Culture and the Corresponding Nutrient Growth Medium in the Presence of Ni
The effect of the presence of nickel in the calcium carbonate supersaturated solutions (18.10 < SRcalcite < 31.36) on the kinetics of calcium carbonate precipitation was investigated in the presence of 20 mL of active A. obliquus culture (2.2 × 108 cells). The total nickel concentration in the supersaturated solutions was 6.0 μM. The experimental conditions are summarized in Table 4. Characteristic plots of the recordings of the pH value of the supersaturated solutions during calcium carbonate precipitation for two supersaturation ratios are shown in Figure 7. All pH time profiles corresponding to the precipitation of calcium carbonate show a characteristic period during which pH remained constant, suggesting that the solutions were stable during the respective time period. Following this time period, defined as the induction time, the solutions’ pH decreases concomitant to the formation of calcium carbonate precipitate. The formation of calcium carbonate is accompanied by a decrease in the solutions’ supersaturation, thus, reaching a plateau value, which corresponds to practically no precipitation. In this situation the pH value remains constant. From the change in the pH–time curves’ slope, the induction time, preceding the onset of calcium carbonate precipitation was inversely proportional to the SRcalcite value.

Table 4.
Precipitation of calcium carbonate in the presence of 6.0 μM of nickel under illumination and 20 mL of active A. obliquus culture (including nutrient medium). Calcium (total) concentration (=carbonate concentration), ratio of supersaturation, relative supersaturation, induction time, and precipitation rate of calcium carbonate; pH 8.50, 0.15 M of NaCl.
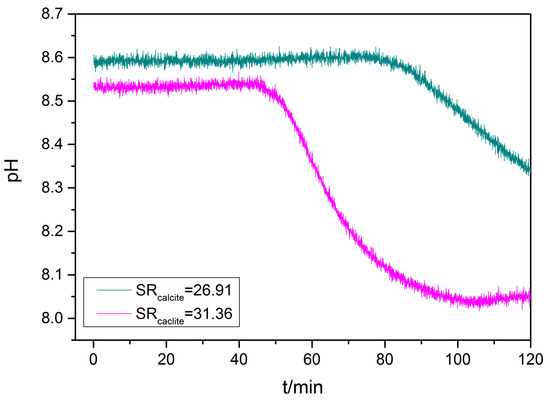
Figure 7.
Precipitation of calcium carbonate in the presence of 6.0 μM of nickel (under illumination in the presence of 20 mL of active A. obliquus culture (including nutrient medium). pH variation as a function of time; 25 °C, pH 8.50, 0.15 Μ of NaCl. At higher supersaturation (red line), precipitation starts earlier and at a higher rate.
The rates of calcium carbonate precipitation were calculated from the calcium concentration time profiles, corresponding to the pH time profiles, and the results are summarized in Table 4.
The induction times, tind, measured in the presence of nickel in the solutions were significantly shorter in comparison with the induction times measured in its absence, as shown in Figure 8.
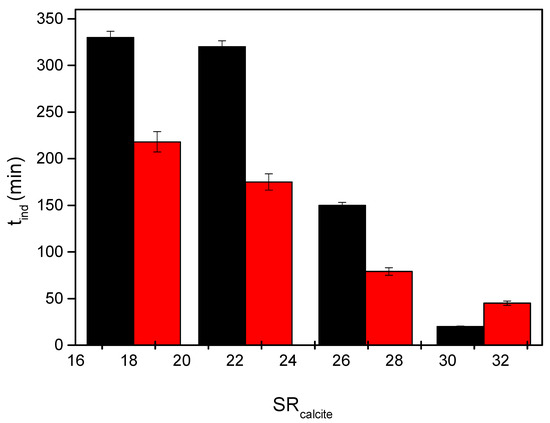
Figure 8.
Induction times preceding the precipitation of calcium carbonate as a function of supersaturation ratio with respect to calcite in the presence of nickel under illumination conditions in the presence of 20 mL of active microalgae culture (including nutrient medium); 25 °C, pH 8.50, 0.15 Μ of NaCl; black bars in the absence of Ni; red bars in the presence of 6.0 μM of Ni.
It should be noted that the differences are clear at lower supersaturations, while at the very high values where induction times were short, the differences are not significant.
The calcium carbonate precipitation rate was significantly reduced in the presence of nickel, in comparison with the corresponding rates measured in the presence of metabolically active A. obliquus culture (Figure 9).
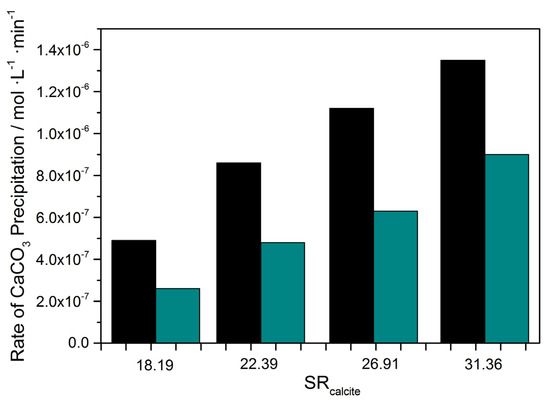
Figure 9.
Calcium carbonate precipitation rate as a function of supersaturation ratio; 25 °C, pH 8.50, 0.15 M of NaCl; (■) only A. obliquus culture; (green bar) in the presence of 6.0 μM of Ni and A. obliquus culture.
In Figure 8 and Figure 9, it is shown that the presence of nickel in the supersaturated calcium carbonate solutions inoculated with A. obliquus culture shortened the time that was needed for the formation of the critical nucleus but reduced the rates of the concomitant precipitation of the solid phase. This rate reduction is possibly due either to the poisoning of the active sites for the crystal growth of calcium carbonate or to the formation of transient calcium carbonate polymorphs, which dissolve with the rapid desupersaturation of the solutions during precipitation and transform to the thermodynamically most stable calcite by a dissolution–precipitation process. Hydroxyl ions released during the dissolution of unstable calcium carbonate polymorphs (vaterite or aragonite) may neutralize the protons that are released during calcite precipitation, and as a result, the overall process proceeds at a slower rate.
The inhibition of the calcium carbonate calculated according to Equations (8) or (9) is presented in Table 5. As may be seen, depending on the supersaturation, the reduction in the rate of precipitation reached ca. 90%. The higher the supersaturation ratio value, the lower the inhibition for the same total nickel concentration.

Table 5.
% Inhibition of the precipitation of calcium carbonate and the corresponding values of the supersaturation ratio with respect to calcite in the presence of 6 μM of nickel, under illumination and in the presence of 20 mL of active A. obliquus culture; 25 °C, pH 8.50, 0.15 M of NaCl.
3.1.3. Precipitation of Calcium Carbonate in Supersaturated Solutions in the Presence of Metabolically Active A. obliquus Cultures and 10 μM of Zn, Cd, Ni, Cu
The method for investigating the precipitation of calcium carbonate in the presence of A. obliquus was modified to first allow the growth of the A. obliquus culture, since the pH of the solutions containing suspensions of A. obliquus culture increased as a result of equilibria shown in Equations (1)–(3). When the solution pH reached a rather stable value (8.2 ± 0.2), the supersaturated solutions were prepared by the addition of sodium bicarbonate stock solution as needed for the desired supersaturation ratio value, and the pH was adjusted to 8.50.
The variation of the solution pH as a function of time from the beginning of the process is shown in Figure 10. In the two plots (Figure 10a,b), two domains may be distinguished. One lasted from the beginning to 48 h, during which the pH value increased due to photosynthesis (Equilibria shown in Equations (1)–(3)). This was confirmed by measurements of the produced chlorophyll in the solutions during this time period. The second domain corresponded to the time lapsed from 48 h to 120 h. The 48th hour was the time in which the carbonate ions were introduced in the solutions to account for the desired supersaturation with respect to calcite. At this point, calcium carbonate precipitation started without the induction time. The variation of the calcium concentration in the supersaturated solutions, concomitant with the precipitation of calcium carbonate, as a function of time, is shown in Figure 11.
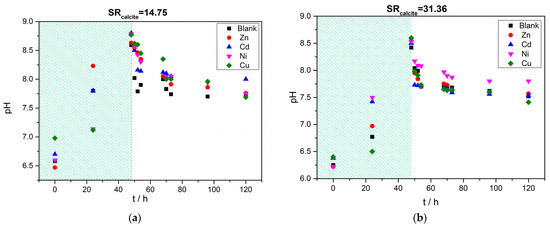
Figure 10.
Precipitation of calcium carbonate in supersaturated solutions in the presence of 10 μM of Zn ( ), Cd(
), Cd( ), Ni (
), Ni ( ), Cu (
), Cu ( ), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd(
), Cd( ), Ni (
), Ni ( ), Cu (
), Cu ( ), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
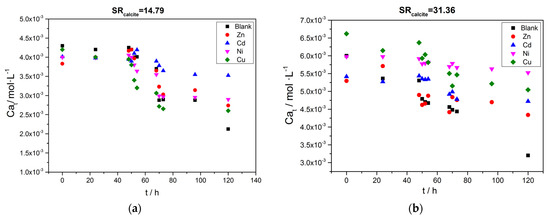
Figure 11.
Precipitation of calcium carbonate in supersaturated solutions in the presence of 10 μM of Zn ( ), Cd (
), Cd ( ), Ni (
), Ni ( ), Cu(
), Cu( ); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd (
), Cd ( ), Ni (
), Ni ( ), Cu(
), Cu( ); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
The continuous increase in total chlorophyll concentration in the solutions, shown in Figure 12, confirmed the ongoing growth of the A. obliquus culture in the supersaturated solutions. The solution supersaturation did not have any significant effect on the growth of the microalgae within the range tested in the present work.
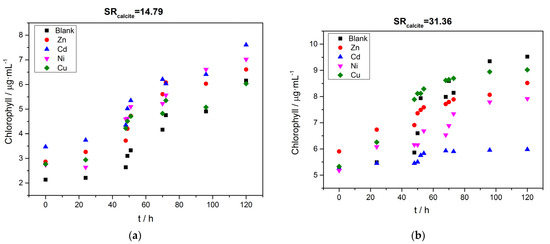
Figure 12.
Total chlorophyll concentration in the supersaturated solutions as a function of time during the precipitation of calcium carbonate in the presence of 10 μM of Zn ( ), Cd (
), Cd ( ), Ni (
), Ni ( ) and Cu (
) and Cu ( ), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd (
), Cd ( ), Ni (
), Ni ( ) and Cu (
) and Cu ( ), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
The growth rates of the A. obliquus culture, calculated, as described before, from logarithmic plots of the chlorophyll concentration produced by growing A. obliquus culture as a function of time, in both the presence and absence of the metals investigated are summarized in Table 6.

Table 6.
Precipitation of calcium carbonate in supersaturated solutions in the presence of 10 μM of heavy metals (Zn or Cd or Ni or Cu), active A. obliquus culture with modified medium, and illumination. Rate of microalgae development; 25 °C, pH 8.50, 0.15 M of NaCl.
The slowest A. obliquus culture growth rate for the lower supersaturation (SR = 14.79) was the rate measured in the presence of Cu, while the highest rate corresponded to the absence of heavy metals (Blank) (Table 6). On the contrary, in the case of higher supersaturation (SR = 31.36), the slowest A. obliquus culture growth rate was calculated in the presence of Cd. Of these rates, the slowest was the growth rate of the A. obliquus culture in the presence of Cd, in agreement with literature reports, according to which the presence of cadmium decreased the growth rate of the microalgae [29]. The growth rates of A. obliquus cultures in the presence of Zn, Cd, Ni and Cu in the supersaturated solutions were all lower than the corresponding, in their absence, even in the presence of suitable nutrients and illumination.
The rates of calcium carbonate precipitation measured are summarized in Table 7. The inhibition—calculated from the reduction in precipitation rates in the presence of Zn, Cd, Ni and Cu in the supersaturated solutions—is shown as well.

Table 7.
Precipitation of calcium carbonate in supersaturated solutions in the presence of 10 μM of Zn, Cd, Ni and Cu. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination, were included in the supersaturated solutions. Supersaturation ratio with respect to calcite, precipitation rate of calcium carbonate and % inhibition (with reference to the blank); 25 °C, pH 8.50, 0.15 M of NaCl.
As shown from the tabulated values, the rate of calcium carbonate precipitation in the presence of 10 μM of the tested heavy metals decreased. The most significant reduction of the rate of precipitation was measured in the presence of nickel. In particular, the inhibition in the case of nickel, regardless of supersaturation, was between 80 and 90%. The inhibition of the calcium carbonate precipitation in the presence of zinc and cadmium in the supersaturated solutions was significantly high (74% and 66%, respectively).
Measurements of the test heavy metals concentrations in the supersaturated solutions during the course of calcium carbonate precipitation showed that they decreased as a function of time, reaching a plateau equilibrium value, as may be seen from the respective plots shown in Figure 13.
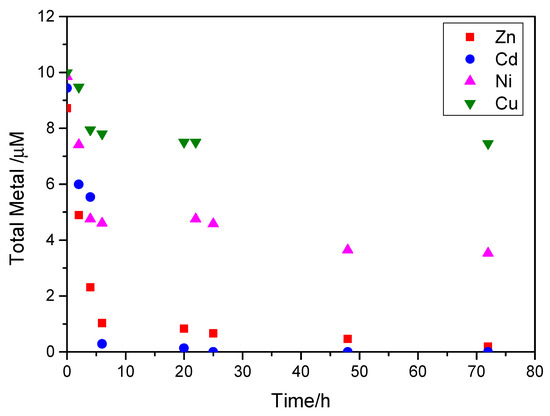
Figure 13.
Concentration of Zn, Cd, Ni and Cd in the supersaturated solutions during precipitation of calcium carbonates, a function of time, in the presence of 20 mL of active A. obliquus culture, under illumination; SRcalcite = 14.79, 25 °C, pH 8.50, 0.15 M of NaCl.
In all cases, after 20 h, the concentration of metals remained stable up to 80 h. The concentrations of zinc and cadmium in the supersaturated solutions decreased by 97% in comparison with their initial value, and the concentration of nickel in the supersaturated solutions decreased by 64%. The smallest change in concentration was in the presence of copper (25%). The reduction in the concentrations of the test metals suggested an uptake from the solid particles suspended in the supersaturated solutions (A. obliquus culture and calcium carbonate precipitated particles). Thermodynamic calculations and a thorough examination of the solids by SEM precluded the formation of any of the metal (Zn, Cd, Ni and Cd) oxides/hydroxides. The different extent of metal-suspended solid particles interactions is associated with the speciation for each metal in the solutions. In the presence of 0.15 M of NaCl, the total dissolved metals are distributed as hydroxo and metal chloride complexes, of which the positively charged were predominant (Figure S1, Supplementary Materials). At pH 8.50 Zn2+ (ca. 55%, with ca. 30% ZnOHCl0 and ca. 10% ZnOH+); CuOH+ (ca. 60% and ca. 30% Cu(OH)20); Ni2+(ca.90%, the rest being NiCl+); and CdCl+(62%, with CdCl20 22% and Cd2+ 15%) are the main species present. The uptake of metals in the form of species present in the supersaturated solutions was responsible for the retardation, because the positively charged ion species poisoned the active sites that were available for the nucleation and growth of calcite crystals. Active sites for the nucleation are the cell membranes (negatively charged because of the ionized -COO- or O- groups), and for the crystal growth the crystal imperfections of the growing crystals [30,31].
The reduction in concentrations of the test metals during the precipitation of calcium carbonate in the supersaturated solutions in the presence of A. obliquus cultures was considered a measure of the removal efficiency of the heavy metals from the solutions that were supersaturated with respect to calcium carbonate. Shown in Figure 14 is the removal efficiency of Zn, Cd, Ni and Cu, which is expressed as % percent of change with respect to their initial concentration in the supersaturated solutions.
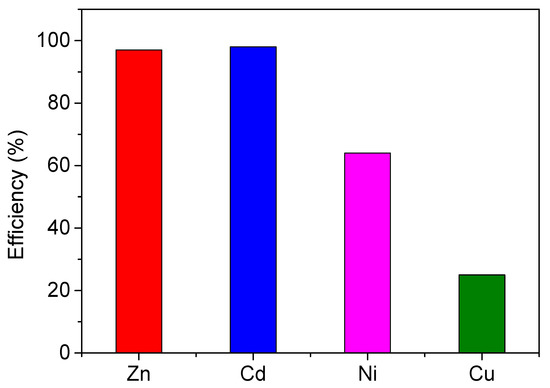
Figure 14.
Heavy metal removal efficiency during the precipitation of calcium carbonate in supersaturated solutions in the presence of 20 mL of active A. obliquus culture; SRcalcite = 14.79, 25 °C, pH 8.50, 0.15 M of NaCl. Initial concentration of test metals, 10 μM.
Cadmium and zinc were strongly taken up by the substrate, with nickel following closely. This resulted in an average reduction in the rate of calcite precipitation by an average of 80 ± 10% for SRcalcite 14.7 and 74 ± 7% for SRcalcite 31.36. The very low Cu uptake corresponded to a similar rate of calcite precipitation reduction of 36% for both SR values. The fact that the suppression of the calcium carbonate precipitation rate was the same or very close for both tested values of SRcalcie, one of which was double in comparison to the other, suggested that the process was dominated by the nucleation of calcium carbonate, which most probably occurred on the A. obliquus matrix. A lower uptake of the metals was associated with lower rates of calcium carbonate precipitation. Zn2+ and Ni2+ as well as CdCl+ (including the significant amount of Cd2+) are easily adsorbed, effectively poisoning the active crystal growth sites, while CuOH+ did not adsorb. It should be noted that at the precipitation conditions the concentration of Cu2+ species was almost negligible, iin contrast with the respective concentrations of the other three metals.
The morphology of the precipitated solids in the presence of heavy metals and active A. obliquus culture was studied with SEM (Figure 15). It has been reported that the presence of heavy metals in the supersaturated calcium carbonate solution stabilizes the thermodynamically unstable phases of calcium carbonate (aragonite and vaterite) [32,33]. In the presence of the active culture of A. obliquus, the organic matrix was expected to stabilize the vaterite [34]. In the present study, however, calcite was the only polymorph identified in the precipitated solids. The calcite particles formed in the presence of zinc and nickel had characteristic pits and a spongy texture. The formation of calcium carbonate crystals of a similar morphology was reported in the presence of water-soluble copolymers, which adsorbed strongly [35]. The formation of porous crystals has been reported in the case of the formation of calcite monocrystals, resulting from the transformation of amorphous calcium carbonate [36]. The presence of the A. obliquus culture in the supersaturated solutions may favor the precipitation of amorphous calcium carbonate at a first stage, which is rapidly transformed to the thermodynamically most stable calcite. Further research is needed in this direction, using methods which do not allow for rapid desupersaturation of the solutions during the precipitation of calcium carbonate. Fast desupersaturation favors the rapid transformation of transient calcium carbonate polymorphs to the thermodynamically most stable polymorph, calcite [37].
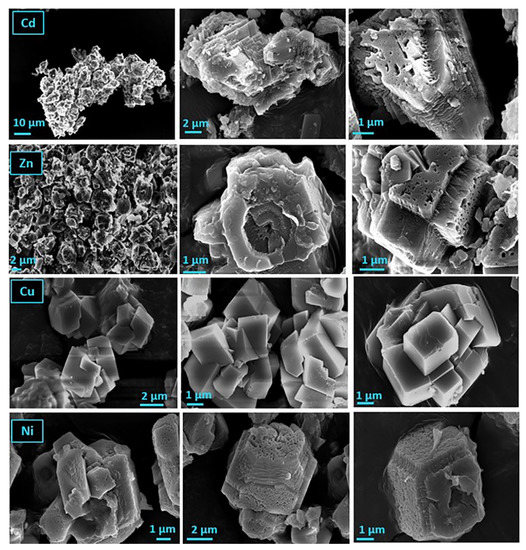
Figure 15.
SEM pictures of calcium carbonate precipitated in supersaturated solutions in the presence of heavy metals (Zn or Cd or Ni or Cu), 20 mL of active A. obliquus culture with modified medium and illumination.; pH 8.5, 25 °C, 0.15 M of NaCl.
3.2. Adsorption
3.2.1. Uptake of Zn, Cd, Ni and Cu by Calcite
The association of the presence of the test HM with the reduction in the rate of calcite precipitation from supersaturated solutions, as well as the significant decrease in their concentration in the solutions during the precipitation, prompted further investigation of the interaction between the precipitating calcite and the metals present in the solutions. First, the uptake of HM by calcite was investigated in the absence of A. obliquus. Assuming that the uptake was due to adsorption on the calcite surface, the experimental data, summarized in Table 8, were fitted to the widely used Langmuir model (Equation (10)).
where Γmax is the surface concentration of each of the adsorbates needed to form a monolayer, and b is a constant related to the free energy of adsorption, ΔGads, through the Equation (11):

Table 8.
Initial concentrations, Co; equilibrium concentration, Ceq; and surface concentration, Γ, for each metal adsorption on calcite. (Ionic strength of 0.15 M of NaCl).
Fitting of the data obtained for the adsorption of Zn, Ni and Cd to the linearized Langmuir isotherm (Equation (10)) gave excellent fit, as may be seen in Figure 16. The surface concentration of the HM on calcite, assuming adsorption, was calculated according to Equation (12):
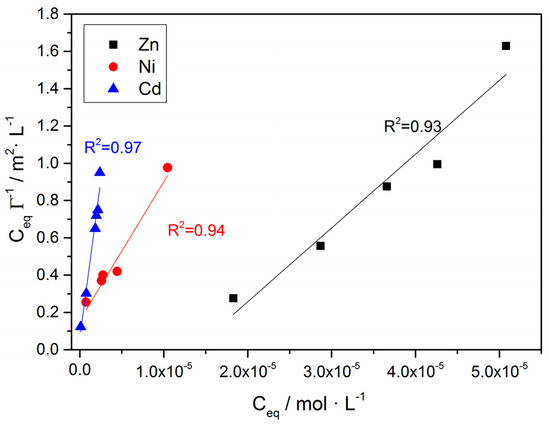
Figure 16.
Fitting of the uptake of Zn, Ni and Cd on calcite in the linearized Langmuir adsorption isotherm (Equation (10)); pH 8.5, 25 °C, 0.15 M of NaC1.
In Equation (12) C0, Ceq are the initial and equilibrium concentrations of the adsorbate, Vs is the volume in which adsorption was measured, m the mass and SSA the specific surface area of the adsorbent. In Table 8, the data obtained for the uptake of Zn, Ni and Cd are summarized. Copper was not included in this analysis because of the low uptake that was measured. It should be noted that the investigation of higher concentrations of this metal was not carried out because of the risk of precipitating several of its hydroxides.
The maximum surface concentrations, Γmax, for complete monolayer coverage of the surface were calculated as equal to 2.53 × 10−5, 1.35 × 10−5 and 2.96 × 10−5 mol·m−2 for zinc, nickel and cadmium, respectively, considering the adsorption of the corresponding hydrated divalent ions flat on the surface.
The removal efficiency of heavy metals by sorption is expressed as follows:
where the initial concentration, Co, and the equilibrium concentration, Ceq, are expressed in mg·L−1. A similar trend was found for Cd and Ni, which showed strong adsorption on calcite. It should be noted that in the respective solutions, the presence of the divalent ions was significant, contrary to Cu, in which the concentration of the divalent species was practically zero because of the formation of hydroxo and chlorocomplexes.
From the comparison shown in the plot of Figure 17, it was concluded that the highest removal efficiency was measured for cadmium (85%).
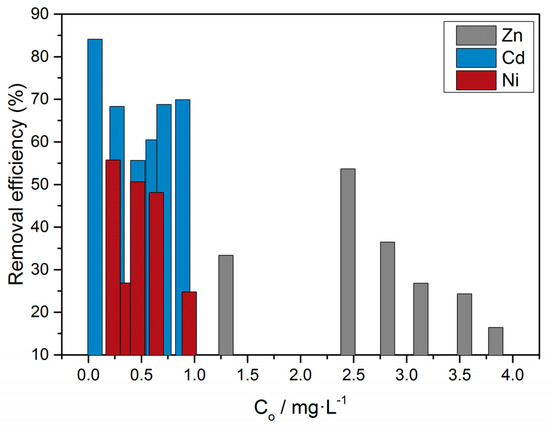
Figure 17.
Removal efficiency from calcite suspensions in aqueous solutions of zinc, nickel and cadmium; 0.15 M of NaC1, pH 8.5, 25 °C.
In Figure 18, SEM photographs of the solids collected at the end of calcite’s equilibration with Zn, Ni and Cd solutions are presented.
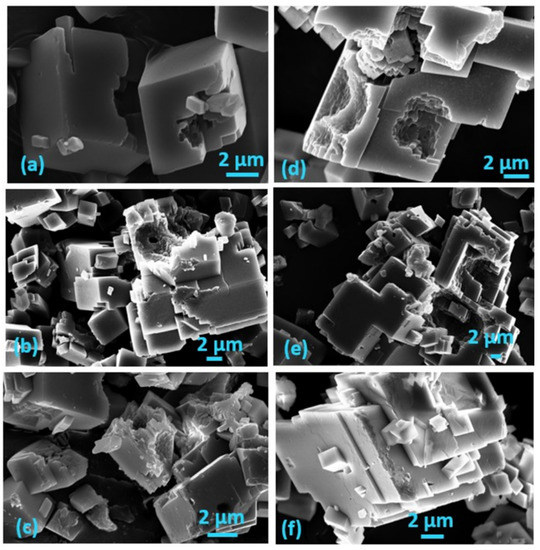
Figure 18.
SEM photographs of calcite following equilibration with solutions containing Zn, Ni and Cd. (a–c) 0.2 µM, (d–f) 10 μM; 25 °C, 0.15 M of NaCl, pH 8.50.
As shown in Figure 18, the size or morphology of the solid remained unchanged, except for minor morphological changes, which mainly consisted in the appearance of pits on the calcite crystals. These pits are indicative of the dissolution of the crystalline solid substrate, which may be the result of the presence of metals, which, in turn, may increase the solubility of the solid because of the formation of surface complexes. A thorough EDS microanalysis of the solids, however, did not show the presence of metals, apparently because their concentration was lower than the detection limit of the analytical instrument (tens of mg per kilogram). No morphological differences were observed, regardless of the metal taken up by the solid. It has been suggested that divalent metal ions including Zn2+, Cd2+ and Ni2+ exchange for Ca2+ ions of calcite [38].
3.2.2. Adsorption of Zinc on Calcite and Microalgae
Adsorption of Zinc on Calcite in Presence of Metabolically Active A. obliquus Culture
Since the presence of the study metals in solutions showed that they are responsible for retarding the growth of A. obliquus cultures (Table 2 and Figure 3), as an example, the effect of the presence of metabolically active A. obliquus culture on the uptake of zinc at equilibrium with calcite suspensions was examined.
Upon equilibration of the suspensions of the calcite crystals with the zinc solutions, in the presence of metabolically active A. obliquus culture, the initial concentration of total zinc in the solution decreased as a function of time, as shown from the data summarized in Table 9 and in Figure 19. The surface concentration of the zinc on the calcite crystals was calculated from Equation (12).

Table 9.
Initial concentration, Co, equilibrium concentration, Ceq, and surface concentration, Γ, for the adsorption of zinc onto calcite crystals in its saturated solutions in the presence of 5 mL of metabolically active A. obliquus culture; 25 °C, pH 8.50, 0.15 M of NaCl.
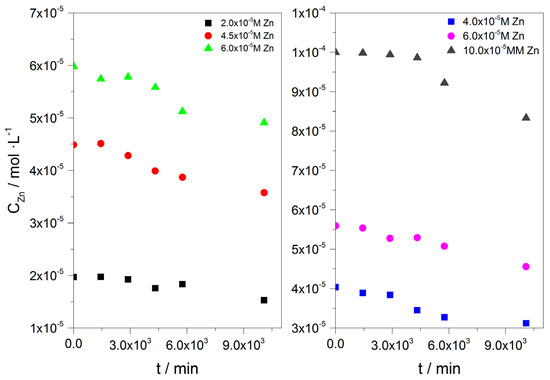
Figure 19.
Zinc (II) concentration as a function of time during the uptake of zinc on calcite crystals in its saturated solutions, in the presence of 5 ml of A. obliquus culture; 25 °C, pH 8.50, 0.15 M of NaC1.
The concentration of zinc tended to decrease over the 7 days equilibration period. However, equilibrium appears to be approached much more slowly in comparison with the adsorption of zinc on calcite in the absence of A. obliquus culture.
Adsorption of Zinc on Calcite and A. obliquus Culture
Next, the adsorption of zinc was studied on calcite with A. obliquus culture grown on it. Moreover, zinc adsorption was investigated on calcite deposited on air-dried (25 °C) A. obliquus culture. The initial concentrations selected for the study were lower to limit the possibility of zinc oxides or other solids undergoing precipitation. The adsorption conditions of zinc on the test adsorbents are summarized in Table 10.

Table 10.
Zinc adsorption in solutions saturated with respect to calcite on different substrates. Substrate, initial zinc concentration and the corresponding substrate solution code name. Temperature of 25 °C, pH 8.50, 0.15 M of NaCl.
“Blank” suspensions, in which only the substrate and electrolyte solution were present, were used for the solid’s characterization by powder XRD.
Zinc concentration measurements (Figure S2) showed that there was no significant change in the zinc concentration, suggesting that zinc was not adsorbed on A. obliquus cultures grown on calcite. It is possible that the active adsorption sites of the substrate were poisoned from the microalgae grown on the flat faces of the rhombohedral crystals. The solid that was collected after equilibration with the zinc solutions consisted exclusively of calcite, according to the XRD analysis.
On the contrary, the equilibration of calcite crystals precipitated on dried A. obliquus culture (specific surface area 2.2 m2·g−1) in the presence of zinc resulted in the reduction in the zinc concentration as a function of time (Figure S3). The equilibrium concentration of zinc and the corresponding initial concentrations, as well as the surface concentration of zinc on the substrate, was calculated (Table S1).
Adsorption of Zinc on Dried (25 °C) A. obliquus Cultures
The last substrate tested for zinc uptake was A. obliquus culture dried at 25 °C. The specific surface area of this solid was 2.64 m2·g−1. Zinc uptake on dried A. obliquus cultures) was significant (Figure S4), and the surface concentration, Γ, of the sorbent per unit area of the sorbent was calculated (Table S2).
The isotherms for the uptake of Zn on calcite grown on air-dried A. obliquus culture and on dried A. obliquus culture are shown in Figure 20.
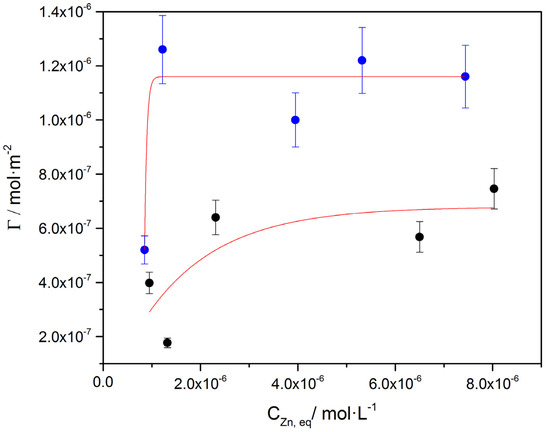
Figure 20.
Uptake of zinc on calcite grown on air-dried A. obliquus culture ( ) and on air-dried A. obliquus culture (
) and on air-dried A. obliquus culture ( ); pH 8.50, 0.15 M of NaCl, 25 °C.
); pH 8.50, 0.15 M of NaCl, 25 °C.
 ) and on air-dried A. obliquus culture (
) and on air-dried A. obliquus culture ( ); pH 8.50, 0.15 M of NaCl, 25 °C.
); pH 8.50, 0.15 M of NaCl, 25 °C.
As shown from the isotherms for zinc uptake, a plateau value is attained for the surface concentration, which corresponds to the limiting adsorption of zinc in each of the substrates. A higher surface concentration was found for the case of active culture. Drying at room conditions—possibly because of membrane damage accompanied by conformational changes—was responsible for this lower limiting surface uptake. It is interesting to note that the data of the above isotherms gave a satisfactory fit to the widely applied Langmuir model, the validity of which, in our case, is questionable. The uptake isotherms shown in Figure 20 suggested the higher affinity (steeper initial segment of the isotherm) of the adsorbate for calcite grown on air-dried A. obliquus culture.
The comparison of the test substrates (Figure 21) with respect to the removal of zinc from aqueous solutions (Equation (13)) showed that the sorbent calcite grown on air-dried microalgae had the highest efficiency, which even reached 70% of the initial concentration, for the lowest zinc concentrations up to 2·10−5 M. At higher zinc concentrations in the solutions (>4·10−5 M), calcite had the highest efficiency (~50%) as a sorbent.
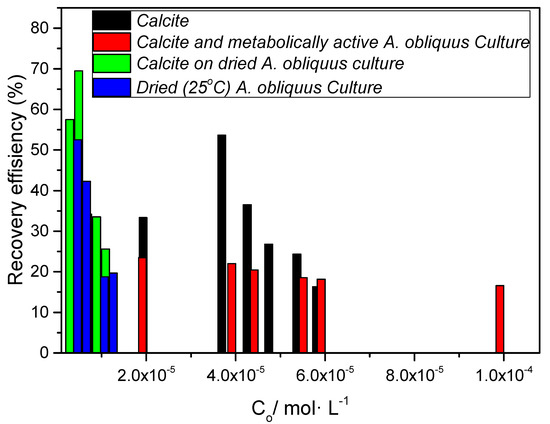
Figure 21.
Zinc adsorption efficiency on various substrates; pH 8.5, 25 °C, 0.15 M of NaC1.
The results of the calcium carbonate precipitation in the presence of A. obliquus active culture in the presence of Ni, Zn, Cd and Cu show that, on the one hand, the formation of calcium carbonate was accelerated; on the other hand, they show that the formation of the solid favored the removal of the metals from the respective supersaturated solutions. In chloride-containing aqueous solutions, it seems that copper removal is low, possibly because the concentration of the free divalent metal species is negligible. The important implication of these findings is that the precipitation of calcium carbonate that is enhanced in the presence of metabolically active microalgae can contribute to the decontamination of natural water systems from heavy metals through the uptake mechanism, which may be described by the Langmuir model.
4. Conclusions
The presence of metabolically active A. obliquus culture in supersaturated solutions with respect to calcium carbonate increased the rate of precipitation. The metabolically inactive (air-dried) culture of A. obliquus, which was grown on calcite crystals, exhibited a lower acceleration of calcium carbonate precipitation, showing that the conformation of microalga cell membranes is important. The presence of Zn, Ni, Cd and Cu in the supersaturated solutions significantly reduced the rates of calcium carbonate precipitation). Similar behavior, i.e., a reduction in the rates of precipitation in the presence of metabolically active A. obliquus, was found in the presence of Zn, Ni, Cd and Cu at concentration levels, which precluded the formation of hydroxide solid precipitates (10 μM). The trend for the reduction in the rates of calcium carbonate precipitation was Cd > Zn > Ni > Cu. The decrease in the rates of precipitation was associated with the uptake of positively charged ion species, especially with the +2 free metal ions on the A. obliquus culture and on the calcium carbonate crystals forming in the supersaturated solutions. The uptake of Zn, Ni and Cd fitted well in the Langmuir isotherm. All the precipitates consisted of calcite, although the formation of transient calcium carbonate phases cannot be precluded. More detailed studies in carefully controlled supersaturation conditions are needed to clarify this issue. All the metals were transferred from the solution to the solid substrate upon equilibration with calcite, air-dried A. obliquus culture, and calcite that was grown on air-dried A. obliquus culture. The presence of calcite dominated in the metals uptake in the order Ni > Zn > Cd. The highest uptakes of zinc from solutions with concentrations up to 2·10−5 M were found for calcite that was grown on air-dried A. obliquus culture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12101424/s1. Figure S1: Speciation of metals studied in the present work. 0.15 M NaCl, 10 μM Metal, 25 °C; a: Zn, b: Cu, c: Ni, d: Cd. Figure S2. Zinc concentration in solutions equilibrated with suspensions of AO culture grown on calcite as a function of time 25 °C, 0.15 M NaCl, pH = 8.50. Figure S3. Zinc (II) concentration as a function of time for zinc adsorption on calcite, precipitated in a dried AO culture; pH = 8.50, 25 °C, 0.15 M NaCl. Figure S4 Zinc concentration as a function of time during equilibration with suspensions of air-dried (25 °C) AO culture; 25 °C, 0.15 M NaCl, pH = 8.50. Table S1. Initial concentration, Co, equilibrium concentration, Ceq, and surface concentration, Γ for zinc adsorption calcite, precipitated in a dried AO culture (sorbent); 25 °C, 0.15 M NaCl, pH = 8.50. Table S2. Initial concentration, Co, equilibrium concentration, Ceq, and surface concentration, Γ for sorption of zinc in suspensions of air-dried (25 °C) microalgae culture; 25 °C, 0.15 M NaCl, pH = 8.50.
Author Contributions
P.D.N. was involved with the experimental investigation, methodology, data curation and writing-original draft preparation. P.G.K. was involved in conceptualization, supervi-sion, ensuring resources and writing and editing of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding by the General Secretariat for Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI) in the context of the action “1st Proclamation of Scholarships from HFRI for PhD Candidates” Contract No. 1576.
Data Availability Statement
Not Applicable.
Acknowledgments
This work was supported by the General Secretariat for Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI) in the context of the action “1st Proclamation of Scholarships from HFRI for PhD Candidates”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herrera-Estrella, L.R.; Guevara-Garcia, A.A. Heavy Metal Adaptation. eLS Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–9. [Google Scholar]
- Gaur, A.; Adholeya, A. Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr. Sci. 2004, 86, 528–534. [Google Scholar]
- Hafeburg, G.; Kohe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2929. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, H.M.; Bae, J.S.; Ha, M.G.; Jin, J.S.; Hong, T.E.; Kim, J.P.; Jeong, E.D. Removal of heavy metal ions by using calcium carbonate extracted from starfish treated by protease and amylase. J. Anal. Sci. Technol. 2011, 2, 75–82. [Google Scholar] [CrossRef]
- Olade, M.A. Heavy Metal Pollution and the Need for Monitoring: Illustrated for Developing Countries in West Africa. In Lead, Mercury, Cadmium and Arsenic in the Environment; Hutchinson, T.C., Meema, K.M., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1987; pp. 335–341. [Google Scholar]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Freitas, O.M.M.; Martins, R.J.E.; Delerue-Matos, C.M.; Boaventura, R.A.R. Removal of Cd(II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: Kinetic modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wilde, W.E.; Benemann, J.R. Bioremoval of heavy metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The effect of heavy metals on the viability of Tetraselmis marina AC16-MESO and an evaluation of the potential use of this microalga in bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef]
- Santomauro, G.; Baier, J.; Huang, W.; Pezold, S.; Bill, J. Formation of Calcium Carbonate Polymorphs Induced by Living Microalgae. J. Biomater. Nanobiotechnol. 2012, 3, 413–420. [Google Scholar] [CrossRef]
- Chin, Z.W.; Arumugam, K.; Ashari, S.E.; Faizal Wong, F.W.; Tan, J.S.; Ariff, A.B.; Mohamed, M.S. Enhancement of Biomass and Calcium Carbonate Biomineralization of Chlorella vulgaris through Plackett–Burman Screening and Box–Behnken Optimization Approach. Molecules 2020, 25, 3416. [Google Scholar] [CrossRef]
- Mazzone, E.J.; Guentzel, J.L.; Olaizola, M. Carbon removal through algal mediated precipitation of calcium carbonate. Mar. Sci. 2002, 223, 537–538. [Google Scholar]
- Xu, P.; Fan, H.; Leng, L.; Fan, L.; Liu, S.; Chen, P.; Zhou, W. Feasibility of microbially induced carbonate precipitation through a Chlorella-Sporosaricina co-culture system. Algal Res. 2020, 47, 101831. [Google Scholar] [CrossRef]
- Heath, C.R.; Leadbeater, B.C.S.; Callow, M.E. Effect of inhibitors on calcium carbonate deposition mediated by freshwater algae. J. Appl. Phycol. 1995, 7, 367–380. [Google Scholar] [CrossRef]
- Irfan, M.F.; Hossain, S.M.Z.; Khalid, H.; Sadaf, F.; Al-Thawadi, S.; Alshater, A.; Hossain, M.M.; Razzak, S.A. Optimization of bio-cement production from cement kiln dust using microalgae. Biotechnol. Rep. 2019, 23, e00356. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Algal Culturing Techniques. In Physiological Society of America; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Reston, VA, USA, 2013; Chapter A43; p. 497. [Google Scholar]
- Cavalletti, E.; Romano, G.; Palma Esposito, P.; Barra, L.; Chiaiese, P.; Balzano, S.; Angela, S. Copper Effect on Microalgae: Toxicity and Bioremediation Strategies. Toxics 2022, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.D.; Santhanam, P.; Ananth, S.; Shenbaga Devi, A.; Nandakumar, R.; Balaji Prasath, B.; Jeyanthi, S.; Jayalakshmi, T.; Ananth, P. Effect of different dosages of zinc on the growth and biomass in five marine microalgae. Int. J. Fish. Aquac. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Fonseca, S.C.; Castro, P.M.L. Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J. Appl. Phycol. 2011, 23, 97–103. [Google Scholar] [CrossRef]
- Ghizellaoui, S.; Euvrard, M. Assessing the effect of zinc on the crystallization of calcium carbonate. Desalination 2008, 220, 394–402. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef]
- Dalas, E.; Kallitsis, J.; Koutsoukos, P.G. The growth of sparingly soluble salts on polymeric substrates. Colloids Surf. 1991, 53, 197–208. [Google Scholar] [CrossRef]
- Giannimaras, E.K.; Koutsoukos, P.G. Precipitation of Calcium Carbonate in Aqueous Solutions in the Presence of Oxalate Anions. Langmuir 1988, 4, 855–861. [Google Scholar] [CrossRef]
- Wada, N.; Yamashita, K.; Umegaki, T. Effects of divalent cations upon nucleation, growth and transformation of calcium carbonate polymorphs under conditions of double diffusion. J. Cryst. Growth 1995, 148, 297–304. [Google Scholar] [CrossRef]
- Kitano, Y.; Kanamori, N.; Yoshioka, S. Adsorption of zinc and copper ions on calcite and aragonite and its influence on the transformation of aragonite to calcite. Geochem. J. 1976, 10, 175–179. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, C.; Jimenez-Lopez, C.; Rodríguez-Navarro, A.; Gonzalez-Munoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralizarion of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.; Yu, S.H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. CrystEngComm 2011, 13, 2054. [Google Scholar] [CrossRef]
- Aizenberg, J.; Muller, D.A.; Grazul, J.L.; Haman, D.R. Direct Fabrication of Large Micropatterned Single Crystals. Science 2003, 299, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Spanos, N.; Koutsoukos, P.G. Kinetics of Precipitation of Calcium Carbonate in Alkaline pH at Constant Supersaturation. Spontaneous and Seeded Growth. J. Phys. Chem. B 1998, 102, 6679–6684. [Google Scholar] [CrossRef]
- Zachara, J.M.; Cowan, C.E.; Retsch, C.T. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 1991, 55, 1549–1562. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).