1. Introduction
With the increasing depletion of fossil fuels and tremendous environmental pollution, hydrogen energy is extensively concerned as it is a clean, highly efficient and renewable energy alternative [
1,
2,
3,
4,
5,
6]. Electrochemical water splitting is considered to be one of the most promising large-scale hydrogen production methods. However, the industrialization of water electrolysis is still limited due to the lack of cheap and efficient catalysts [
7,
8,
9,
10]. Among the numerous catalysts, transition-metal phosphides with high conductivity, chemical stability, special crystal structure and abundant valence state have been intensively focused. To improve the electrocatalytic performances, an important strategy is increasing the number of active sites by means of generating a larger specific surface area [
11,
12,
13,
14]. Besides, three dimension (3D) basal electrodes, for example, carbon cloth has excellent conductivity, mechanical properties and larger specific surface area [
15]. At present, Pt metal is regarded to be the most efficient electrocatalyst for hydrogen evolution reactions [
9]. However, the resource of Pt is scarce and expensive. In this report, the hyperdispersed Pt nanoparticles for the sake of modifying transition-metal phosphides film is designed and fabricated in order to utilize the catalytic performance of Pt and reduce Pt dosage at the same time. The 3D FeP-Pt film was grown on conductive carbon cloth (CC) by means of hydrothermal methods with high temperature phosphating and electro-deposition [
5,
7,
8]. The results show FeP-Pt/CC has excellent electrocatalytic performances for HER at all pH and has excellent durability and long term stability.
2. Experimental
Firstly, the CC matrix with an area of 1 cm × 1 cm is hydrothermally treated in 68% HNO3 solution at 120 °C, and then it is washed by deionized water and electrochemically oxidized for 30 mins in NaCl solution. The solution of Ferric nitrate, ammonium fluoride and carbamide was prepared in a reactor, and then the treated CC matrix was placed in the solution at 120 °C for 12 h. Iron compound film attached to carbon cloth obtained after cooled and washed. The iron compound film was then dried at 100 °C and subjected to sintering in succession at 300–350 °C for 2–3 h in a tube furnace with argon and sodium hypophosphite to obtain the FeP/CC. Finally, the Platinum nanoparticles were prepared on the FeP/CC by electrodeposition on FeP/CC, and then the 3D FeP-Pt/CC was obtained. It is electrodeposited for 2 h under the voltage of −0.6–−0.7 V in the solution of potassium chloroplatinate and a small amount of boric acid.
The X-ray diffraction (XRD) measurements were carried out by using a Dandong DX-2700B diffractometer with CuKa radiation (λ = 1.5418 Å). The scanning electron microscopy (SEM) images were obtained by scanning electron microscopy (VEGA3, TESCAN, Brno, South Moravia, Czech Republic). The X-ray photoelectron spectroscopes (XPS) were carried out (ESCALAB 250Xi, Al Ka, 150 W, Waltham, MA, USA) to examine the chemical composition and valence state of the as-prepared samples. The electrochemical measurements were performed with a three-electrode system in 0.5 M H2SO4, 1 M PBS(Phosphate buffer solution), 1 M KOH solution using a princeton electrochemical workstation. A saturated calomel electrode (SCE) and a graphite rod were used as the reference electrode and counter electrode, respectively in acidic and neutral solutions. Hg/HgO is used as reference electrodes in alkaline solution. The as-prepared FeP-Pt/CC was used as the working electrode. The linear scan voltammetry (LSV) measurements were carried at a scanning rate of 5 mVs−1. The electrochemical impedance spectra (EIS) were obtained at −200 mV vs reference electrode with a frequency range from 0.1 Hz to 100,000 Hz, 5 mg 20% Pt/C catalyst, add 100 μL 5w% Nafion solution and 900 μL ethanol and mix with ultrasound for at least 30 min; 1 μL of solution suck each time and drop it onto the glassy carbon electrode (with a diameter of 3 mm), drop it again after drying, and until the loading capacity is 0.212 mg/cm2. All the measurements were corrected using iR compensation.
3. Results and Discussion
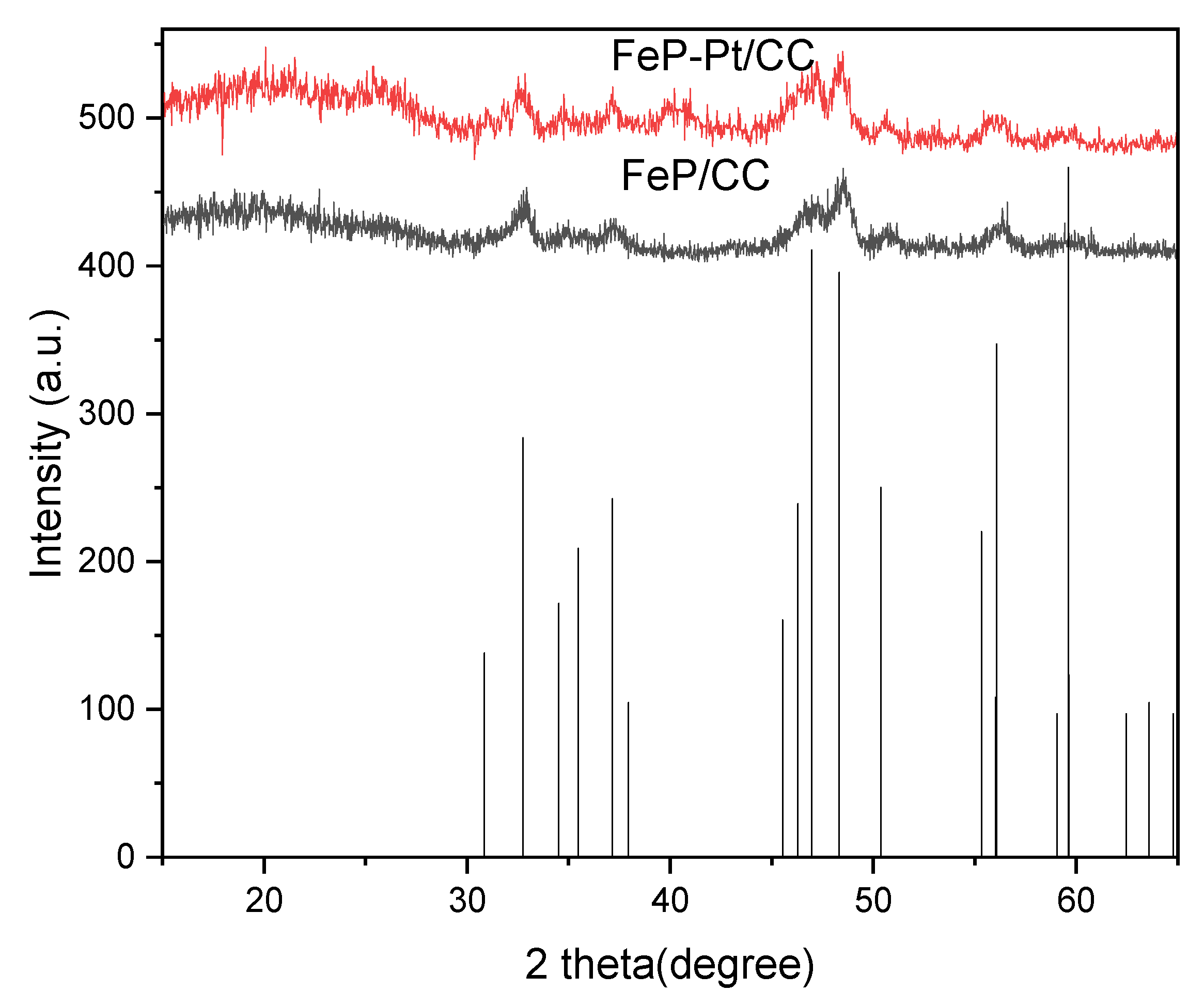
The XRD patterns of the FeP-Pt/CC and FeP/CC are shown in
Figure 1. It can be seen from
Figure 1 that all the samples before and after electro-deposition (FeP/CC and FeP-Pt/CC) can be well indexed as the orthorhombic FeP (JCPDS No. 390809) phase. It is evidenced that the FeP was well prepared. It can be seen from the figure that after the electrodeposition of platinum, the crystallinity and purity of the film decreased. In addition, the diffraction peaks of Platinum shown in the XRD pattern of FeP-Pt/CC in
Figure 1 at 39.77°, 46.26° indicate that there has Pt metal in FeP-Pt/CC.
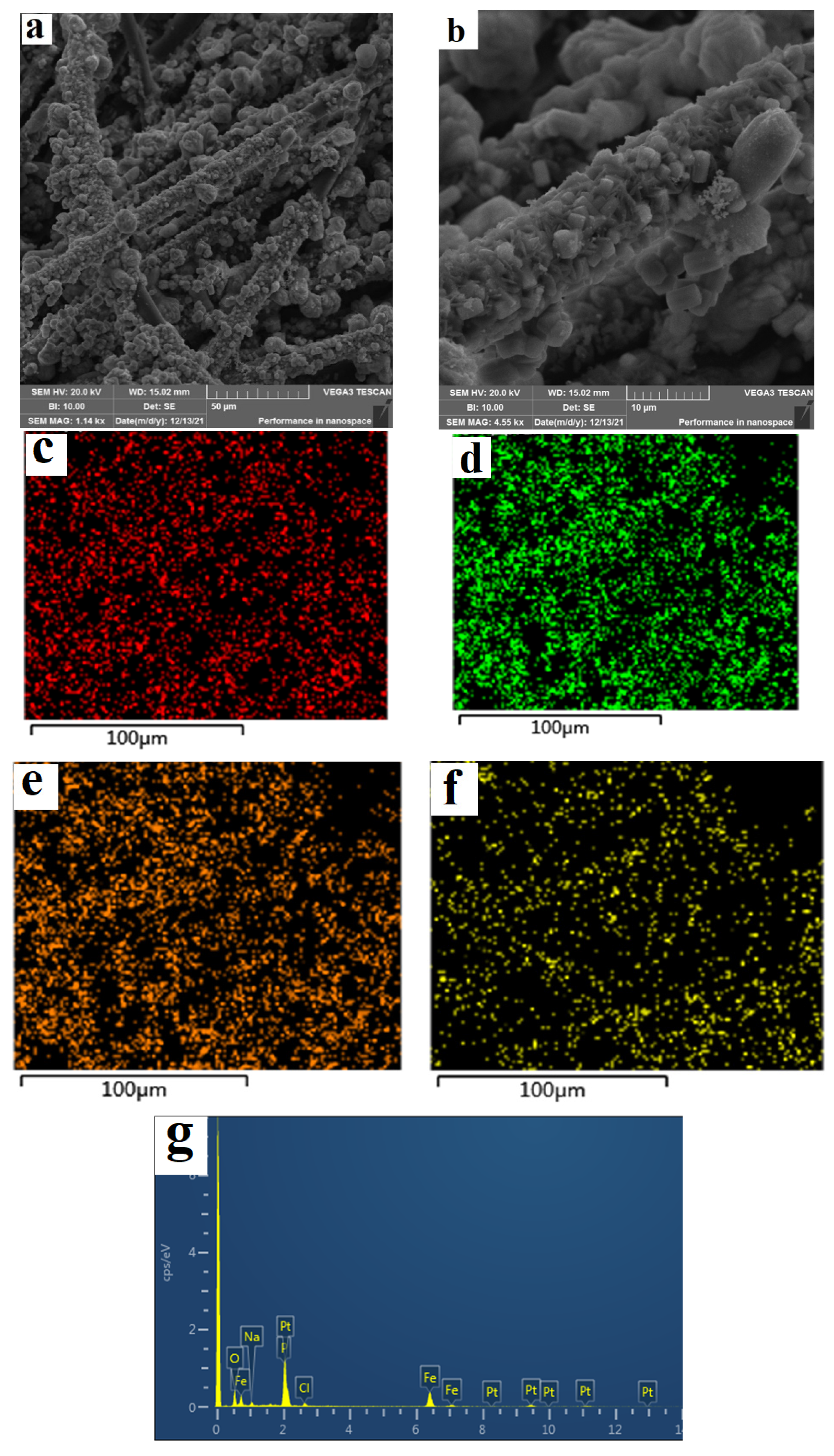
The SEM and edx mapping images of the FeP-Pt/CC and FeP/CC films are shown in
Figure 2 and
Figure 3. It can be seen from the scanning electron microscope that the surface grains of the film before electrodeposition are mainly composed of rods with a diameter of less than 1 micron, and massive grains with a size of several microns appear after electrodeposition. According to the EDX diagram, the molar ratio of Fe:P:Pt is about 16.45:18.9:4.87 and there are some impurities of NaCl. According to EDX mapping photos, Fe,P,Pt has similar element distribution, indicating the dispersion uniformity of Pt, and a small amount of oxidation points can be seen. It can also be seen from
Figure 3 that there have not been Pt elements in the FeP/CC particles and the molar ratio of Fe:P is 18.58:25.76. It is evidenced that the FeP-Pt/CC was successfully obtained.
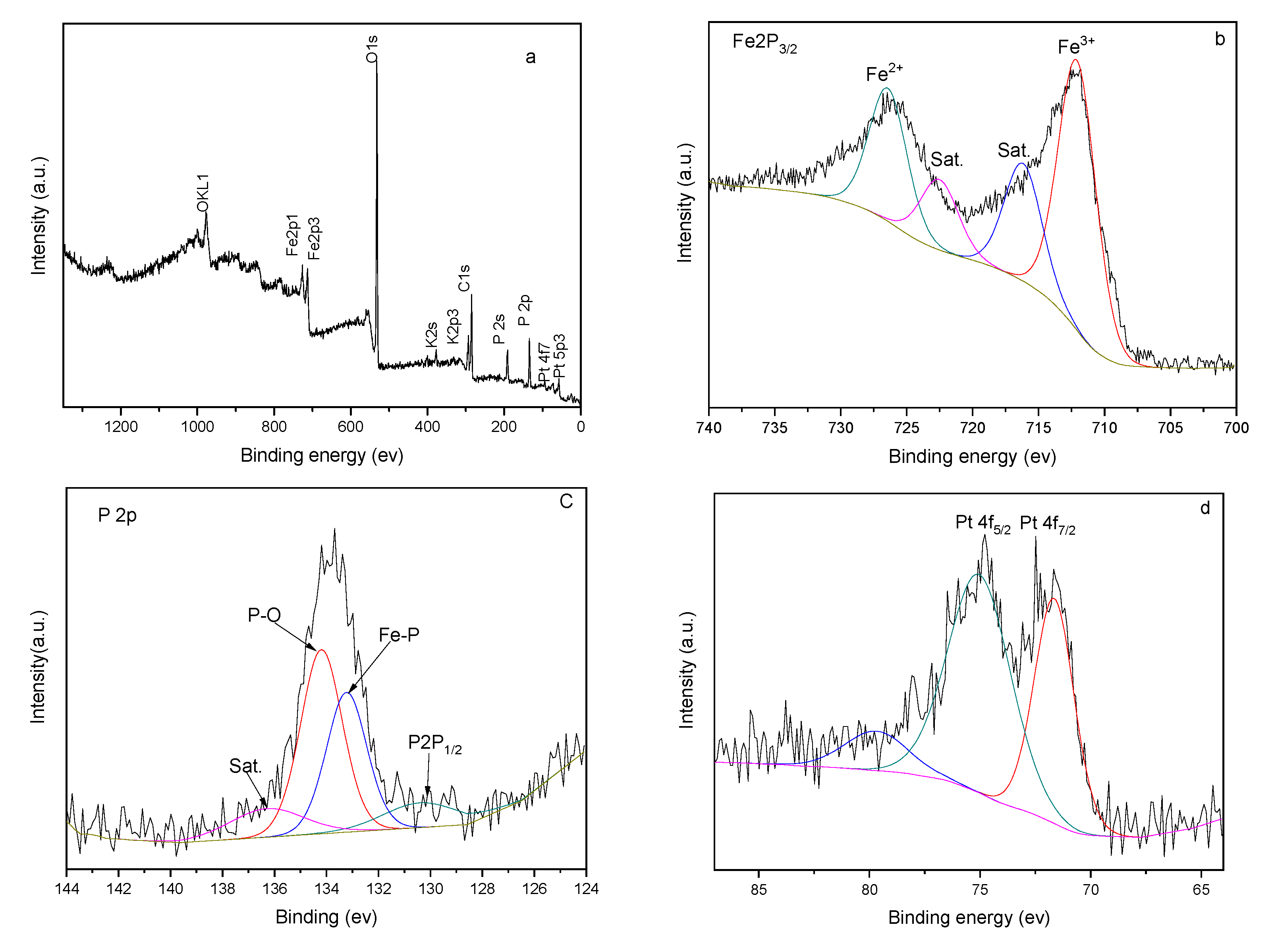
In order to further verify the composition of the FeP-Pt/CC film surface, the X-ray photoelectron spectroscopy (XPS) survey is used.
Figure 4 presents the XPS results of the FeP-Pt/CC. As shown in the figure, the total spectrum suggests the co-existence of Carbon, Oxygen, Ferrum, Phosphorus and Platinum element in the material. The Fe 2p
3/2 corresponds to peak at 712 eV and the Fe2p
1/2 corresponds to peak at 726 eV. The P 2p corresponds to one peak at 134 eV and P 2s corresponds to the peak at 192 eV; 71.6 eV and 75.16 eV of the peak corresponds to zero valences of Pt. Besides, the C 1s and O 1s correspond to the peaks at 280 eV and 531.9 eV, respectively. The OKL1 and OKL2 correspond to peaks at 990 eV and 1000 eV. It is well evidenced that the FeP-Pt/CC was successfully fabricated with a small amount of precious Pt.
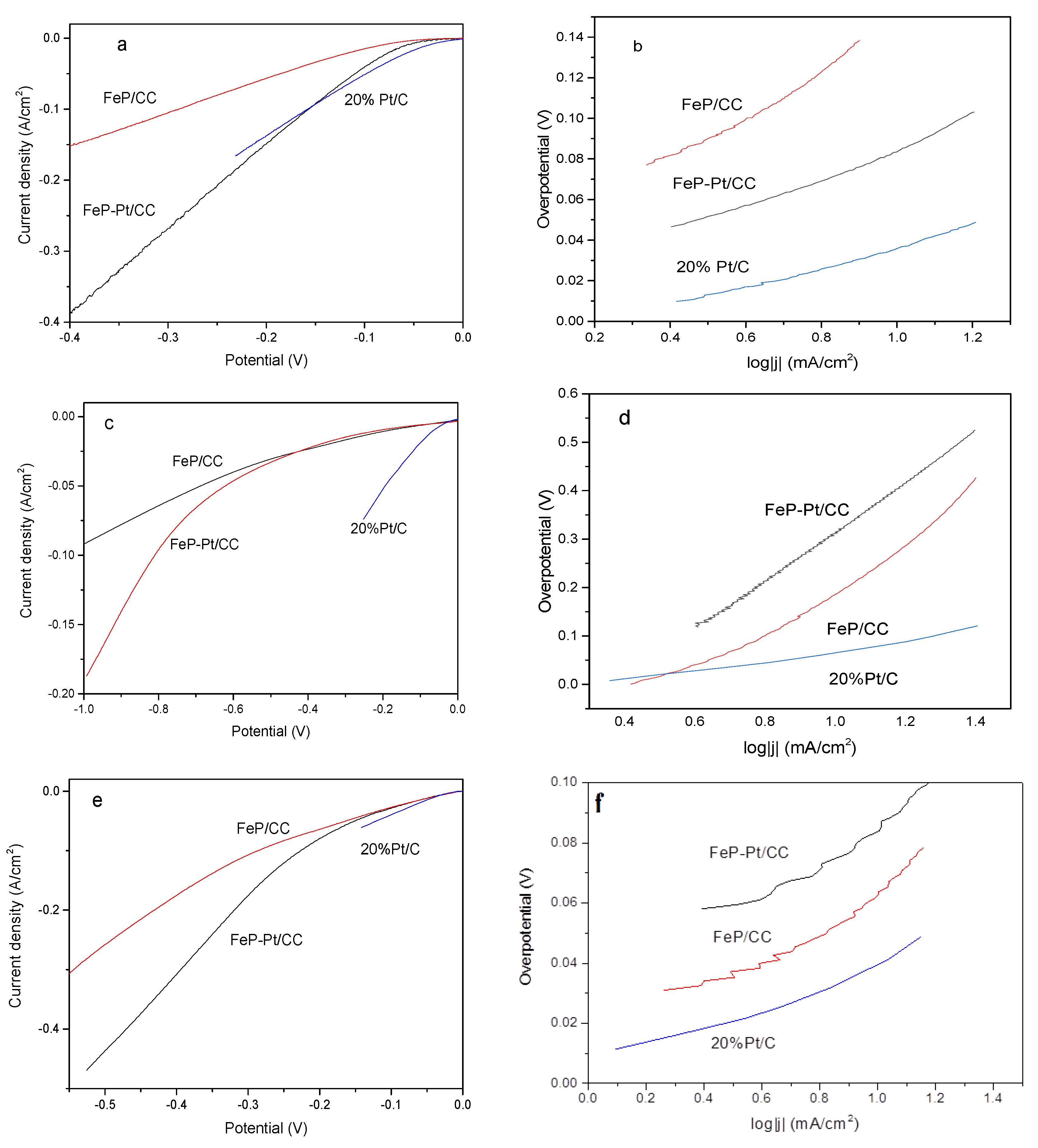
The electrocatalytic performances of FeP-Pt/CC and FeP/CC were investigated by a Princeton electrochemical workstation. All HER measurements were carried out at 25 °C. The results are given in
Figure 5. It can be seen from
Figure 5a that FeP-Pt/CC and FeP/CC possesses over-potentials of −58 mV and −110 mV reach the cathode current density of 10 mA·cm
−2 in 0.5 M H
2SO
4, while 20% Pt/C has the smallest η
10 (−36 mV). Their corresponding Tafel slope is 49.6, 70.6, 108.6 mV/dec. Tafel slopes suggest that the Volmer reaction is fast and the rate-limiting step is the Heyrovsky reaction. It shows that the addition of platinum greatly improves the hydrogen evolution performance of FeP. However, in the phosphate buffer solution, the hydrogen evolution performance is poor, η
10 of FeP-Pt/CC, FeP/CC and Pt/C is −214, −187, −60 mV, respectively. The corresponding Tafel slope are 256, 510, 105.5 mV/dec. At a higher potential, platinum can improve the HER performance of FeP greatly, even more than 20% Pt/C. A neutral-effective electrocatalyst has apparently the best benefit of environmental benignity and very broad application prospects. Alkaline-efficient electrocatalysts are the most important and widely used technology in the industry. From
Figure 5e, we can find that 20%Pt/C exhibits excellent electrocatalytic activity in 1 m KOH. η
10 of FeP-Pt/CC and FeP/CC are −42.6, −44 mV, respectively. The corresponding Tafel slopes are 80, 60.5 mV/dec. LSV of FeP-Pt/CC has been very close to Pt/C catalyst. It shows that FeP-Pt/CC has a good application prospect.
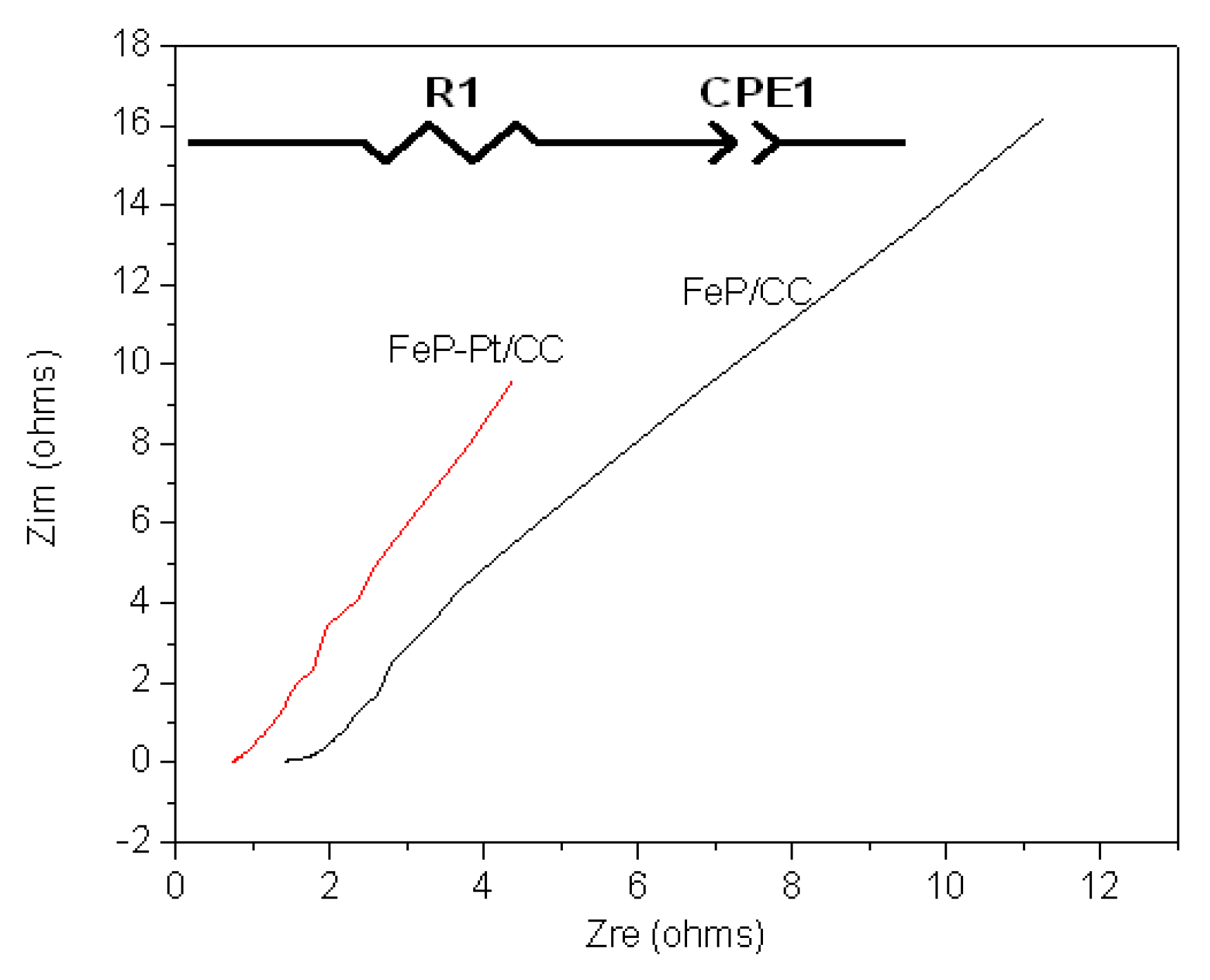
To further investigate the electrocatalytic performances of the materials, electrochemical impedance spectroscopy is also carried out.
Figure 6 shows the results of Nyquist plots of FeP-Pt/CC and FeP/CC at −0.2 V in 0.5 M H
2SO
4.
Table 1 shows the element values in the equivalent circuit of the AC impedance spectrum. It can be found from
Figure 6 that the Nyquist plot shows a line with an angle of inclination of 45°, which suggests the phase angle of reactive ions concentration fluctuation on the electrode surface is 45 degrees lags behind the AC current. In addition, it can be seen from
Table 1 that the R1 (reactive resistance) of FeP-Pt/CC is 0.75 Ω, which is very much less than that of FeP/CC (1.6 Ω). These features indicate that the electrode reaction is completely controlled by the diffusion step. It is estimated that the reason is Platinum particles reduce the reactive resistance and make the surface rougher.
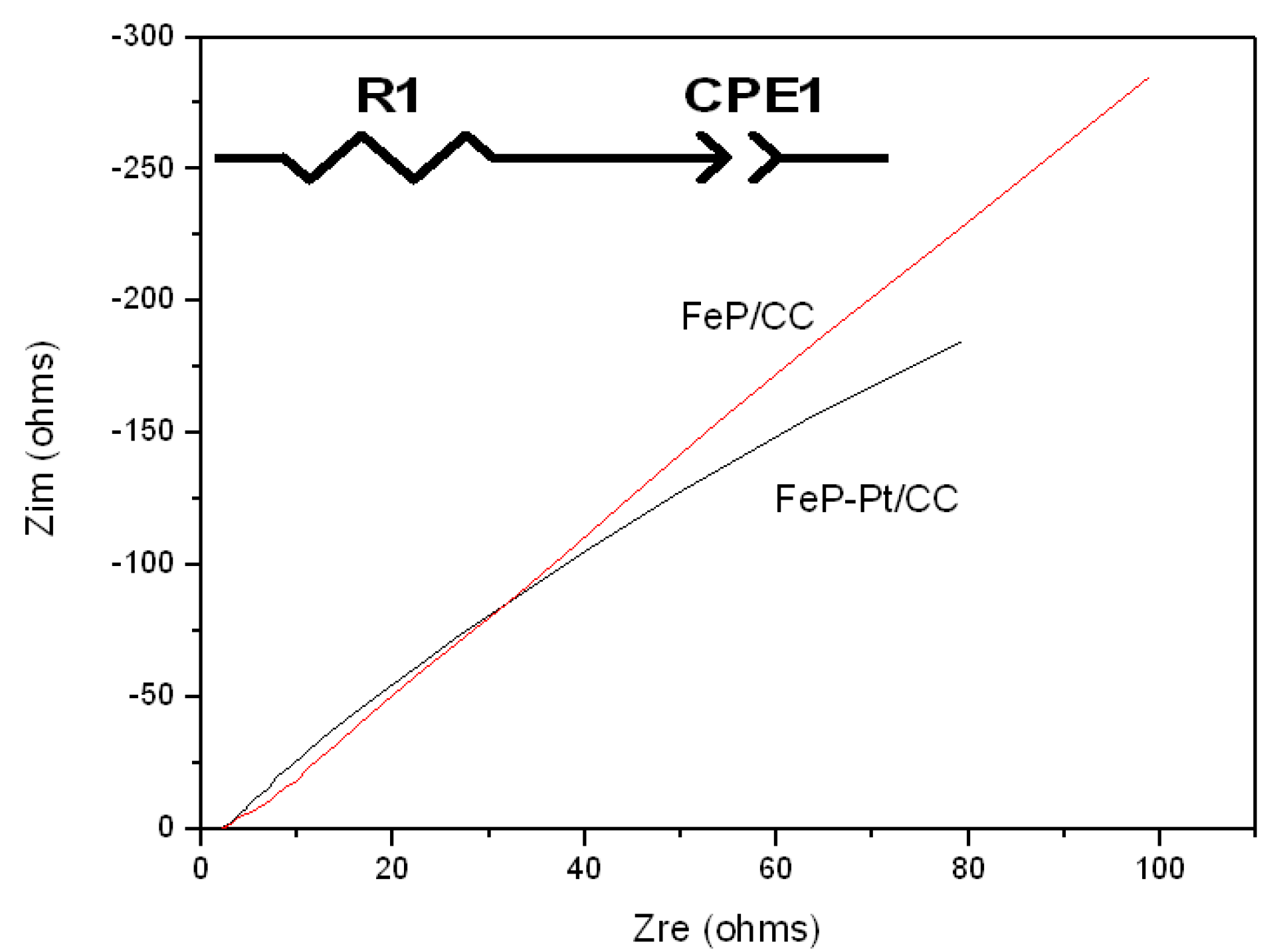
Figure 7 shows the results of Nyquist plots of FeP-Pt/CC and FeP/CC at −0.2 V in 1 M KOH with an inset of the equivalent electrical circuit. It can be seen from
Figure 7 that the inclined straight lines show an angle of nearly 45 degrees, this suggests that there is a thick and compact passivation film on the surface of the electrode, and the ion migration is greatly inhibited. These results indicate that a dense passivation film is easily formed on the surface of iron in a strongly alkaline solution. It can also be deduced that the circuit diagram is a series connection of resistor R1 and constant phase element (CPE). Here the constant phase element CPE has two values, CPE-P and CPE-T.
Table 2 shows the element values in the equivalent circuit of the AC impedance spectrum. As is shown in
Table 2, the CPE-P values of FeP-Pt/CC and FeP/CC are 0.7778 Ω
−1·cm
−2·s
−n and 0.7689 Ω
−1·cm
−2·s
−n, respectively. It can be illustrated that the rough and porous electrode surface produces double-layer capacitance and there exists a dispersion effect on the electrode surface. The resistance should decrease because platinum has better conductivity than FeP after platinum plating. In addition, the increase of CPE value indicates the increase of capacitance effect and the increase of film thickness and roughness.
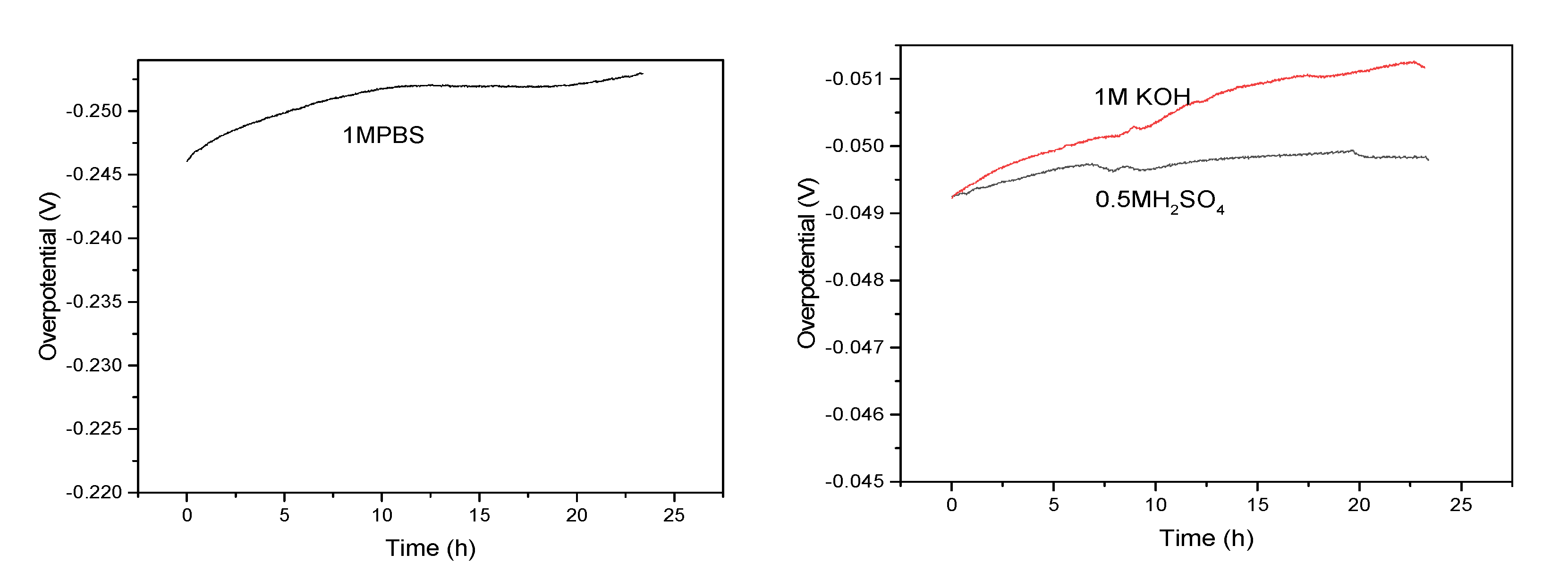
In addition, the durability and long term stability of materials were further investigated.
Figure 8 shows the V-T curve of FeP-Pt/CC at 10 mA/cm
2 in 0.5 M H
2SO
4, 1 M PBS, 1 M KOH, respectively. Compared with other solutions, potential changes little in the 0.5 M H
2SO
4. In general, the potential changes little after 20 h in various solutions, which can be applied in practical production.