Electrochemical In Situ Fabrication of Titanium Dioxide Nanotubes on a Titanium Wire as a Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Preparation of SPME Fiber
2.4. The Procedure of SPME-HPLC
3. Results and Discussion
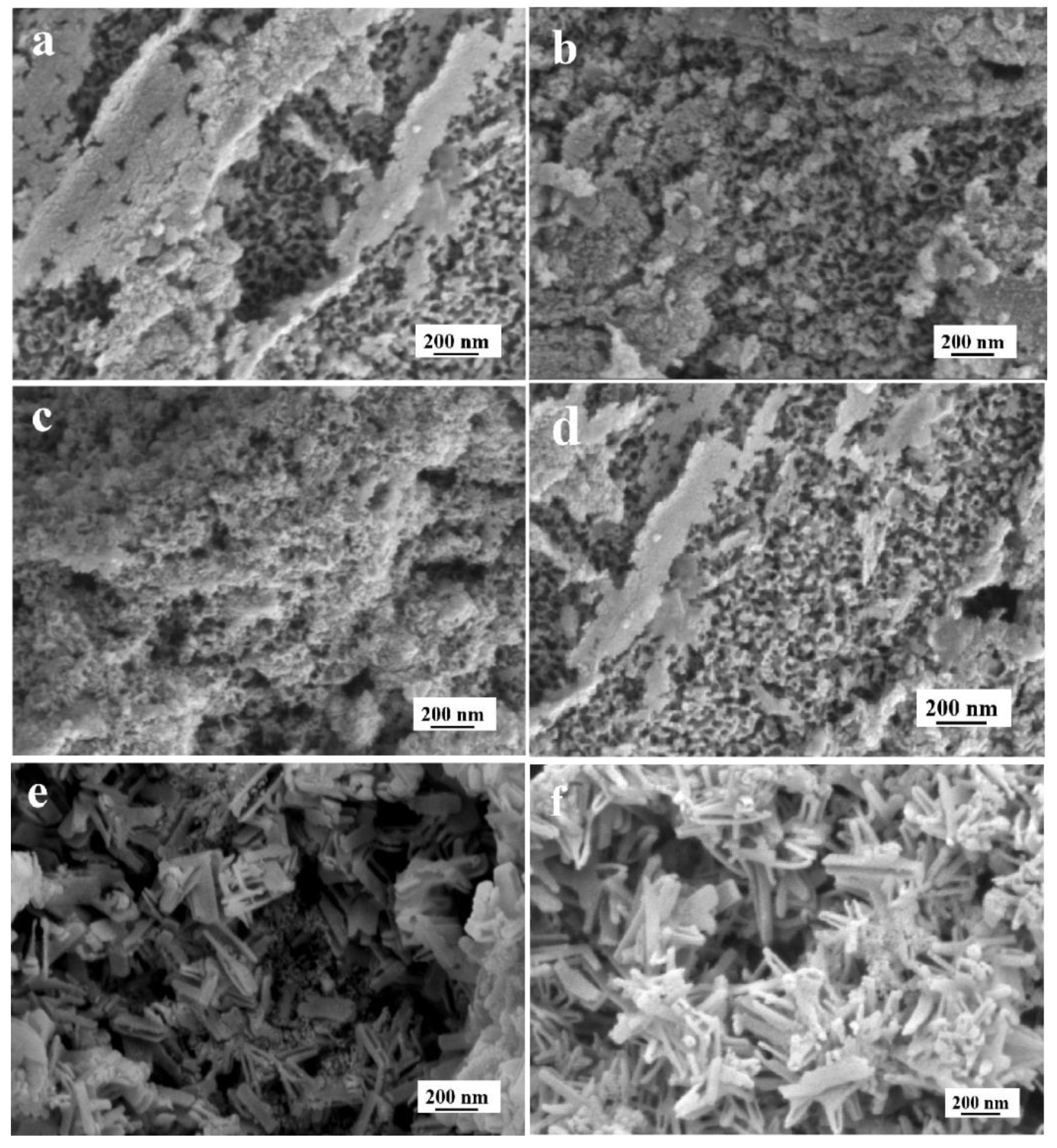
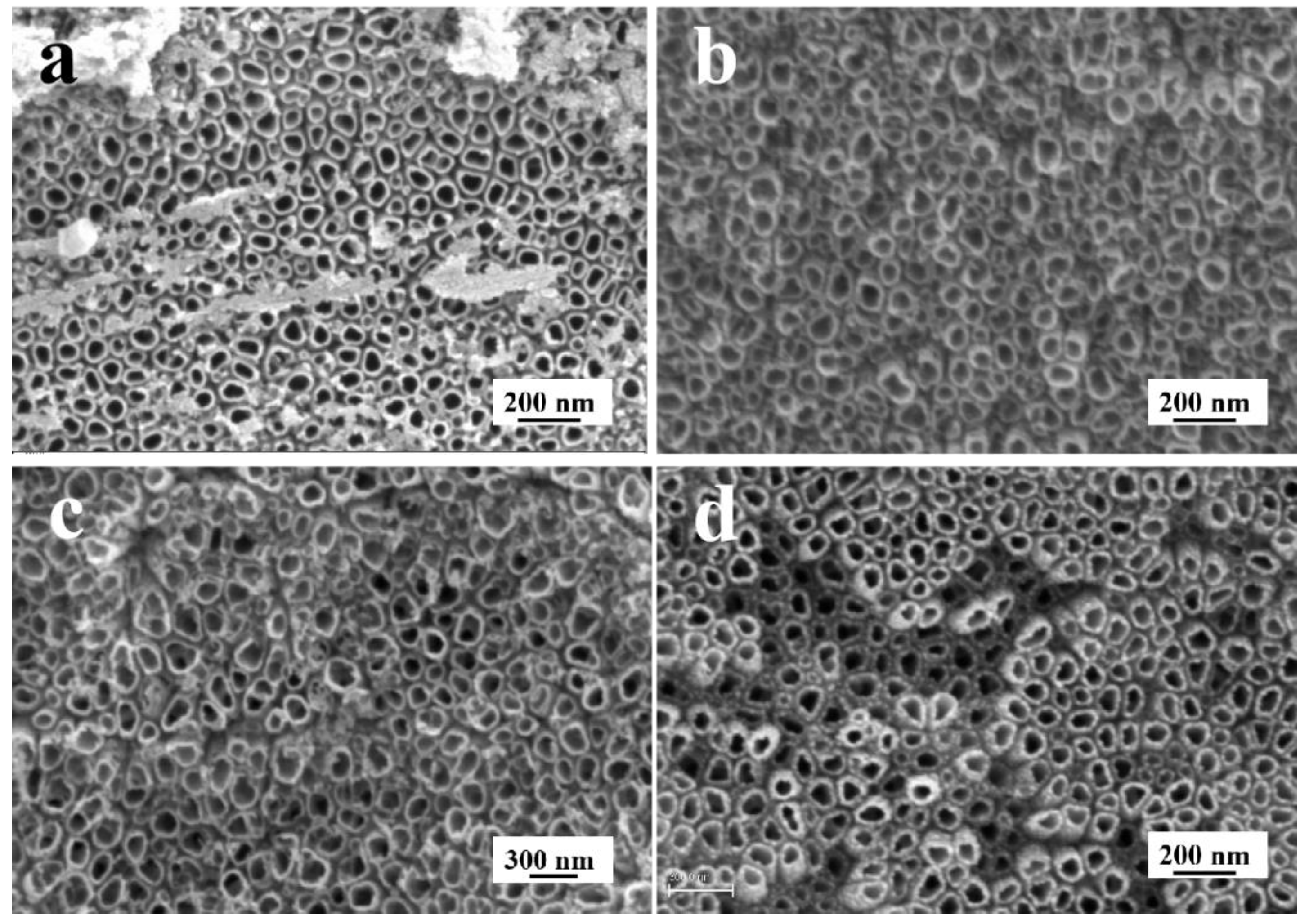
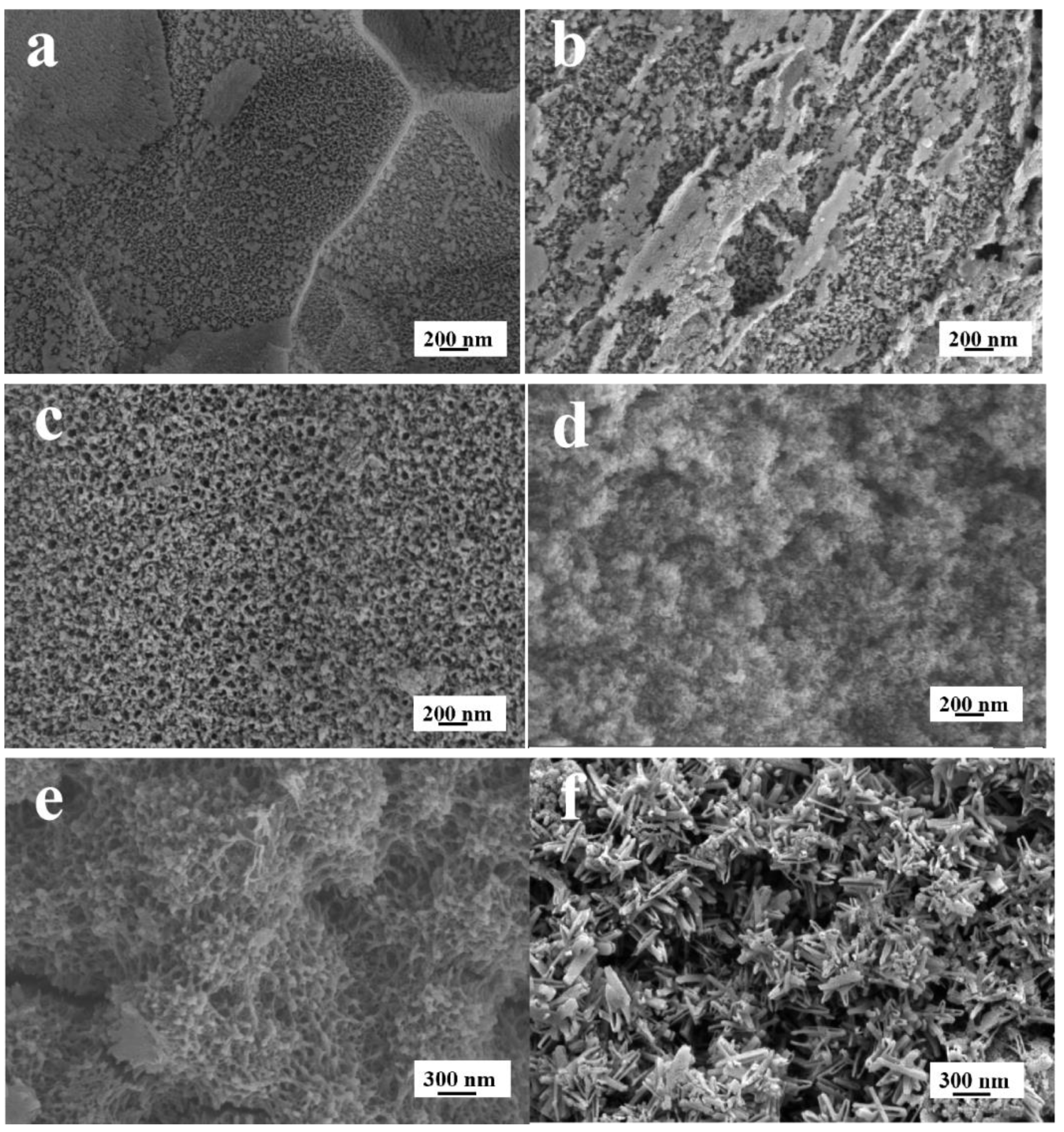
3.1. The Effect of NH4F on TiO2NTs Coating
3.1.1. Aqueous Solution System
3.1.2. Organic Electrolyte Solution System
3.2. The Effect of Ethylene Glycol
3.3. The Effect of Voltages
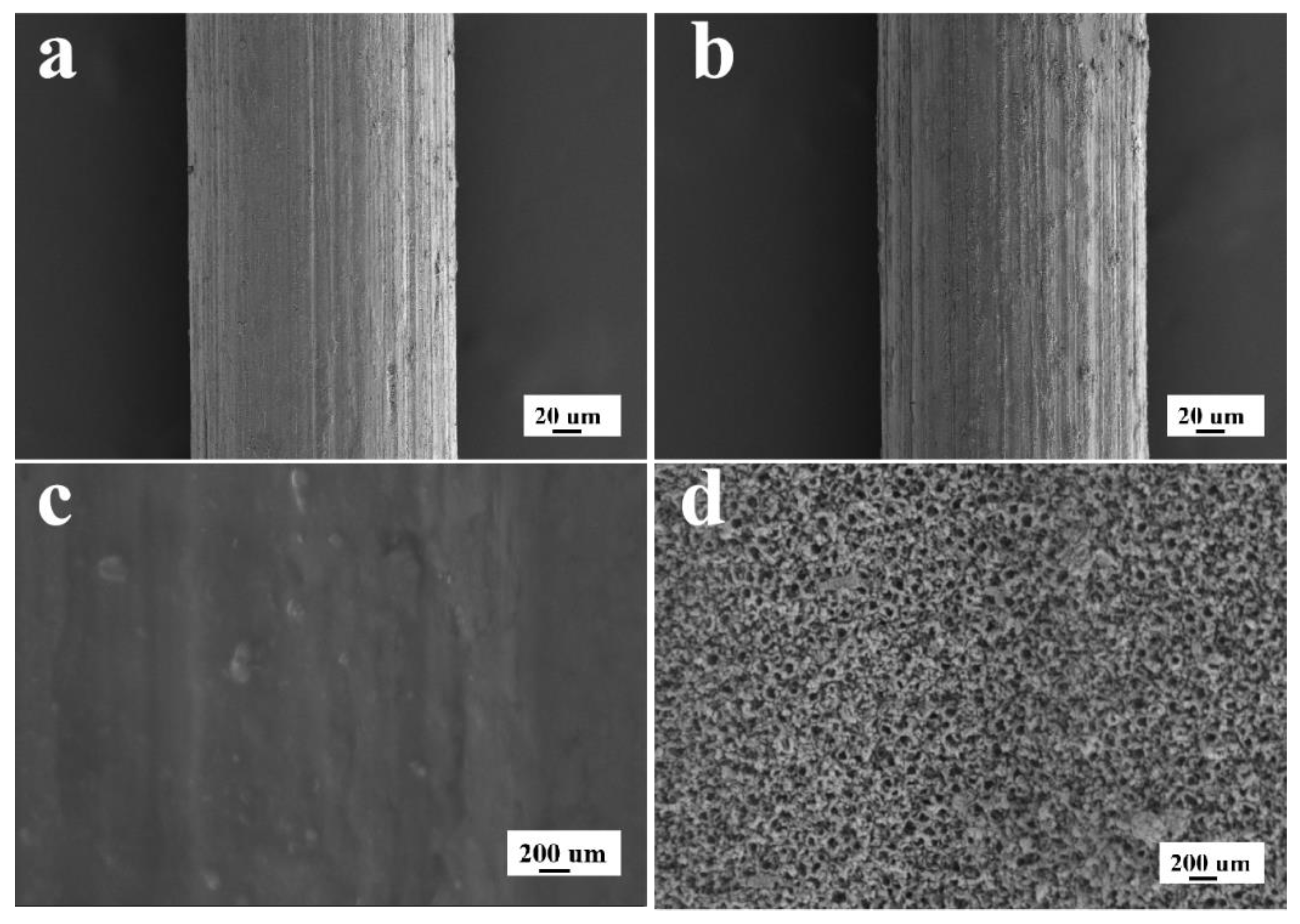
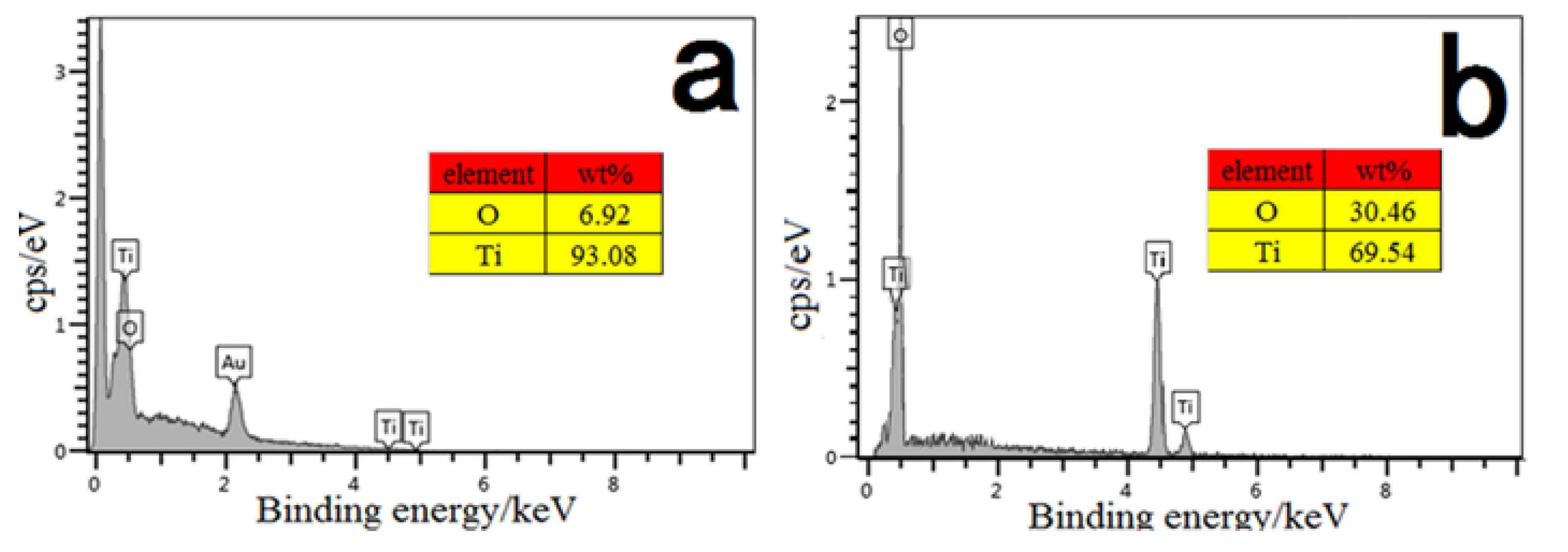
3.4. The Characterization of the TiO2NTs
3.5. The Extraction Mechanism of TiO2NTs Fiber
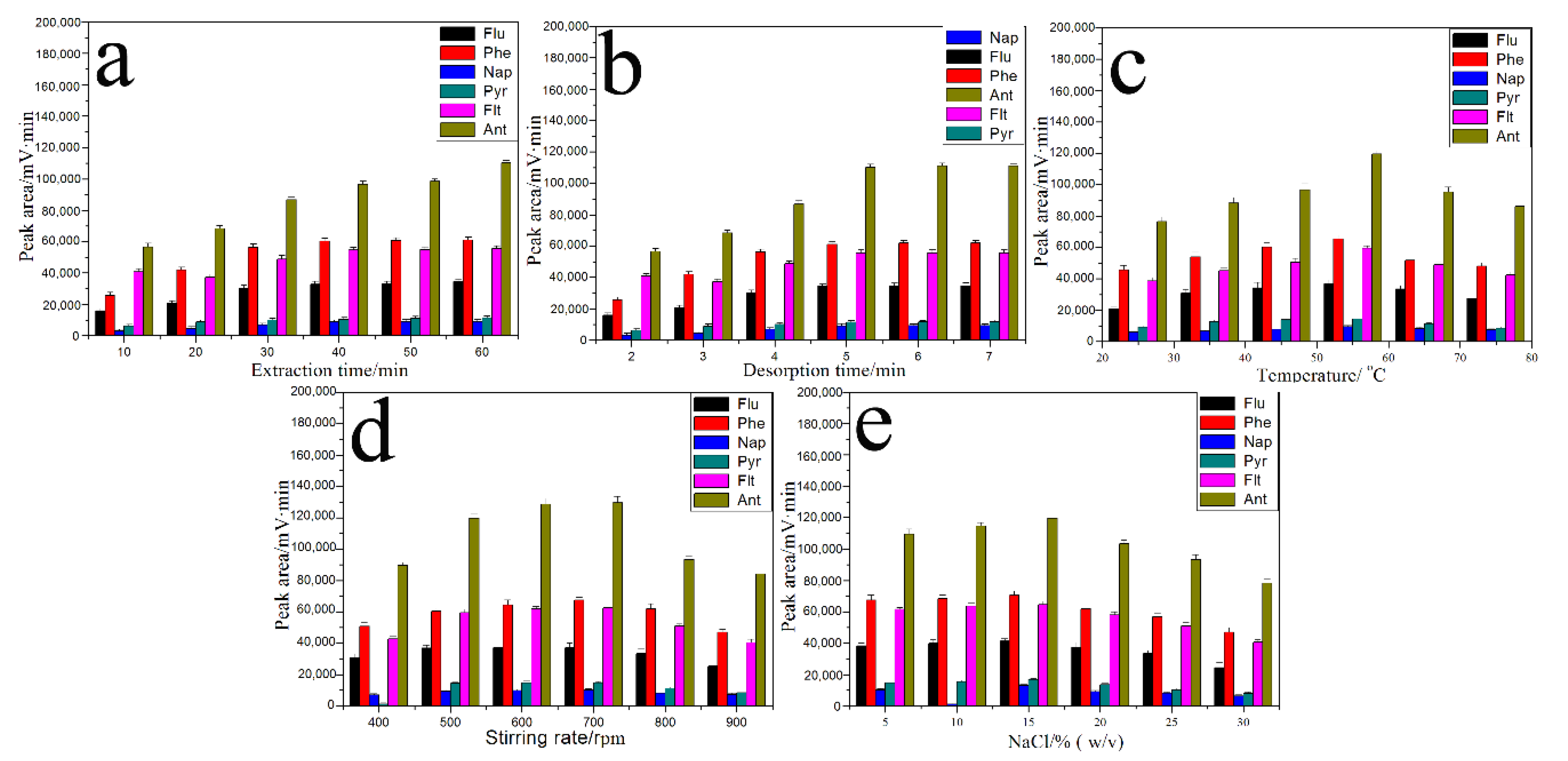
3.6. The Optimization of SPME Conditions for PAHs
3.6.1. Extraction and Desorption Time
3.6.2. Extraction Temperature
3.6.3. Stirring Speed
3.6.4. Ionic Strength
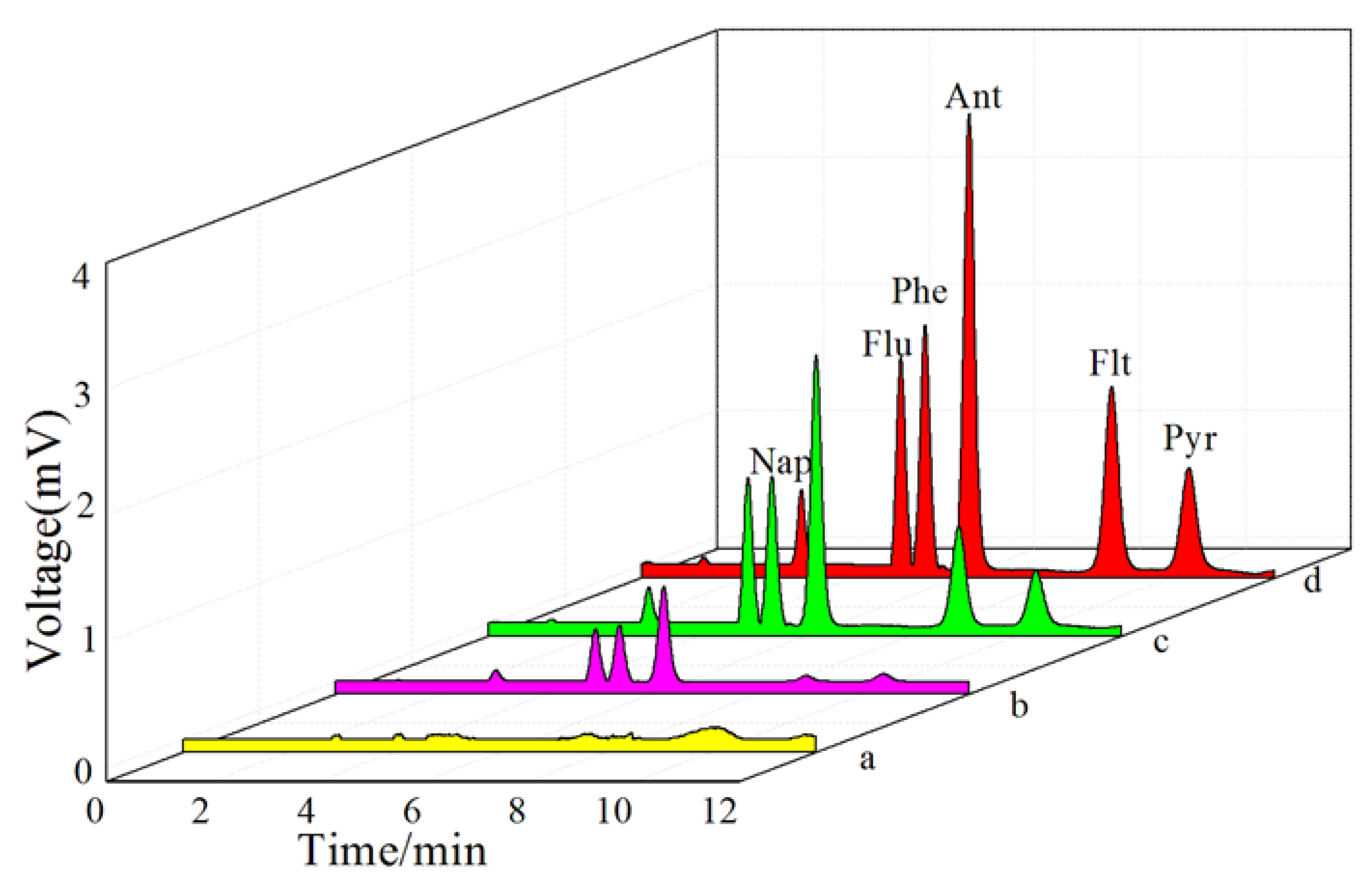
3.7. Analytical Performance
3.8. Spiked Real Samples Analysis
3.9. The Stability of the TiO2NTs/Ti Fiber
3.10. A Comparison of the Developed Method with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alpendurada, M.D.F. Solid-Phase Microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef]
- Bagheri, H.; Piri-Moghadam, H.; Naderi, M. Towards greater mechanical, thermal and chemical stability in solid-phase microextraction. TrAC Trends Anal. Chem. 2012, 34, 126–139. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Rutkowska, M.; Szczepańska, N.; Namieśnik, J.; Płotka-Wasylka, J. Solid phase microextraction: Apparatus, sorbent materials, and application. Crit. Rev. Anal. Chem. 2018, 49, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.J.; Qiu, H.D.; Liu, X.; Jiang, S.X. The development of SPME fibers with metal wires as supporting substrates. TrAC Trends Anal. Chem. 2014, 44, 44–58. [Google Scholar]
- Sun, M.; Feng, J.J.; Bu, Y.N.; Wang, X.J. Graphene coating bonded onto stainless steel wire as a solid-phase microextraction fiber. Talanta 2015, 134, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Piryaei, M.; Abolghasemi, M.M. Anodized aluminum wire as a solid-phase microextraction fiber for rapid determination of volatile constituents in medicinal plant. Anal. Chim. Acta 2011, 701, 1–5. [Google Scholar] [CrossRef]
- Djozan, D.; Abdollahi, L. Anodized zinc wire as a solid-phase microextraction fiber. Chromatographia 2003, 57, 799–804. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhang, Y.D.; Zhang, M.; Zhen, Q.; Wang, X.M.; Du, X.Z. Gold nanoparticle modified NiTi composite nanosheet coating for efficient and selective solid phase microextraction of polycyclic aromatic hydrocarbons. Anal. Methods 2016, 8, 6064–6073. [Google Scholar] [CrossRef]
- Feng, J.J.; Sun, M.; Liu, H.M.; Li, J.B.; Liu, X.; Jiang, S.X. Au nanoparticles as a novel coating for solid-phase microextraction. J. Chromatogr. A 2010, 1217, 8079–8086. [Google Scholar] [CrossRef]
- Mehdinia, A.; Aziz-Zanjani, M.O. Recent advances in nanomaterials utilized infiber coatings for solid-phase microextraction. TrAC Trends Anal. Chem. 2013, 42, 205–215. [Google Scholar] [CrossRef]
- Aziz-Zanjani, M.O.; Mehdinia, A. Electrochemically prepared solid-phase microextraction coatings—A review. Anal. Chim. Acta 2013, 781, 1–13. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Gong, D.W.; Grimes, C.A.; Varghese, O.; Hu, W.C.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef] [Green Version]
- García-Valverde, M.T.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Titanium-dioxide nanotubes as sorbents in (micro)extraction techniques. TrAC Trends Anal. Chem. 2014, 62, 37–45. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.Q.; Lu, J.L.; Wu, X.L. Electrodeposition preparation of Ag nanoparticles loaded TiO2 nanotube arrays with enhanced photocatalytic performance. Appl. Surf. Sci. 2014, 288, 513–517. [Google Scholar] [CrossRef]
- Cabanas-Polo, S.; Boccaccini, A.R. Electrophoretic deposition of nanoscale TiO2: Technology and applications. J. Eur. Ceram. Soc. 2015, 36, 265–283. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Goldmann, W.H. Insights into theranostic properties of Titanium dioxide for nanomedicine. Nano-Micro. Lett. 2020, 12, 106–140. [Google Scholar]
- Xu, X.; Chen, B.; Hu, J.P.; Sun, B.W.; Liang, X.H.; Li, N.; Yang, S.Y.; Zhang, H.; Huang, W.; Yu, T. Heterostructured TiO2 spheres with tunable interiors and shells toward improved packing density and pseudocapacitive sodium storage. Adv. Mater. 2019, 31, 1904589. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, Y.D.; Herath, H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar-Their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Pulleyblank, C.; Cipullo, S.; Campo, P.; Kelleher, B.; Coulonet, F. Analytical progress and challenges for the detection of oxygenated polycyclic aromatic hydrocarbon transformation products in aqueous and soil environmental matrices: A review. Crit. Rev. Env. Sci. Technol. 2019, 49, 357–409. [Google Scholar] [CrossRef]
- Gorshkov, A.G.; Izosimova, O.N.; Kustova, O.V. Determination of priority polycyclic aromatic hydrocarbons in water at the trace level. J. Anal. Chem. 2019, 74, 771–777. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Liu, P.; Li, S.L.; Zhang, X.J.; Chen, M.D. Progress in the analytical research methods of polycyclic aromatic hydrocarbons (PAHs). J. Liq. Chromatogr. Relat. Technol. 2020, 43, 425–444. [Google Scholar] [CrossRef]
- Riddle, S.G.; Robert, M.A.; Jakober, C.A.; Hannigan, M.P.; Kleeman, M.J. Size distribution of trace organic species emitted from light-duty gasoline vehicles. Environ. Sci. Technol. 2007, 41, 7464–7471. [Google Scholar] [CrossRef] [PubMed]
- Zencak, Z.; Klanova, J.; Holoubek, I.; Gustafsson, O. Source apportionment of atmospheric PAHs in the western balkans by natural abundance radiocarbon analysis. Environ. Sci. Technol. 2007, 41, 3850–3855. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yan, X.P.; Kim, K.N.; Wang, S.W.; Liu, M.G. Exploration of coordination polymer as sorbent for flow injection solid-phase extraction on-line coupled with high-performance liquid chromatography for determination of polycyclic aromatic hydrocarbons in environmental materials. J. Chromatogr. A 2006, 1116, 172–178. [Google Scholar] [CrossRef]
- Li, K.; Li, H.F.; Liu, L.B.; Hashi, Y.; Maeda, T.; Lin, J.M. Solid-phase extraction with C30 bonded silica for analysis of polycyclic aromatic hydrocarbons in airborne particulate matters by gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1154, 74–80. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Xu, X.; Wang, L.; Lin, L.H.; Wang, D.H. Simultaneous determination of forty-two parent and halogenated polycyclic aromatic hydrocarbons using solid-phase extraction combined with gas chromatography-mass spectrometry in drinking water. Ecotoxicol. Environ. Saf. 2019, 181, 241–247. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Wang, Z.Y.; Wang, X.M.; Du, X.Z. Tailoring the selectivity of titania nanowire arrays grown on titanium fibers by self-assembled modification of trichlorophenylsilane for solid-phase microextraction of polycyclic aromatic hydrocarbons. Microchim. Acta 2019, 186, 536. [Google Scholar] [CrossRef]
- Burkhardt, M.R.; Zaugg, S.D.; Burbank, T.L.; Olson, M.C.; Iverson, J.L. Pressurized liquid extraction using water/isopropanol coupled with solid-phase extraction clean up for semivolatile organic compounds, polycyclic aromatic hydrocarbons (PAH), and alkylate PAH homolog groups in sediment. Anal. Chim. Acta 2005, 549, 104–116. [Google Scholar] [CrossRef]
- Hartonen, K.; Bøwadt, S.; Dybdahl, H.P.; Nylund, K.; Sporring, S.; Lund, H.; Oreld, F. Nordic laboratory intercomparison of supercritical fluid extraction for the determination of total petroleum hydrocarbon, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in soil. J. Chromatogr. A 2002, 958, 239–248. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Marten-Jahns, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P. Filling of TiO2 nanotubes by self-doping and electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]
- Valota, A.; LeClere, D.J.; Skeldon, P.; Curioni, M.; Hashimoto, T.; Berger, S.; Kunze, J.; Schmuki, P.; Thompson, G.E. Influence of water content on nanotubular anodic titania formed in fluoride/glycerol electrolytes. Electrochim. Acta 2009, 54, 4321–4327. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, M.; Wang, X.Q.; Luo, C.N.; Feng, J.J. A nanospherical metal–organic framework UiO-66 for solid-phase microextraction of polycyclic aromatic hydrocarbons. Chromatographia 2018, 81, 1053–1061. [Google Scholar] [CrossRef]
- Fang, L.; Hou, L.X.; Zhang, Y.H.; Wang, Y.K.; Yan, G.H. Synthesis of highly hydrophobic rutile Titania-silica nanocomposites by an improved hydrolysis co-precipitation method. Ceram. Int. 2017, 43, 5592–5598. [Google Scholar] [CrossRef]
- Huang, Y.N.; Chen, J.; Li, Z.; Wang, L.; Guan, M.; Qiu, H.D. Porous graphene-coated stainless-steel fiber for direct immersion solid-phase microextraction of polycyclic aromatic hydrocarbons. Anal. Methods 2019, 11, 213–218. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.G.; Zhang, M.; Wang, X.M.; Du, X.Z. In situ anodic growth of rod-like TiO2 coating on a Ti wire as a selective solid-phase microextraction fiber. RSC Adv. 2014, 4, 53820–53827. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, F.F.; Zhang, R.; Wang, Z.; Du, X.Z. A new strategy for electrochemical fabrication of manganese dioxide coatings based on silica nanoparticles deposited on titanium fibers for selective and highly efficient solid-phase microextraction. New J. Chem. 2019, 43, 5055–5064. [Google Scholar] [CrossRef]
- Doong, R.A.; Chang, S.M.; Sun, Y.C. Solid-phase microextraction for determining the distribution of sixteen US Environmental Protection Agency polycyclic aromatic hydrocarbons in watersamples. J. Chromatogr. A 2000, 879, 177–188. [Google Scholar] [CrossRef]
- Feng, J.J.; Sun, M.; Liu, H.M.; Li, J.B.; Liu, X.; Jiang, S.X. A novel silver-coated solid- phase microextraction metal fiber based on electroless plating technique. Anal. Chim. Acta 2011, 701, 174–180. [Google Scholar] [CrossRef]
- Lucio-Gutiérrez, R.; Salazar-Cavazos, J.M.D.; de Torres, N.H.W.; Castro-Ríos, R. Solid-phase microextraction followed by high-performance liquid chromato-graphy with fluorimetric and UV detection for the determination of polycyclic aromatic hydrocarbons in water. Anal. Lett. 2008, 41, 119–136. [Google Scholar] [CrossRef]
- Liu, H.X.; Liu, L.; Li, Y.; Wang, X.M.; Du, X.Z. Preparation of a robust and sensitive gold-coated fiber for solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental waters. Anal. Lett. 2014, 47, 1759–1771. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.L.; Wang, T.E.; Li, Y.; Wang, X.M.; Du, X.Z. Phenyl-functionalization of titanium dioxide-nanosheets coating fabricated on a titanium wire for selective solid-phase microextraction of polycyclic aromatic hydrocarbons from environment water samples. Talanta 2015, 144, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Yang, Y.X.; Li, Y.; Wang, X.M.; Du, X.Z. Growth of cedar-like Au nanoparticles coating on an etched stainless steel wire and its application for selective solid-phase microextraction. Anal. Chim. Acta 2015, 876, 55–62. [Google Scholar] [CrossRef]

| PAHs | Linearity (μg·L−1) | R2 | Recovery a % | RSD (%) a | LODs/(μg·L−1) | LOQs/(μg·L−1) | |
|---|---|---|---|---|---|---|---|
| Single Fiber (n = 5) | Fiber-to-Fiber (n = 3) | ||||||
| Nap | 0.2–500 | 0.9989 | 90.4 | 4.7 | 7.6 | 0.04 | 0.15 |
| Flu | 0.2–500 | 0.9985 | 107 | 3.8 | 6.8 | 0.05 | 0.17 |
| Phe | 0.2–500 | 0.9992 | 118 | 4.2 | 5.2 | 0.04 | 0.13 |
| Ant | 0.2–500 | 0.9980 | 92.4 | 4.0 | 7.4 | 0.03 | 0.12 |
| Flt | 0.2–500 | 0.9987 | 87.4 | 4.3 | 7.9 | 0.04 | 0.14 |
| Pyr | 0.2–500 | 0.9991 | 92.8 | 3.5 | 6.3 | 0.05 | 0.18 |
| Water Samples | PAHs | Original/(μg·L−1) | Spiked with 5 μg·L−1 | Spiked with 10 μg·L−1 | ||||
|---|---|---|---|---|---|---|---|---|
| Detected/(μg·L−1) | Recovery/% | RSD/% | Detected/(μg·L−1) | Recovery/% | RSD/% | |||
| Tap water | Nap | ND a | 4.85 | 97.0 | 7.1 | 9.12 | 91.2 | 7.9 |
| Flu | ND | 5.38 | 108 | 5.6 | 11.3 | 113 | 6.7 | |
| Phe | ND | 4.22 | 84.4 | 6.3 | 11.72 | 117.2 | 7.1 | |
| Ant | ND | 4.06 | 81.2 | 5.4 | 8.73 | 87.3 | 5.8 | |
| Flt | ND | 5.95 | 119 | 6.8 | 11.68 | 116.8 | 7.3 | |
| Pyr | ND | 4.41 | 88.2 | 4.3 | 9.05 | 90.5 | 5.5 | |
| River water | Nap | 3.69 | 9.26 | 106.6 | 7.8 | 12.27 | 89.6 | 8.7 |
| Flu | 2.57 | 6.83 | 90.2 | 4.9 | 11.85 | 94.3 | 5.8 | |
| Phe | 3.43 | 7.85 | 93.1 | 6.2 | 15.21 | 113.3 | 6.9 | |
| Ant | 1.12 | 4.81 | 78.6 | 5.9 | 10.16 | 91.4 | 6.1 | |
| Flt | 1.34 | 7.02 | 110.7 | 6.5 | 12.05 | 106.3 | 7.3 | |
| Pyr | 0.82 | 6.78 | 116.5 | 4.4 | 11.24 | 103.9 | 5.1 | |
| Wastewater | Nap | 1.97 | 6.23 | 89.4 | 8.4 | 13.02 | 108.8 | 8.9 |
| Flu | 3.12 | 8.65 | 106.5 | 6.2 | 12.36 | 94.2 | 7.6 | |
| Phe | 2.62 | 6.87 | 90.2 | 7.9 | 11.24 | 89.1 | 8.3 | |
| Ant | 2.34 | 6.63 | 90.3 | 7.3 | 11.14 | 90.3 | 8.5 | |
| Flt | 0.36 | 6.34 | 118.3 | 8.1 | 11.76 | 113.5 | 8.7 | |
| Pyr | 0.89 | 6.53 | 110.9 | 5.2 | 11.98 | 111.0 | 6.3 | |
| Rain water | Nap | 1.82 | 7.17 | 105.1 | 7.9 | 11.37 | 96.2 | 8.6 |
| Flu | ND | 4.89 | 97.8 | 5.3 | 8.52 | 85.2 | 6.1 | |
| Phe | 0.88 | 5.07 | 86.2 | 7.3 | 11.76 | 108.1 | 8.2 | |
| Ant | 3.75 | 8.28 | 94.6 | 5.8 | 15.22 | 110.7 | 6.7 | |
| Flt | 1.54 | 6.94 | 106.1 | 6.9 | 10.99 | 95.23 | 7.6 | |
| Pyr | ND | 5.07 | 101.4 | 4.6 | 10.78 | 107.8 | 5.1 | |
| Methods a | Analytes | Time (min) | Linear Ranges (μg⋅L−1) | LOD (μg⋅L−1) | RSD (%) | Recovery (%) | Refs |
|---|---|---|---|---|---|---|---|
| PDMS-SPME-GC-MS | Nap, Flu, Phe, Ant, Flt, Pyr | 90 | 0.1–100 | 0.03–0.24 | <19 | 69–105 | [41] |
| AuNPs-SPME-GC-FID | Nap, Flu, Ant, Flt | 50 | 0.05–300 | 0.025–0.25 | 2.49–7.90 | 78.4–119.9 | [42] |
| PDMS/DVB-SPME-HPLC-UV | Nap, Flu, Ant, Phe, Pyr | 60 | 0.04–15 | 0.005–0.027 | 0.97–2.21 | 81.23–89.11 | [43] |
| AuMPs-SPME-HPLC-UV | Nap, Flu, Ant, Phe, Pyr | 50 | 0.20–500 | 0.016–0.22 | 2.03–11.7 | 86.0–112.9 | [44] |
| ph-TiO2NS-SPME-HPLC-UV | Nap, Flu, Phe | 40 | 0.05–300 | 0.008–0.043 | 6.13–9.45 | 86.2–112 | [45] |
| AuNPs-SPME-HPLC-UV | Nap, Phe | 20 | 0.1–300 | 0.008–0.037 | 3.49–9.26 | 82.74–110.0 | [46] |
| TiO2NTs/Ti-SPME-HPLC-UV | Nap, Flu, Phe, Ant, Flt, Pyr | 50 | 0.20–500 | 0.03–0.05 | 3.5–7.9 | 87.4–118 | Present method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Wei, Y.; Chen, J.; Shang, Q. Electrochemical In Situ Fabrication of Titanium Dioxide Nanotubes on a Titanium Wire as a Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Crystals 2021, 11, 1384. https://doi.org/10.3390/cryst11111384
Ma M, Wei Y, Chen J, Shang Q. Electrochemical In Situ Fabrication of Titanium Dioxide Nanotubes on a Titanium Wire as a Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Crystals. 2021; 11(11):1384. https://doi.org/10.3390/cryst11111384
Chicago/Turabian StyleMa, Mingguang, Yunxia Wei, Jie Chen, and Qiong Shang. 2021. "Electrochemical In Situ Fabrication of Titanium Dioxide Nanotubes on a Titanium Wire as a Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons" Crystals 11, no. 11: 1384. https://doi.org/10.3390/cryst11111384
APA StyleMa, M., Wei, Y., Chen, J., & Shang, Q. (2021). Electrochemical In Situ Fabrication of Titanium Dioxide Nanotubes on a Titanium Wire as a Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Crystals, 11(11), 1384. https://doi.org/10.3390/cryst11111384






