Characterization of GAGG Doped with Extremely Low Levels of Chromium and Exhibiting Exceptional Intensity of Emission in NIR Region
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
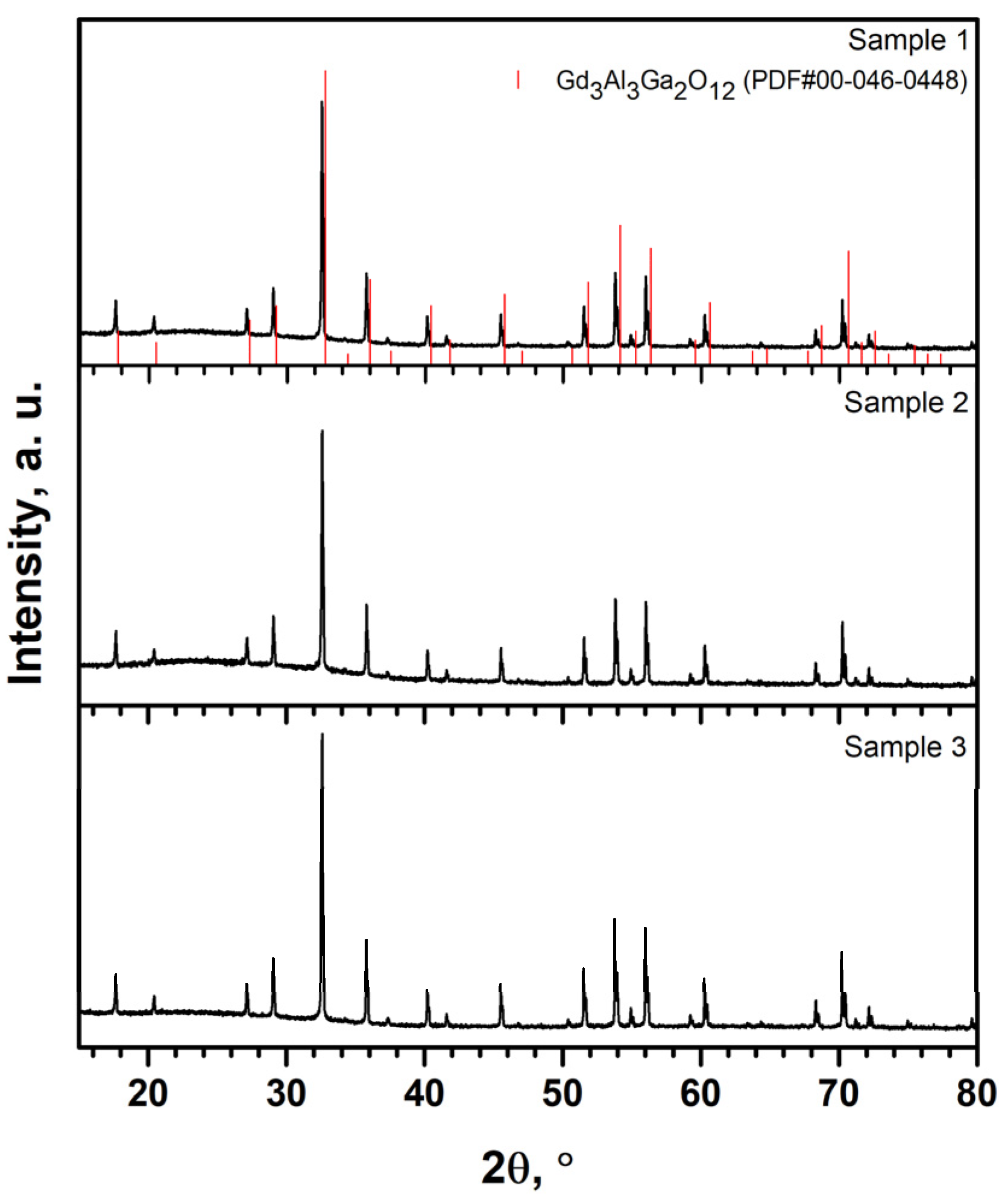
3.1. X-ray Diffraction Analysis
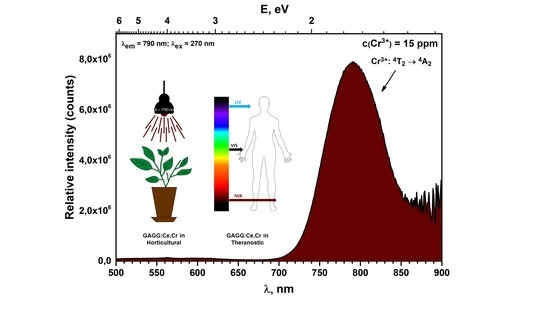
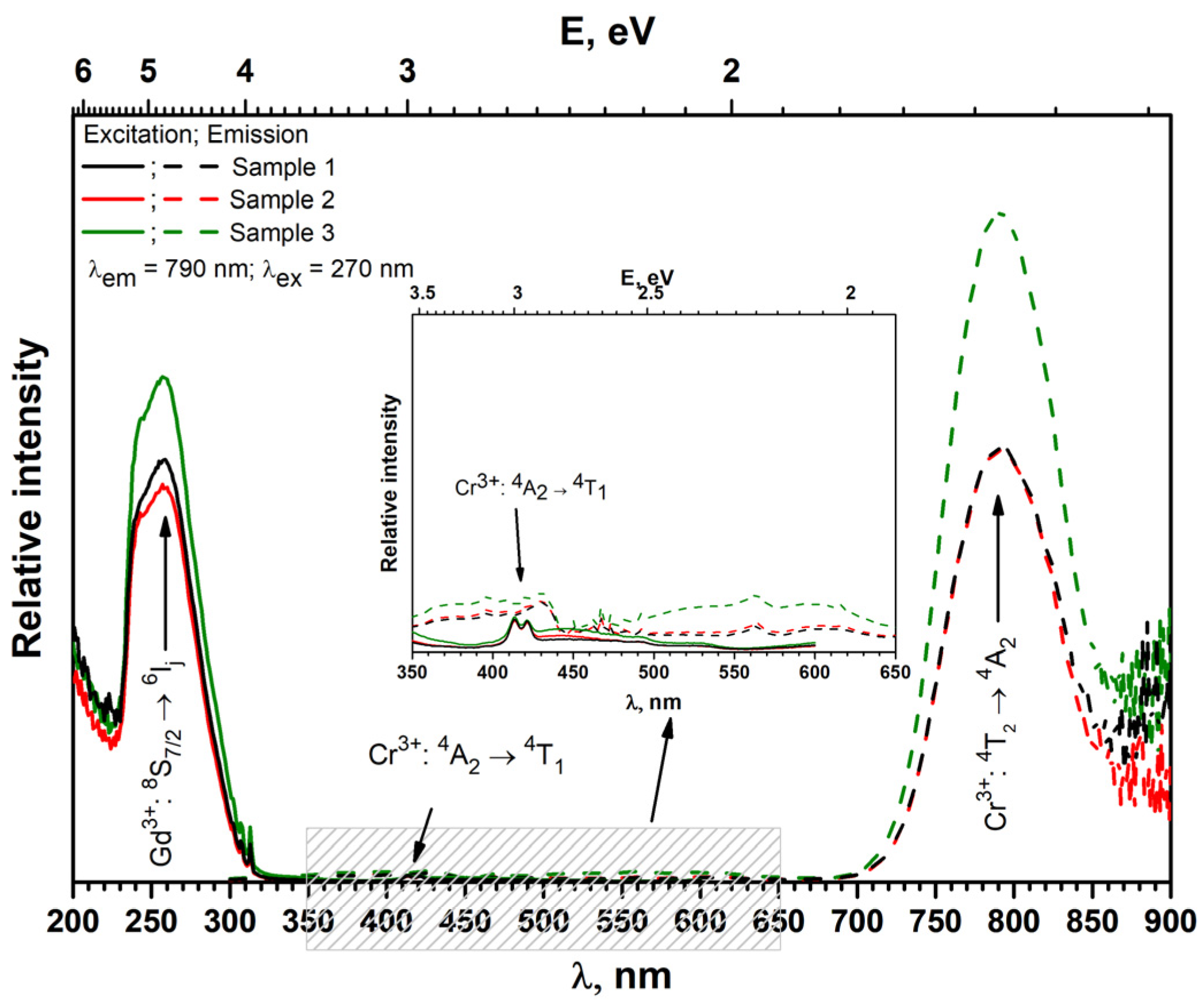
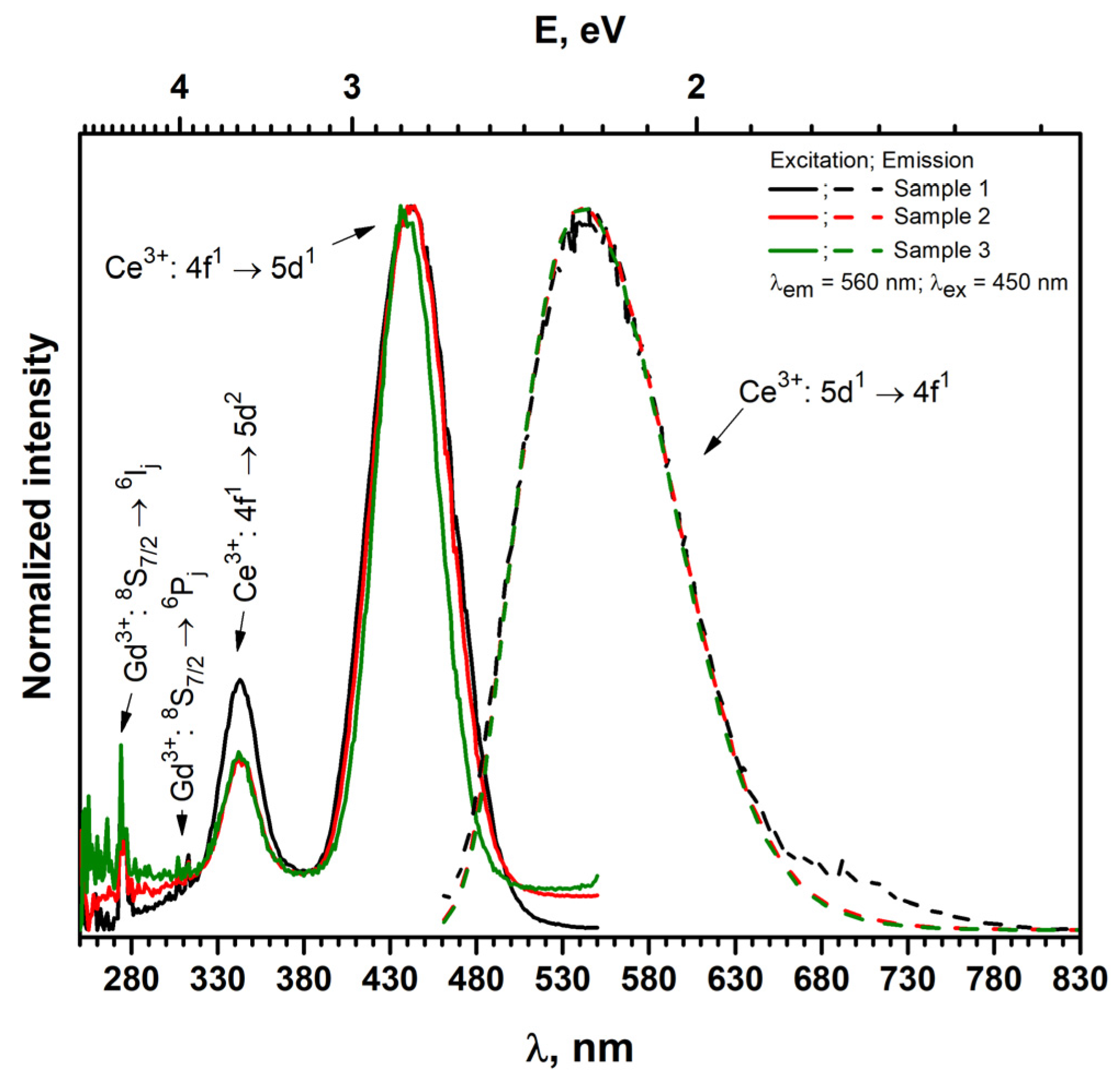
3.2. Luminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Paper, C.; Cherepy, N.; Livermore, L.; Seeley, Z.M.; Livermore, L.; Beck, P.; Livermore, L.; Hunter, S.L.; Livermore, L. High Energy Resolution Transparent Ceramic Garnet Scintillators. Int. Soc. Opt. Photonics 2014, 2. [Google Scholar] [CrossRef]
- Sakthong, O.; Chewpraditkul, W.; Wanarak, C.; Kamada, K. Nuclear Instruments and Methods in Physics Research A Scintillation Properties of Gd3Al2Ga3O12:Ce3þ Single Crystal Scintillators. Nucl. Inst. Methods Phys. Res. A 2014, 751, 1–5. [Google Scholar] [CrossRef]
- Belli, P.; Bernabei, R.; Cerulli, R.; Dai, C.J.; Danevich, F.A.; Incicchitti, A.; Kobychev, V.V.; Ponkratenko, O.A.; Prosperi, D.; Tretyak, V.I.; et al. Performances of a CeF3 Crystal Scintillator and Its Application to the Search for Rare Processes. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 498, 352–361. [Google Scholar] [CrossRef]
- Van Eijk, C.W.E. Scintillator-Based Detectors; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 8. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature Quenching of Yellow Ce3+ Luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Khaidukov, N.; Zorenko, T.; Iskaliyeva, A.; Paprocki, K.; Batentschuk, M.; Osvet, A.; Van Deun, R.; Zhydaczevskii, Y.; Suchocki, A.; Zorenko, Y. Synthesis and Luminescent Properties of Prospective Ce3+ Doped Silicate Garnet Phosphors for White LED Converters. J. Lumin. 2017, 192, 328–336. [Google Scholar] [CrossRef]
- Ueda, J.; Dorenbos, P.; Bos, A.J.J.; Meijerink, A.; Tanabe, S. Insight into the Thermal Quenching Mechanism for Y3Al5O12:Ce3+ through Thermoluminescence Excitation Spectroscopy. J. Phys. Chem. C 2015, 119, 25003–25008. [Google Scholar] [CrossRef]
- Auffray, E.; Korjik, M.; Lucchini, M.T.; Nargelas, S.; Sidletskiy, O.; Tamulaitis, G.; Tratsiak, Y.; Vaitkevičius, A. Free Carrier Absorption in Self-Activated PbWO4 and Ce-Doped Y3(Al0.25Ga0.75)3O12 and Gd3Al2Ga3O12 Garnet Scintillators. Opt. Mater. 2016, 58, 461–465. [Google Scholar] [CrossRef][Green Version]
- Seminko, V.; Maksimchuk, P.; Bespalova, I.; Masalov, A.; Viagin, O.; Okrushko, E.; Kononets, N.; Malyukin, Y. Defect and Intrinsic Luminescence of CeO2 Nanocrystals. Phys. Status Solidi 2017, 254, 1600488. [Google Scholar] [CrossRef]
- Zatsepin, D.A.; Boukhvalov, D.W.; Zatsepin, A.F.; Kuznetsova, Y.A.; Mashkovtsev, M.A.; Rychkov, V.N.; Shur, V.Y.; Esin, A.A.; Kurmaev, E.Z. Electronic Structure, Charge Transfer, and Intrinsic Luminescence of Gadolinium Oxide Nanoparticles: Experiment and Theory. Appl. Surf. Sci. 2018, 436, 697–707. [Google Scholar] [CrossRef]
- Kanai, T.; Satoh, M.; Miura, I. Characteristics of a Nonstoichiometric Gd3+δ(Al,Ga) 5-ΔO12:Ce Garnet Scintillator. J. Am. Ceram. Soc. 2008, 91, 456–462. [Google Scholar] [CrossRef]
- Geller, S. Magnetic Interactions and Distribution of Ions in the Garnets. J. Appl. Phys. 1960, 30. [Google Scholar] [CrossRef]
- Yanagida, T.; Kamada, K.; Fujimoto, Y.; Yagi, H.; Yanagitani, T. Comparative Study of Ceramic and Single Crystal Ce:GAGG Scintillator. Opt. Mater. 2013, 35, 2480–2485. [Google Scholar] [CrossRef]
- Fedorov, A.; Gurinovich, V.; Guzov, V.; Dosovitskiy, G.; Korzhik, M.; Kozhemyakin, V.; Lopatik, A.; Kozlov, D.; Mechinsky, V.; Retivov, V. Sensitivity of GAGG Based Scintillation Neutron Detector with SiPM Readout. Nucl. Eng. Technol. 2020, 52, 2306–2312. [Google Scholar] [CrossRef]
- Kitaura, M.; Sato, A.; Kamada, K.; Kurosawa, S.; Ohnishi, A.; Sasaki, M.; Hara, K. Photoluminescence Studies on Energy Transfer Processes In. Opt. Mater. 2015, 41, 45–48. [Google Scholar] [CrossRef]
- Kitaura, M.; Sato, A.; Kamada, K.; Ohnishi, A.; Sasaki, M. Phosphorescence of Ce-Doped Gd3Al2Ga3O12 Crystals Studied Using Luminescence Spectroscopy. J. Appl. Phys. 2014, 115, 10–18. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Gundacker, S.; Lecoq, P.; Benaglia, A.; Nikl, M.; Kamada, K.; Yoshikawa, A.; Auffray, E. Timing Capabilities of Garnet Crystals for Detection of High Energy Charged Particles. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 852, 1–9. [Google Scholar] [CrossRef]
- Gundacker, S.; Turtos, R.M.; Auffray, E.; Lecoq, P. Precise Rise and Decay Time Measurements of Inorganic Scintillators by Means of X-Ray and 511 KeV Excitation. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 891, 42–52. [Google Scholar] [CrossRef]
- Ogieg, J.M.; Katelnikovas, A.; Zych, A.; Ju, T.; Meijerink, A.; Ronda, C.R. Luminescence and Luminescence Quenching in Gd3 (Ga,Al)5O12 Scintillators Doped with Ce3+. J. Phys. Chem. A 2013, 117, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Tanabe, S.; Nakanishi, T. Analysis of Ce3+ Luminescence Quenching in Solid Solutions between Y3Al5O12 and Y3Ga 5O12 by Temperature Dependence of Photoconductivity Measurement. J. Appl. Phys. 2011, 110, 053102. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, M.; Su, Q. Tailored Photoluminescence of YAG:Ce Phosphor through Various Methods. J. Phys. Chem. Solids 2004, 65, 845–850. [Google Scholar] [CrossRef]
- Yoshino, M.; Kamada, K.; Kochurikhin, V.V.; Ivanov, M.; Nikl, M.; Okumura, S.; Yamamoto, S.; Yeom, J.Y.; Shoji, Y.; Kurosawa, S.; et al. Li+, Na+ and K+ Co-Doping Effects on Scintillation Properties of Ce:Gd3Ga3Al2O12 Single Crystals. J. Cryst. Growth 2018, 491, 1–5. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yamamoto, S.; Yeom, J.; Kamada, K.; Yoshikawa, A. Development of High Resolution Phoswich Depth-of-Interaction Block Detectors Utilizing Mg Co-Doped New Scintillators. Nucl. Inst. Methods Phys. Res. A 2017. [Google Scholar] [CrossRef]
- Meng, F.; Koschan, M.; Wu, Y.; Melcher, C.L. Relationship between Ca2+ Concentration and the Properties of Codoped Gd3Ga3Al2O12:Ce Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 797, 138–143. [Google Scholar] [CrossRef]
- Kamada, K.; Shoji, Y.; Kochurikhin, V.V.; Nagura, A.; Okumura, S.; Yamamoto, S.; Yeom, J.Y.; Kurosawa, S.; Pejchal, J.; Yokota, Y.; et al. Single Crystal Growth of Ce:Gd3(Ga,Al)5O12 with Various Mg Concentration and Their Scintillation Properties. J. Cryst. Growth 2017, 468, 407–410. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Babin, V.; Bohacek, P.; Gundacker, S.; Kamada, K.; Nikl, M.; Petrosyan, A.; Yoshikawa, A.; Auffray, E. Effect of Mg2+ Ions Co-Doping on Timing Performance and Radiation Tolerance of Cerium Doped Gd3Al2Ga3O12 Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 816, 176–183. [Google Scholar] [CrossRef]
- Auffray, E.; Augulis, R.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Gulbinas, V.; Koschan, M.; Lucchini, M.; Melcher, C.; Nargelas, S.; et al. Excitation Transfer Engineering in Ce-Doped Oxide Crystalline Scintillators by Codoping with Alkali-Earth Ions. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–10. [Google Scholar] [CrossRef]
- Ueda, J.; Kuroishi, K.; Tanabe, S. Yellow Persistent Luminescence in Ce3+-Cr3+-Codoped Gadolinium Aluminum Gallium Garnet Transparent Ceramics after Blue-Light Excitation. Appl. Phys. Express 2014, 7. [Google Scholar] [CrossRef]
- Asami, K.; Ueda, J.; Tanabe, S. Trap Depth and Color Variation of Ce3+-Cr3+ Co-Doped Gd3(Al,Ga)5O12 Garnet Persistent Phosphors. Opt. Mater. 2016, 62, 171–175. [Google Scholar] [CrossRef]
- Gotoh, T.; Jeem, M.; Zhang, L.; Okinaka, N.; Watanabe, S. Synthesis of Yellow Persistent Phosphor Garnet by Mixed Fuel Solution Combustion Synthesis and Its Characteristic. J. Phys. Chem. Solids 2020, 142, 109436. [Google Scholar] [CrossRef]
- Tamulaitis, G.; Vaitkevičius, A.; Nargelas, S.; Augulis, R.; Gulbinas, V.; Bohacek, P.; Nikl, M.; Borisevich, A.; Fedorov, A.; Korjik, M.; et al. Subpicosecond Luminescence Rise Time in Magnesium Codoped GAGG:Ce Scintillator. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 870, 25–29. [Google Scholar] [CrossRef]
- Ueda, J.; Kuroishi, K.; Tanabe, S. Bright Persistent Ceramic Phosphors of Ce3+-Cr3+-Codoped Garnet Able to Store by Blue Light. Appl. Phys. Lett. 2014, 104, 5–9. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C.; Ostertag, M. The Afterglow Mechanism of Chromium-Doped Gadolinium Gallium Garnet. J. Alloys Compd. 1993, 200, 17–18. [Google Scholar] [CrossRef]
- Yamada, A.; Tanigawa, T.; Suyama, T.; Matsuno, T.; Kunitake, T. Red:Far-Red Light Ratio and Far-Red Light Integral Promote or Retard Growth and Flowering in Eustoma Grandiflorum (Raf.) Shinn. Sci. Hortic. 2009, 120, 101–106. [Google Scholar] [CrossRef]
- Jia, Z.; Yuan, C.; Liu, Y.; Wang, X.J.; Sun, P.; Wang, L.; Jiang, H.; Jiang, J. Strategies to Approach High Performance in Cr3+-Doped Phosphors for High-Power NIR-LED Light Sources. Light Sci. Appl. 2020, 9, 2047–7538. [Google Scholar] [CrossRef]
- He, S.; Zhang, L.; Wu, H.; Wu, H.; Pan, G.; Hao, Z.; Zhang, X.; Zhang, L.; Zhang, H.; Zhang, J. Efficient Super Broadband NIR Ca2LuZr2Al3O12:Cr3+,Yb3+ Garnet Phosphor for Pc-LED Light Source toward NIR Spectroscopy Applications. Adv. Opt. Mater. 2020, 8, 1901684. [Google Scholar] [CrossRef]
- Ferrauto, G.; Carniato, F.; Di Gregorio, E.; Botta, M.; Tei, L. Photoacoustic Ratiometric Assessment of Mitoxantrone Release from Theranostic ICG-Conjugated Mesoporous Silica Nanoparticles. Nanoscale 2019, 11, 18031–18036. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Shi, Z.; Quan, G.; Chen, T.; Guo, S.; Hu, Z.; Liu, P. Preparation and Luminescence Properties of Li2MgZrO4:Mn4+ Red Phosphor for Plant Growth. J. Lumin. 2017, 188, 577–581. [Google Scholar] [CrossRef]
- Inkrataite, G.; Zabiliute-Karaliune, A.; Aglinskaite, J.; Vitta, P.; Kristinaityte, K.; Marsalka, A.; Skaudzius, R. Study of YAG:Ce and Polymer Composite Properties for Application in LED Devices. Chempluschem 2020, 85, 1504–1510. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Jiang, J.; Wu, Y.; Jiang, H. Effects of Ga Substitution for Al on the Fabrication and Optical Properties of Transparent Ce:GAGG-Based Ceramics. J. Eur. Ceram. Soc. 2017, 37, 4109–4114. [Google Scholar] [CrossRef]
- Panatarani, C.; Faizal, F.; Florena, F.F.; Jumhur, D.; Made Joni, I. The Effects of Divalent and Trivalent Dopants on the Luminescence Properties of ZnO Fine Particle with Oxygen Vacancies. Adv. Powder Technol. 2020, 31, 2605–2612. [Google Scholar] [CrossRef]
- Dickens, P.T.; Haven, D.T.; Friedrich, S.; Lynn, K.G. Scintillation Properties and Increased Vacancy Formation in Cerium and Calcium Co-Doped Yttrium Aluminum Garnet. J. Cryst. Growth 2019, 507, 16–22. [Google Scholar] [CrossRef]
- Mao, M.; Zhou, T.; Zeng, H.; Wang, L.; Huang, F.; Tang, X.; Xie, R.J. Broadband Near-Infrared (NIR) Emission Realized by the Crystal-Field Engineering of Y3-XCaxAl5-XSixO12:Cr3+ (x = 0-2.0) Garnet Phosphors. J. Mater. Chem. C 2020, 8, 1981–1988. [Google Scholar] [CrossRef]
- Butkute, S.; Gaigalas, E.; Beganskiene, A.; Ivanauskas, F.; Ramanauskas, R.; Kareiva, A. Sol-Gel Combustion Synthesis of High-Quality Chromium-Doped Mixed-Metal Garnets Y3Ga5O12 and Gd3Sc2Ga3O12. J. Alloys Compd. 2018, 739, 504–509. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Liu, F.; Chen, H.; Wang, H.; Zhao, E.; Jiang, Y.; Chan, T.S.; Wang, C.H.; Zhang, W.; et al. Charge Deformation and Orbital Hybridization: Intrinsic Mechanisms on Tunable Chromaticity of Y3Al5O12:Ce3+ Luminescence by Doping Gd3+ for Warm White LEDs. Sci. Rep. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Yamaji, A.; Kawaguchi, N.; Kamada, K.; Totsuka, D.; Fukuda, K.; Yamanoi, K.; Nishi, R.; Kurosawa, S.; et al. Study of the Correlation of Scintillation Decay and Emission Wavelength. Radiat. Meas. 2013, 55, 99–102. [Google Scholar] [CrossRef]
- Malysa, B.; Meijerink, A.; Jüstel, T. Temperature Dependent Luminescence Cr3+-Doped GdAl3(BO3)4 and YAl3(BO3)4. J. Lumin. 2016, 171, 246–253. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, X.; Wu, B.; Jiang, S.; Li, L.; Luo, X.; Pang, Y. The Broad Emission at 785 Nm in YAG:Ce3+, Cr3+ Phosphor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 76–80. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Koshimizu, M.; Watanabe, K.; Sato, H.; Yagi, H.; Yanagitani, T. Positive Hysteresis of Ce-Doped GAGG Scintillator. In Optical Materials; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 36, pp. 2016–2019. [Google Scholar] [CrossRef]
- Liu, W.-R.; Lin, C.C.; Chiu, Y.-C.; Yeh, Y.-T.; Jang, S.-M.; Liu, R.-S.; Cheng, B.-M. Versatile Phosphors BaY2Si3O10:RE (RE = Ce3+, Tb3+, Eu3+) for Light-Emitting Diodes. Opt. Express 2009, 17, 18103. [Google Scholar] [CrossRef]
- Spassky, D.; Kozlova, N.; Zabelina, E.; Kasimova, V.; Krutyak, N.; Ukhanova, A.; Morozov, V.A.; Morozov, A.V.; Buzanov, O.; Chernenko, K.; et al. Influence of the Sc Cation Substituent on the Structural Properties and Energy Transfer Processes in GAGG:Ce Crystals. CrystEngComm 2020, 22, 2621–2631. [Google Scholar] [CrossRef]
- Katelnikovas, A.; Bettentrup, H.; Dutczak, D.; Kareiva, A.; Jüstel, T. On the Correlation between the Composition of Pr3 Doped Garnet Type Materials and Their Photoluminescence Properties. J. Lumin. 2011, 131, 2754–2761. [Google Scholar] [CrossRef]
- Zhou, D.; Tao, L.; Yu, Z.; Jiao, J.; Xu, W. Efficient Chromium Ion Passivated CsPbCl3:Mn Perovskite Quantum Dots for Photon Energy Conversion in Perovskite Solar Cells. J. Mater. Chem. C 2020, 8, 12323–12329. [Google Scholar] [CrossRef]
- Malysa, B.; Meijerink, A.; Jüstel, T. Temperature Dependent Cr3+ Photoluminescence in Garnets of the Type X3Sc2Ga3O12 (X = Lu, Y, Gd, La). J. Lumin. 2018, 202, 523–531. [Google Scholar] [CrossRef]
- Dantelle, G.; Boulon, G.; Guyot, Y.; Testemale, D.; Guzik, M.; Kurosawa, S.; Kamada, K.; Yoshikawa, A. Research on Efficient Fast Scintillators: Evidence and X-Ray Absorption Near Edge Spectroscopy Characterization of Ce4+ in Ce3+, Mg2+-Co-Doped Gd3Al2Ga3O12 Garnet Crystal. Phys. Status Solidi Basic Res. 2020, 257, 1900510. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Buganov, O.; Auffray, E.; Bohacek, P.; Korjik, M.; Kozlov, D.; Nargelas, S.; Nikl, M.; Tikhomirov, S.; Tamulaitis, G.; et al. Measurement of Non-Equilibrium Carriers Dynamics in Ce-Doped YAG, LuAG and GAGG Crystals with and without Mg-Codoping. J. Lumin. 2018, 194, 1–7. [Google Scholar] [CrossRef]
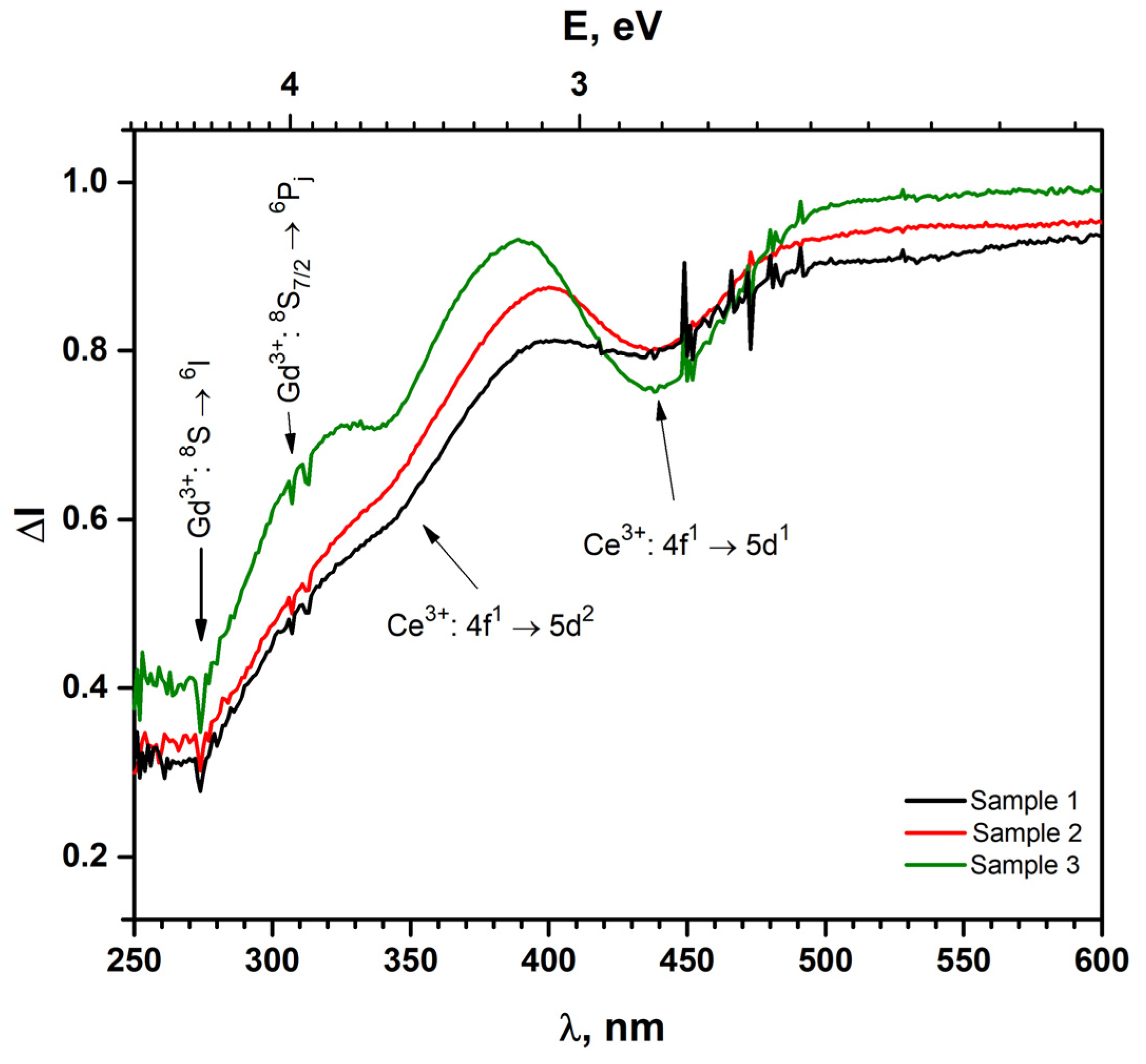
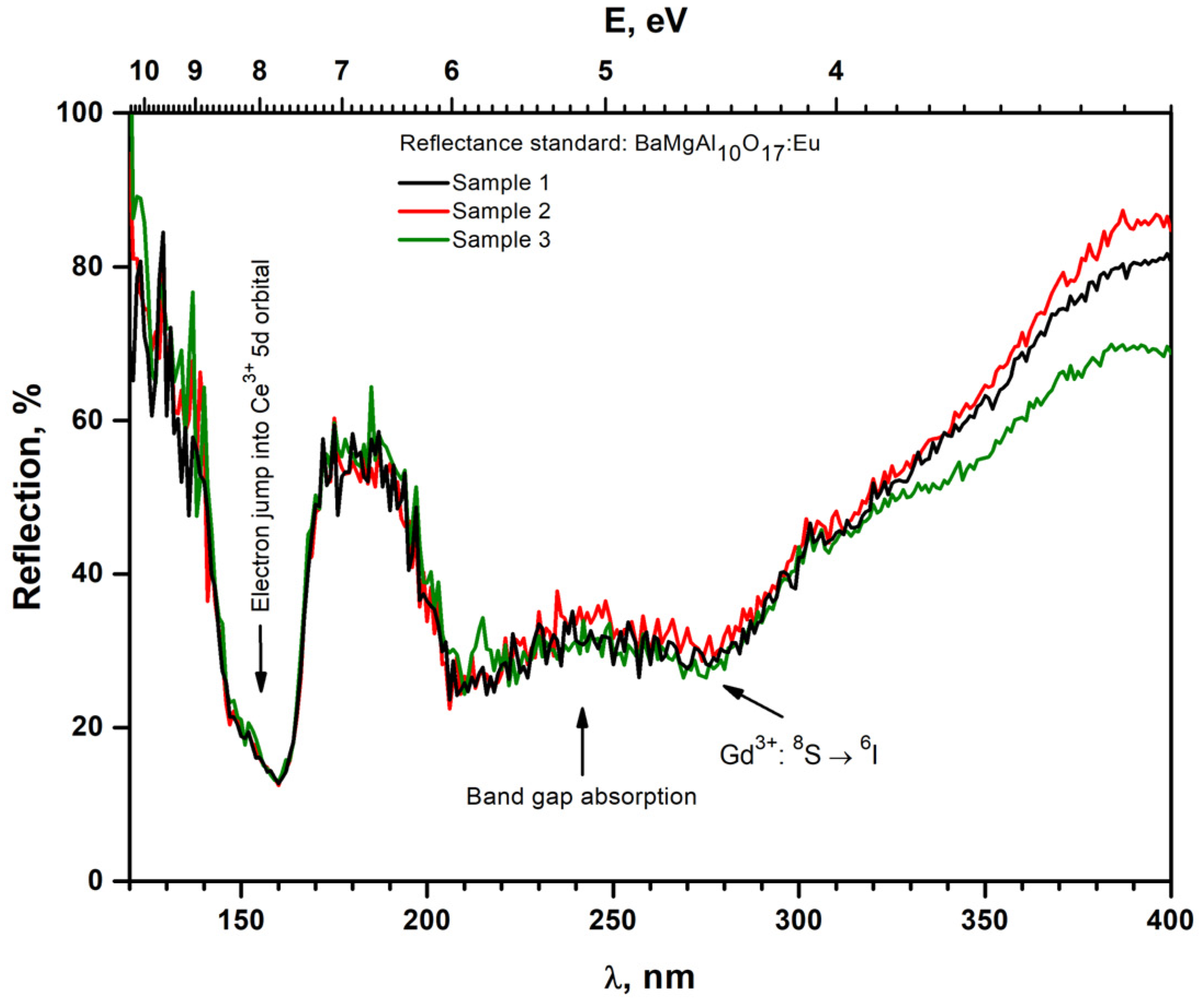
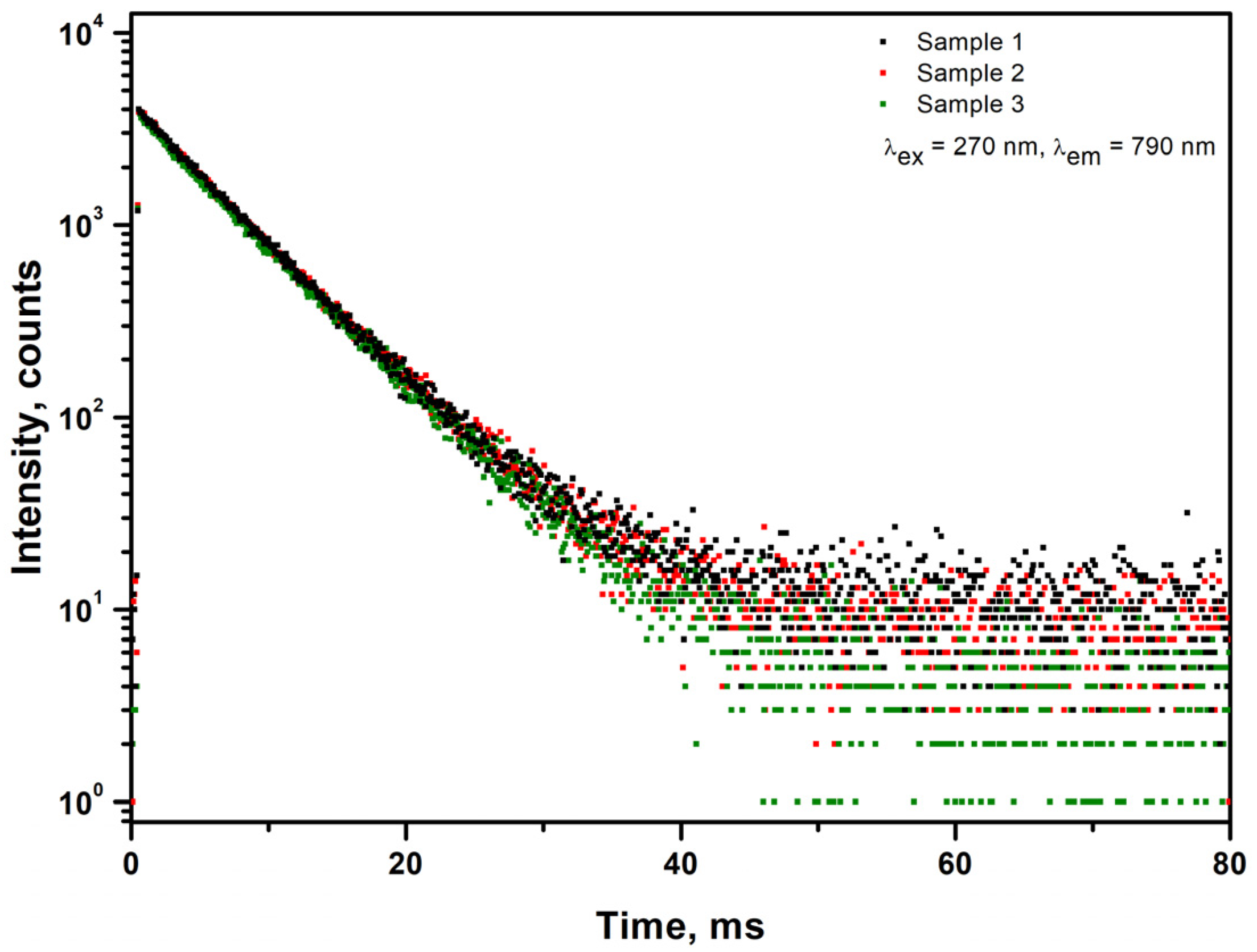
| Name of Sample | Formula of Sample |
|---|---|
| Sample 1 | GAGG: Ce 0.05%, Ca 100 ppm, Mg 7 ppm, Cr 15 ppm |
| Sample 2 | GAGG: Ce 0.05%, Ca 94 ppm, Mg 8 ppm, Cr 15 ppm |
| Sample 3 | GAGG: Ce 0.05%, Ca 57 ppm, Mg 9 ppm, Cr 15 ppm |
| Sample | Decay Time (ms) |
|---|---|
| GAGG: Ce 0.05%, Ca 100 ppm, Mg 7 ppm, Cr 15 ppm (Sample 1) | 5.9 |
| GAGG: Ce 0.05%, Ca 94 ppm, Mg 8 ppm, Cr 15 ppm (Sample 2) | 6 |
| GAGG: Ce 0.05%, Ca 57 ppm, Mg 9 ppm, Cr 15 ppm (Sample 3) | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkrataite, G.; Laurinavicius, G.; Enseling, D.; Zarkov, A.; Jüstel, T.; Skaudzius, R. Characterization of GAGG Doped with Extremely Low Levels of Chromium and Exhibiting Exceptional Intensity of Emission in NIR Region. Crystals 2021, 11, 673. https://doi.org/10.3390/cryst11060673
Inkrataite G, Laurinavicius G, Enseling D, Zarkov A, Jüstel T, Skaudzius R. Characterization of GAGG Doped with Extremely Low Levels of Chromium and Exhibiting Exceptional Intensity of Emission in NIR Region. Crystals. 2021; 11(6):673. https://doi.org/10.3390/cryst11060673
Chicago/Turabian StyleInkrataite, Greta, Gerardas Laurinavicius, David Enseling, Aleksej Zarkov, Thomas Jüstel, and Ramunas Skaudzius. 2021. "Characterization of GAGG Doped with Extremely Low Levels of Chromium and Exhibiting Exceptional Intensity of Emission in NIR Region" Crystals 11, no. 6: 673. https://doi.org/10.3390/cryst11060673
APA StyleInkrataite, G., Laurinavicius, G., Enseling, D., Zarkov, A., Jüstel, T., & Skaudzius, R. (2021). Characterization of GAGG Doped with Extremely Low Levels of Chromium and Exhibiting Exceptional Intensity of Emission in NIR Region. Crystals, 11(6), 673. https://doi.org/10.3390/cryst11060673