Pyridine-Based Three-Ring Bent-Shape Supramolecular Hydrogen Bond-Induced Liquid Crystalline Complexes: Preparation and Density Functional Theory Investigation
Abstract
1. Introduction
2. Results and Discussion
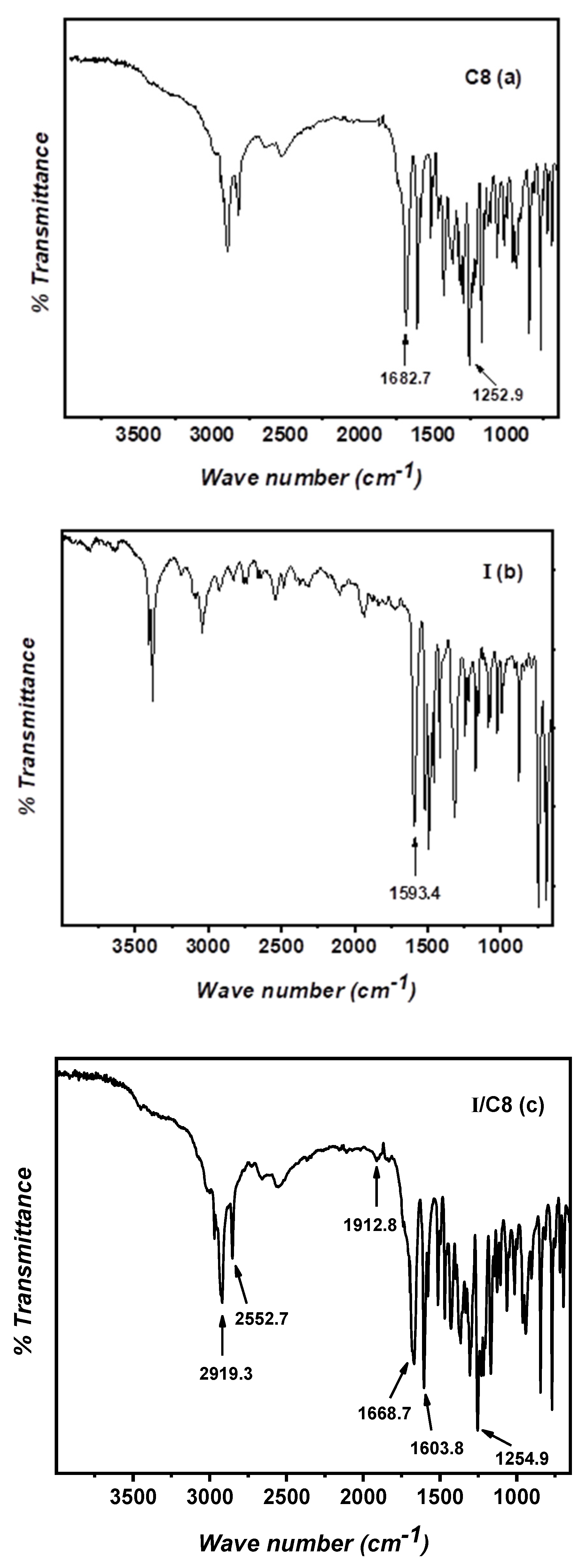
2.1. Fourier-Transform Infrared (FT–IR) Characterizations
2.2. Theoretical Calculations (DFT)
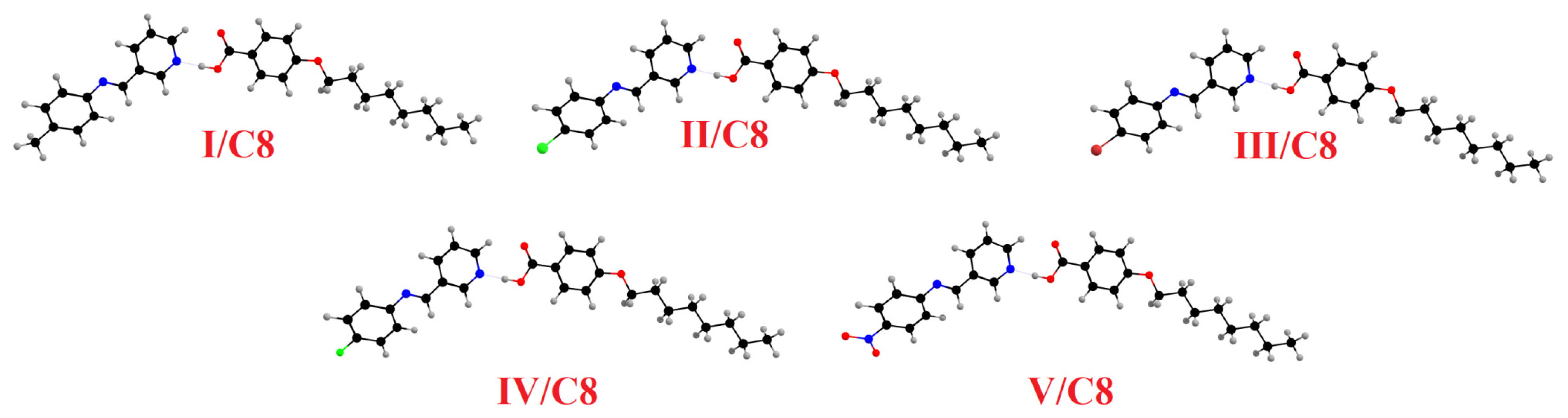
Molecular Geometry
2.3. Molecular Electrostatic Potential (MEP)
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Shimizu, K.; Ferreira da Silva, J. Halogen and hydrogen bonding interplay in the crystal packing of halometallocenes. Molecules 2018, 23, 2959. [Google Scholar] [CrossRef]
- Borissova, A.O.; Antipin, M.Y.; Perekalin, D.S.; Lyssenko, K.A. Crucial role of Ru⋯H interactions in the crystal packing of ruthenocene and its derivatives. CrystEngComm 2008, 10, 827–832. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.H.; Park, Y.W.; Lagerwall, J.P.; Scalia, G. Ultralong ordered nanowires from the concerted self-assembly of discotic liquid crystal and solvent molecules. Langmuir 2015, 31, 9432–9440. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.A.; Alrefay, B.S.; Haddad, B.A.; Albalawi, R.F.; Aljuhani, R.H.; Aloqebi, L.D.; Alsenani, S.F. Induced phases of new H-bonded supramolecular liquid crystal complexes; mesomorphic and geometrical estimation. Molecules 2020, 25, 1549. [Google Scholar] [CrossRef]
- Scholte, A.; Hauche, S.; Wagner, M.; Prehm, M.; Poppe, S.; Chen, C.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. A self-assembled liquid crystal honeycomb of highly stretched (3-1-1)-hexagons. Chem. Commun. 2020, 56, 62–65. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.; Yao, K.; Chen, Y. Self-assembly of diblock polythiophenes with discotic liquid crystals on side chains for the formation of a highly ordered nanowire morphology. ACS Appl. Mater. Interfaces 2013, 5, 8321–8328. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wen, W.; Yang, Z.; Chen, A. Liquid Crystalline Nanowires by Polymerization Induced Hierarchical Self-Assembly. Macromolecules 2019, 53, 465–472. [Google Scholar] [CrossRef]
- Chen, J.-W.; Huang, C.-C.; Chao, C.-Y. Supramolecular liquid-crystal gels formed by polyfluorene-based π-conjugated polymer for switchable anisotropic scattering device. ACS Appl. Mater. Interfaces 2014, 6, 6757–6764. [Google Scholar] [CrossRef]
- Foelen, Y.; van der Heijden, D.A.; Del Pozo, M.; Lub, J.; Bastiaansen, C.W.; Schenning, A.P. An Optical Steam Sterilization Sensor Based On a Dual-Responsive Supramolecular Cross-Linked Photonic Polymer. ACS Appl. Mater. Interfaces 2020, 12, 16896–16902. [Google Scholar] [CrossRef]
- Todisco, M.; Fraccia, T.P.; Smith, G.P.; Corno, A.; Bethge, L.; Klussmann, S.; Paraboschi, E.M.; Asselta, R.; Colombo, D.; Zanchetta, G. Nonenzymatic polymerization into long linear RNA templated by liquid crystal self-assembly. ACS Nano 2018, 12, 9750–9762. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Am. Chem. Soc. 1931, 53, 3225–3237. [Google Scholar] [CrossRef]
- Steiner, T.; Saenger, W. Role of CH. cntdot.. cntdot.. cntdot. O hydrogen bonds in the coordination of water molecules. Analysis of neutron diffraction data. J. Am. Chem. Soc. 1993, 115, 4540–4547. [Google Scholar] [CrossRef]
- Watt, S.W.; Dai, C.; Scott, A.J.; Burke, J.M.; Thomas, R.L.; Collings, J.C.; Viney, C.; Clegg, W.; Marder, T.B. Structure and Phase Behavior of a 2: 1 Complex between Arene-and Fluoroarene-Based Conjugated Rigid Rods. Angew. Chem. Int. Ed. 2004, 43, 3061–3063. [Google Scholar] [CrossRef]
- Arikainen, E.O.; Boden, N.; Bushby, R.J.; Lozman, O.R.; Vinter, J.G.; Wood, A. Complimentary polytopic interactions. Angew. Chem. 2000, 112, 2423–2426. [Google Scholar] [CrossRef]
- Lozman, O.R.; Bushby, R.J.; Vinter, J.G. Complementary polytopic interactions (CPI) as revealed by molecular modelling using the XED force field. J. Chem. Soc. Perkin Trans. 2001, 2, 1446–1452. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Nakazawa, T.; Ishihara, M.; Yamaguchi, M.; Sugihara, Y.; Murata, I. Synthesis and Properties of 3-Substituted 8 H-3-Azaheptalen-8-ones. Chem. Lett. 1990, 19, 91–92. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kanie, K. Hydrogen-bonded liquid crystalline materials: Supramolecular polymeric assembly and the induction of dynamic function. Macromol. Rapid Commun. 2001, 22, 797–814. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Horton, P.N.; Hursthouse, M.B.; Legon, A.C.; Bruce, D.W. Halogen bonding: A new interaction for liquid crystal formation. J. Am. Chem. Soc. 2004, 126, 16–17. [Google Scholar] [CrossRef]
- Price, D.J.; Willis, K.; Richardson, T.; Ungar, G.; Bruce, D.W. Hydrogen bonded liquid crystals from nitrophenols and alkoxystilbazoles. J. Mater. Chem. 1997, 7, 883–891. [Google Scholar] [CrossRef]
- Treybig, A.; Eissflog, W.W.; Plass, M.; Kresse, H. Hydrogen-bond induced liquid crystalline phases in compounds with a carbonyl group as proton acceptor. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1997, 300, 127–141. [Google Scholar] [CrossRef]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.; Skoulios, A. Liquid crystalline behaviour of hydrogen bonded complexes of a non-mesogenic anil with pn-alkoxybenzoic acids. Liq. Cryst. 1997, 22, 51–60. [Google Scholar] [CrossRef]
- Lin, H.-C.; Ko, C.-W.; Guo, K.; Cheng, T.-W. Supramolecular liquid crystals containing isoquinoline hydrogen-bonded acceptors. Liq. Cryst. 1999, 26, 613–618. [Google Scholar] [CrossRef]
- Demus, D.; Goodby, J.W.; Gray, G.W.; Spiess, H.W.; Vill, V. Handbook of Liquid Crystals, Volume 2A: Low Molecular Weight Liquid Crystals I: Calamitic Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Torgova, S.I.; Strigazzi, A. Hydrogen bonding and its role in the liquid crystal formation and properties. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1999, 336, 229–245. [Google Scholar] [CrossRef]
- Tal’roze, R.; Shatalova, A.; Shandryuk, G. Development and stabilization of liquid crystalline phases in hydrogen-bonded systems. Polym. Sci. Ser. B 2009, 51, 57–83. [Google Scholar] [CrossRef]
- He, W.-L.; Wang, L.; Yang, Z.; Yang, H.; Xie, M.-W. Synthesis and optical behaviour of hydrogen-bonded liquid crystals based on a chiral pyridine derivative. Liq. Cryst. 2011, 38, 1217–1225. [Google Scholar] [CrossRef]
- Muniprasad, M.; Srinivasulu, M.; Chalapathi, P.; Potukuchi, D. Influence of chemical moieties and the flexible chain for the tilted smectic phases in linear hydrogen bonded liquid crystals with Schiff based pyridene derivatives. J. Mol. Struct. 2012, 1015, 181–191. [Google Scholar] [CrossRef]
- Pai, P.; Kulkarni, S.D.; Srinivasulu, M.; Baral, M.; Apoorva, M.; Bhagavath, P. Enhanced conductivity in nanoparticle doped hydrogen bonded binary mixture. J. Mol. Liq. 2019, 296, 111754. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, X.; Yang, H. Synthesis and mesomorphic properties of two series of hydrogen-bonded liquid crystals based on laterally fluorinated benzoic acid and 4, 4′-bipyridine with a molar ratio of 2:1. Mol. Cryst. Liq. Cryst. 2012, 557, 1–10. [Google Scholar] [CrossRef]
- Miranda, M.D.; Chávez, F.V.; Maria, T.M.; Eusebio, M.E.S.; Sebastião, P.; Silva, M.R. Self-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: Solvent-free synthesis by mechanochemistry. Liq. Cryst. 2014, 41, 1743–1751. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Voutsas, E.C.; Boulougouris, G.C.; Economou, I.G.; Tassios, D.P. Water/hydrocarbon phase equilibria using the thermodynamic perturbation theory. Ind. Eng. Chem. Res. 2000, 39, 797–804. [Google Scholar] [CrossRef]
- Thote, A.J.; Gupta, R.B. Hydrogen-bonding effects in liquid crystals for application to LCDs. Ind. Eng. Chem. Res. 2003, 42, 1129–1136. [Google Scholar] [CrossRef]
- Crisp, G.T.; Jiang, Y.-L. Intramolecular hydrogen bonding of nucleobases. Tetrahedron Lett. 2002, 43, 3157–3160. [Google Scholar] [CrossRef]
- Lammers, J.N. Phase behavior of glycol in gas pipeline calculated. Oil Gas J. 1991, 89, 15. [Google Scholar]
- Kataoka, H.; Shigeno, N.; Munakata, M.; Urabe, T. Reflective Guest-Host Liquid-Crystal Display Device. U.S. Patent 6016178A, 18 January 2000. [Google Scholar]
- Matsude, M. Reflective Liquid Crystal Display Device Having Nematic Liquid Crystal and Dichroic Dye. Japanese Patent JP 19564, 2000. [Google Scholar]
- Acree, W.E., Jr.; Chickos, J.S. Phase change enthalpies and entropies of liquid crystals. J. Phys. Chem. Ref. Data 2006, 35, 1051–1330. [Google Scholar] [CrossRef]
- Oweimreen, G.; Morsy, M. DSC studies on p-cyanophenyl p-(n-alkyl) benzoate liquid crystals: Evidence for polymorphism and conformational change. Thermochim. Acta 1999, 325, 111–118. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Heat capacities and entropies of organic compounds in the condensed phase. Volume III. J. Phys. Chem. Ref. Data 1996, 25, 1–525. [Google Scholar] [CrossRef]
- Oweimreen, G.; Morsy, M. DSC studies on p-(n-alkyl)-p′-cyanobiphenyl (RCB’s) and p-(n-alkoxy)-p′-cyanobiphenyl (ROCB’s) liquid crystals. Thermochim. Acta 2000, 346, 37–47. [Google Scholar] [CrossRef]
- Yousif, Y.Z.; Al-hamdani, A.J. Liquid-crystalline behaviour of some bis (4-alkyloxyphenyl) thiazolo [5,4-d] dithiazoles. Liq. Cryst. 1993, 15, 451–460. [Google Scholar] [CrossRef]
- Tsuji, K.; Sorai, M.; Suga, H.; Seki, S. Heat Capacity and Thermodynamic Properties of p′-Substituted p-n-Hexyloxybenzylideneaniline. I. p-n-Hexyloxybenzylideneamno-p′-Benzonitrile (HBAB). Mol. Cryst. Liq. Cryst. 1979, 55, 71–88. [Google Scholar] [CrossRef]
- Chidichimo, G.; Salerno, G.; Veltri, L.; Gabriele, B.; Nicoletta, F.P. Synthesis and mesomorphic properties of new liquid crystalline stilbene derivatives containing vinyloxyalkoxy chains. Liq. Cryst. 2004, 31, 733–737. [Google Scholar] [CrossRef]
- Wang, X.; Cui, W.; Li, B.; Zhang, X.; Zhang, Y.; Huang, Y. Supramolecular self-assembly of two-component systems comprising aromatic amides/Schiff base and tartaric acid. Front. Chem. Sci. Eng. 2020, 14, 1112–1121. [Google Scholar] [CrossRef]
- Cramer, J.; Jiang, X.; Schönemann, W.; Silbermann, M.; Zihlmann, P.; Siegrist, S.; Fiege, B.; Jakob, R.P.; Rabbani, S.; Maier, T. Enhancing the enthalpic contribution of hydrogen bonds by solvent shielding. RSC Chem. Biol. 2020, 1, 281–287. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Cui, W.; Wang, X.; Li, B.; Zhang, Y.; Yang, J. Novel liquid crystalline organogelators based on terephthalic acid and terephthalaldehyde derivatives: Properties and promotion through the formation of halogen bonding. New J. Chem. 2020, 44, 614–625. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Zhang, J.; Li, K.; Cao, M.; Mo, L.; Hu, G.; Chen, Y.; Yu, H.; Yang, H. Photoresponsive iodine-bonded liquid crystals based on azopyridine derivatives with a low phase-transition temperature. Liq. Cryst. 2019, 46, 37–44. [Google Scholar] [CrossRef]
- Saunders, M.; Hyne, J.B. Study of hydrogen bonding in systems of hydroxylic compounds in carbon tetrachloride through the use of NMR. J. Chem. Phys. 1958, 29, 1319–1323. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.; Oh, J.; Park, S.; Park, S.; Ishii, Y. Synthesis of 13C-,15N-labeled graphitic carbon nitrides and NMR-based evidence of hydrogen-bonding assisted two-dimensional assembly. Chem. Mater. 2017, 29, 5080–5089. [Google Scholar] [CrossRef]
- Lam, R.K.; Smith, J.W.; Saykally, R.J. Communication: Hydrogen Bonding Interactions in Water-Alcohol Mixtures from X-ray Absorption Spectroscopy; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Pothoczki, S.; Pethes, I.; Pusztai, L.; Temleitner, L.; Csókás, D.; Kohara, S.; Ohara, K.; Bakó, I. Hydrogen bonding and percolation in propan-2-ol--water liquid mixtures: X-ray diffraction experiments and computer simulations. arXiv 2020, arXiv:2001.11923. [Google Scholar]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.M.; Storey, J.M.D.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Sherif, S.; Nafee, H.A.A. Mohamed Hagar New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine Derivatives. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Alhaddad, O.; Ahmed, H.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef] [PubMed]
- Babkov, L.; Korolevich, M.; Moiseikina, E. Hydrogen bonding, IR spectrum, and the structure of methyl-β-D-glucopyranoside. J. Struct. Chem. 2012, 53, 55–62. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Alnoman, R.; Ahmed, H.A.; Hagar, M. Synthesis, optical, and geometrical approaches of new natural fatty acids’ esters/Schiff base liquid crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behaviour of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1611–1620. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef]
- Abdullah Alshabanah, L.; Al-Mutabagani, L.A.; Ahmed, H.A.; Hagar, M. Induced wide nematic phase by seven-ring supramolecular H-bonded systems: Experimental and computational evaluation. Molecules 2020, 25, 1694. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Abu Al-Ola, K.A.; Hagar, M.; Ahmed, H.A. Chair-and V-Shaped of H-bonded supramolecular complexes of azophenyl nicotinate derivatives; mesomorphic and DFT molecular geometry aspects. Molecules 2020, 25, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Hagar, M.; Alnoman, R.B.; Jaremko, M.; Emwas, A.-H.; Ahmed, H.A. Mesomorphic, Optical and DFT Aspects of Near to Room-Temperature Calamitic Liquid Crystal. Crystals 2020, 10, 1044. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Abdullah Alshabanah, L.; Ahmed, H.A.; Abu Al-Ola, K.A.; Hagar, M. New Rod-Like H-Bonded Assembly Systems: Mesomorphic and Geometrical Aspects. Crystals 2020, 10, 795. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Naoum, M.M.; Hagar, M.; Ahmed, H.A. Experimental and computational approaches of newly polymorphic supramolecular H-bonded liquid crystal complexes. Front. Chem. 2020, 8, 571120. [Google Scholar] [CrossRef]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Kirsch, P.; Bremer, M. Understanding fluorine effects in liquid crystals. ChemPhysChem 2010, 11, 357–360. [Google Scholar] [CrossRef]
- Subhapriya, P.; Sadasivam, K.; Mohan, M.M.; Vijayanand, P. Experimental and theoretical investigation of p–n alkoxy benzoic acid based liquid crystals–A DFT approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 123, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Paterson, D.A.; Abberley, J.P.; Harrison, W.T.; Storey, J.M.; Imrie, C.T. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 127–146. [Google Scholar] [CrossRef]
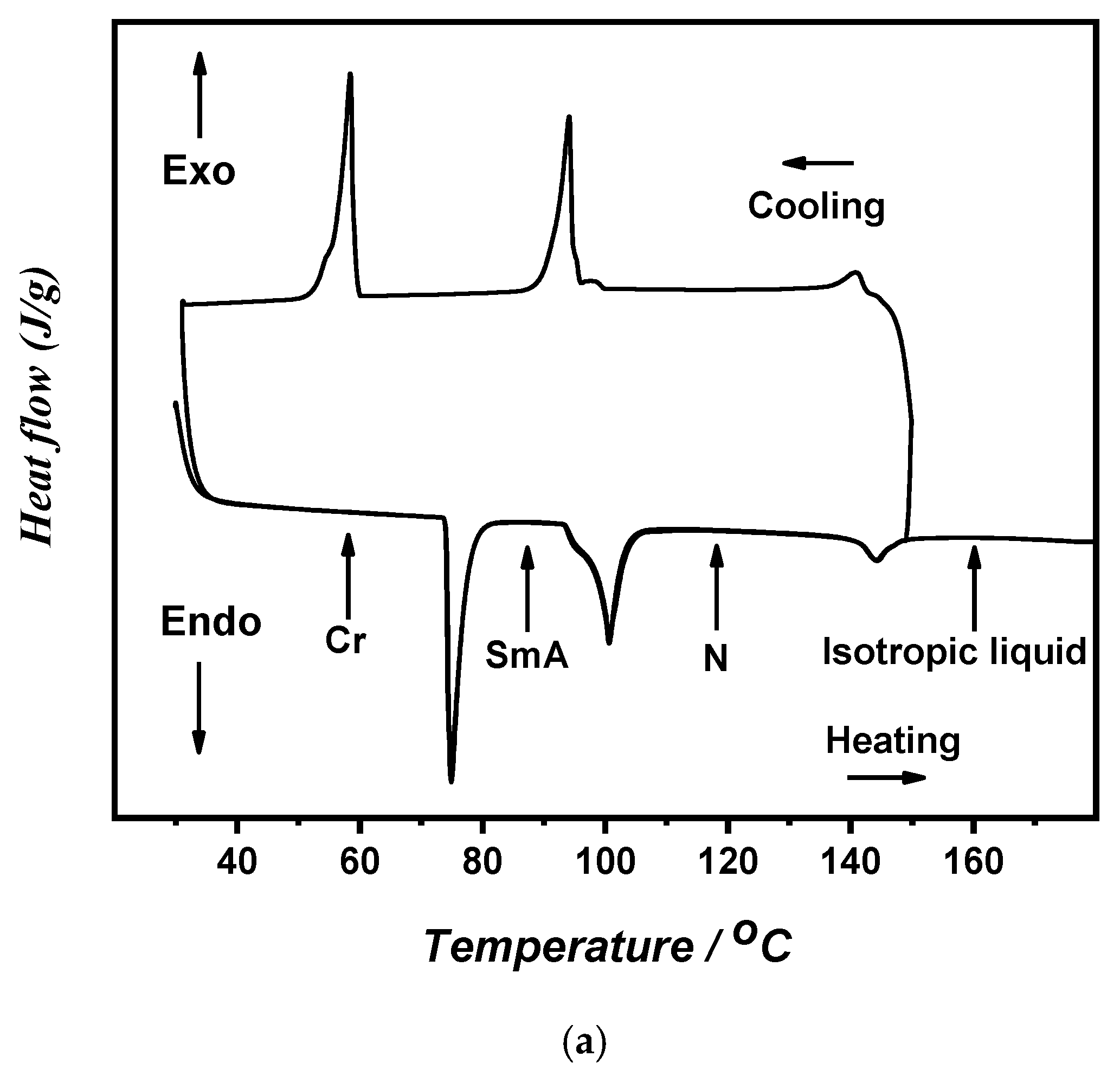

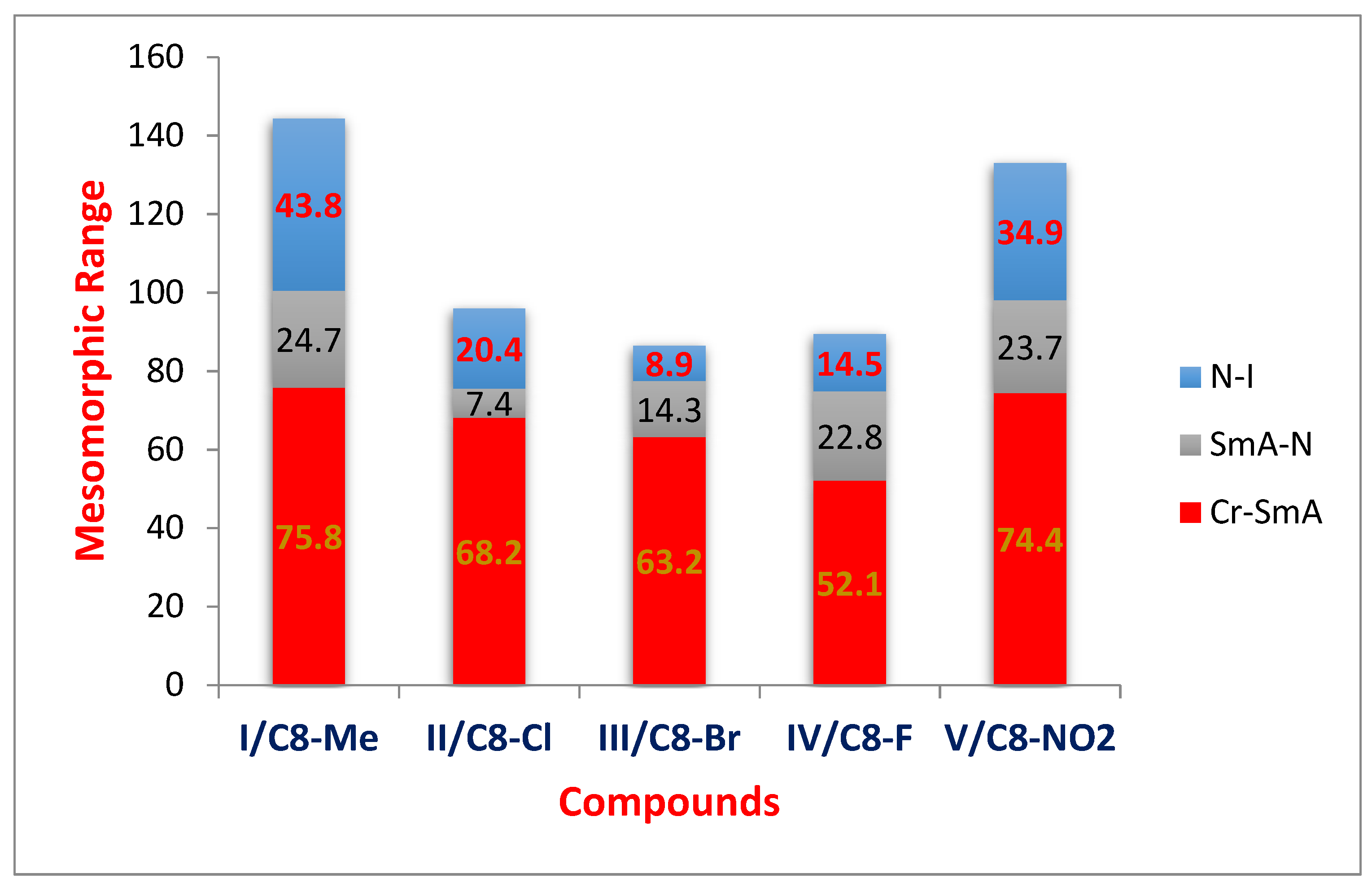

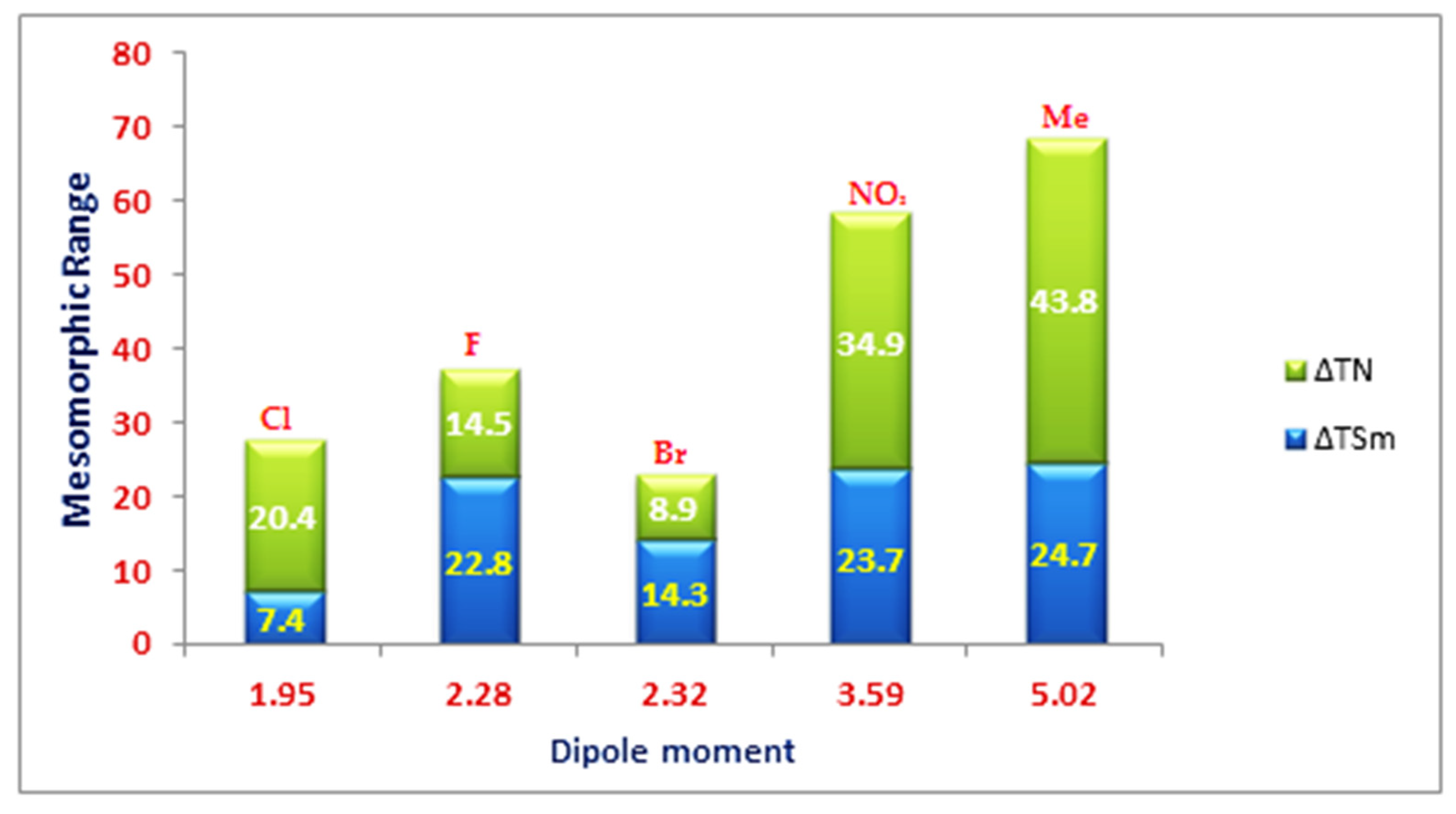
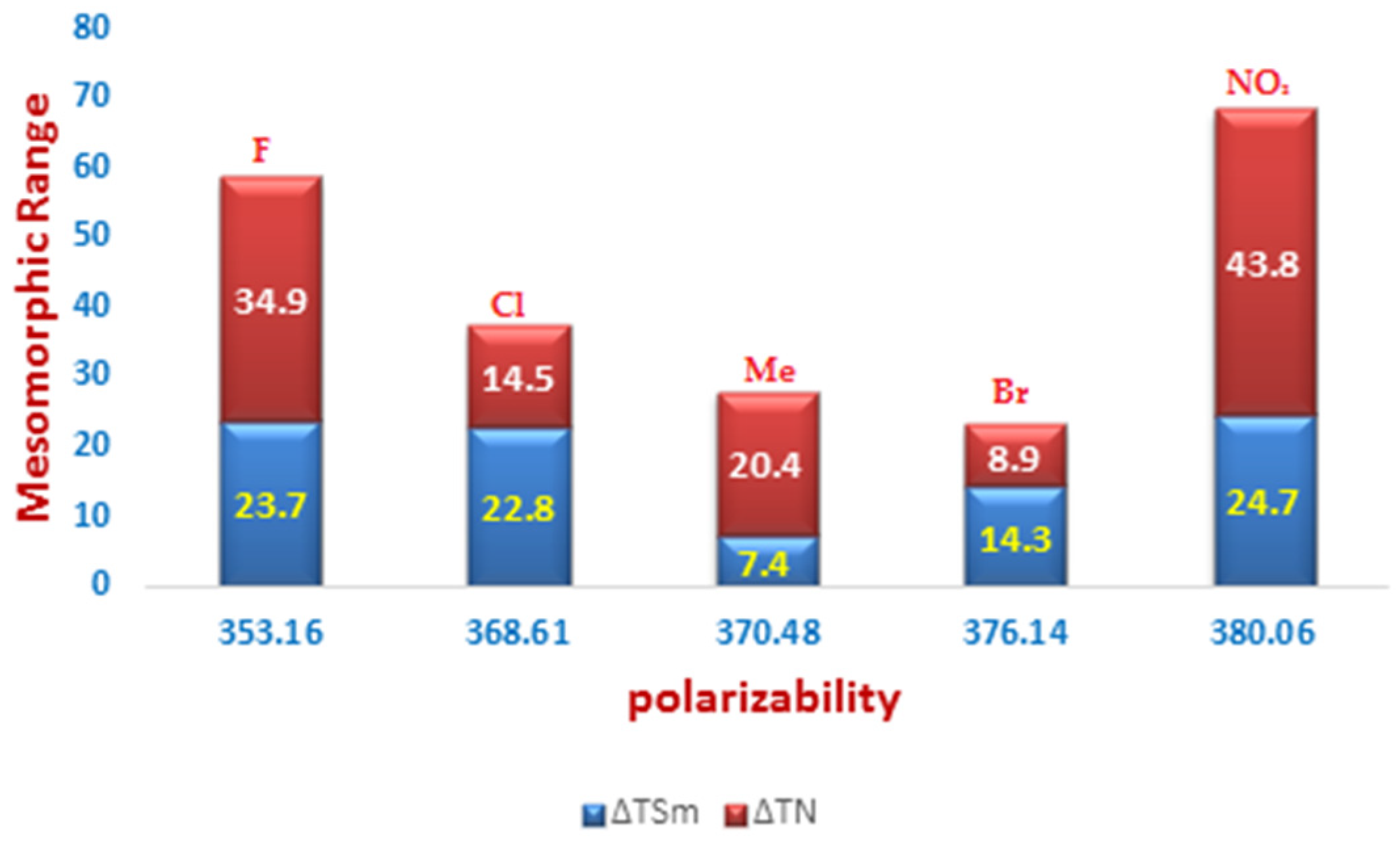



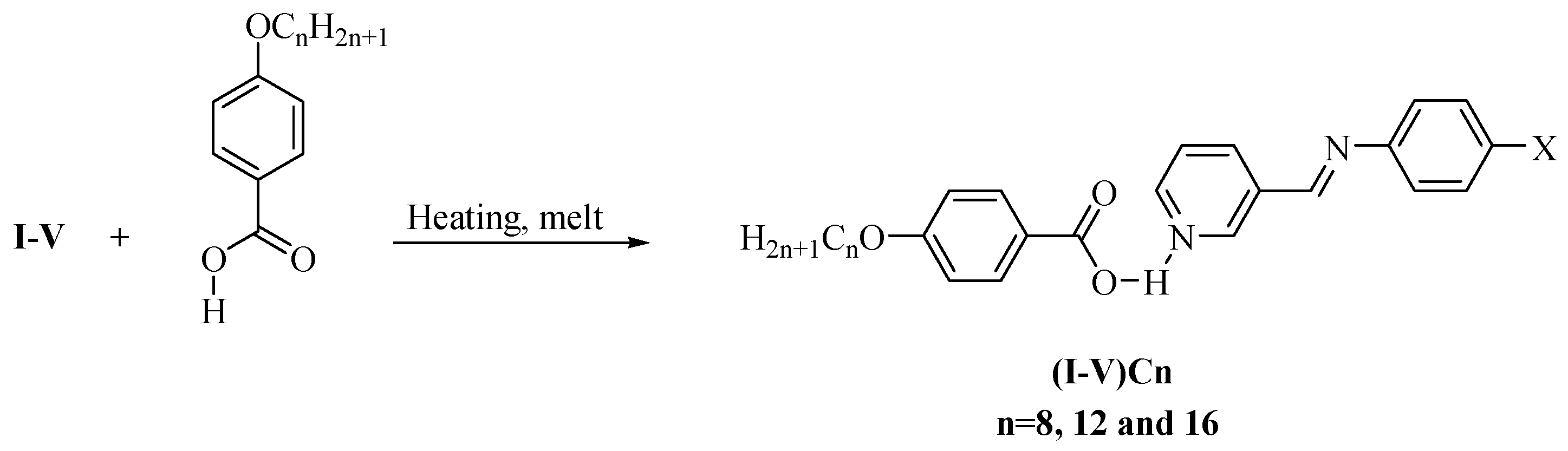
| Compounds | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol | |
|---|---|---|---|---|---|---|---|---|---|
| TCr-SmA | ΔHCr-SmA | TSmA-N | ΔHSmA-N | TSmA-I | ΔHSmA-I | TN-I | ΔHN-I | ||
| I/C8 | C8–Me | 75.8 | 2.01 | 100.5 | 1.66 | 144.3 | 0.34 | ||
| II/C8 | C8–Cl | 68.2 | 4.99 | 75.6 | 1.82 | 96.0 | 0.15 | ||
| III/C8 | C8–Br | 63.2 | 1.12 | 77.5 | 2.35 | 86.4 | 0.06 | ||
| IV/C8 | C8–F | 52.1 | 3.23 | 74.9 | 0.89 | 89.4 | 0.20 | ||
| V/C8 | C8–NO2 | 74.4 | 1.22 | 98.1 | 0.97 | 133.0 | 0.99 | ||
| I/C16 | C16–Me | 54.3 | 4.92 | 91.3 | 11.10 | 121.1 | 6.22 | ||
| II/C16 | C16–Cl | 65.7 | 2.15 | 95.1 | 7.70 | ||||
| III/C16 | C16–Br | 67.5 | 4.05 | 94.1 | 10.68 | 130.3 | 2.70 | ||
| IV/C16 | C16–F | 65.8 | 4.17 | 94.8 | 7.70 | 118.5 | 4.10 | ||
| V/C16 | C16–NO2 | 102.0 | 5.70 | 116.4 | 4.30 | 139.3 | 6.03 | ||
| Compounds | HOMO | LUMO | ΔE |
|---|---|---|---|
| I/C8 | −6.11 | −2.33 | 3.78 |
| II/C8 | −6.18 | −2.61 | 3.57 |
| III/C8 | −6.17 | −2.57 | 3.60 |
| IV/C8 | −6.17 | −2.54 | 3.63 |
| V/C8 | −6.26 | −3.35 | 2.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammady, S.Z.; Aldhayan, D.M.; Hagar, M. Pyridine-Based Three-Ring Bent-Shape Supramolecular Hydrogen Bond-Induced Liquid Crystalline Complexes: Preparation and Density Functional Theory Investigation. Crystals 2021, 11, 628. https://doi.org/10.3390/cryst11060628
Mohammady SZ, Aldhayan DM, Hagar M. Pyridine-Based Three-Ring Bent-Shape Supramolecular Hydrogen Bond-Induced Liquid Crystalline Complexes: Preparation and Density Functional Theory Investigation. Crystals. 2021; 11(6):628. https://doi.org/10.3390/cryst11060628
Chicago/Turabian StyleMohammady, Sayed Z., Daifallah M. Aldhayan, and Mohamed Hagar. 2021. "Pyridine-Based Three-Ring Bent-Shape Supramolecular Hydrogen Bond-Induced Liquid Crystalline Complexes: Preparation and Density Functional Theory Investigation" Crystals 11, no. 6: 628. https://doi.org/10.3390/cryst11060628
APA StyleMohammady, S. Z., Aldhayan, D. M., & Hagar, M. (2021). Pyridine-Based Three-Ring Bent-Shape Supramolecular Hydrogen Bond-Induced Liquid Crystalline Complexes: Preparation and Density Functional Theory Investigation. Crystals, 11(6), 628. https://doi.org/10.3390/cryst11060628







