1,12-Diiodo-Ortho-Carborane: A Classic Textbook Example of the Dihalogen Bond †
Abstract
1. Introduction
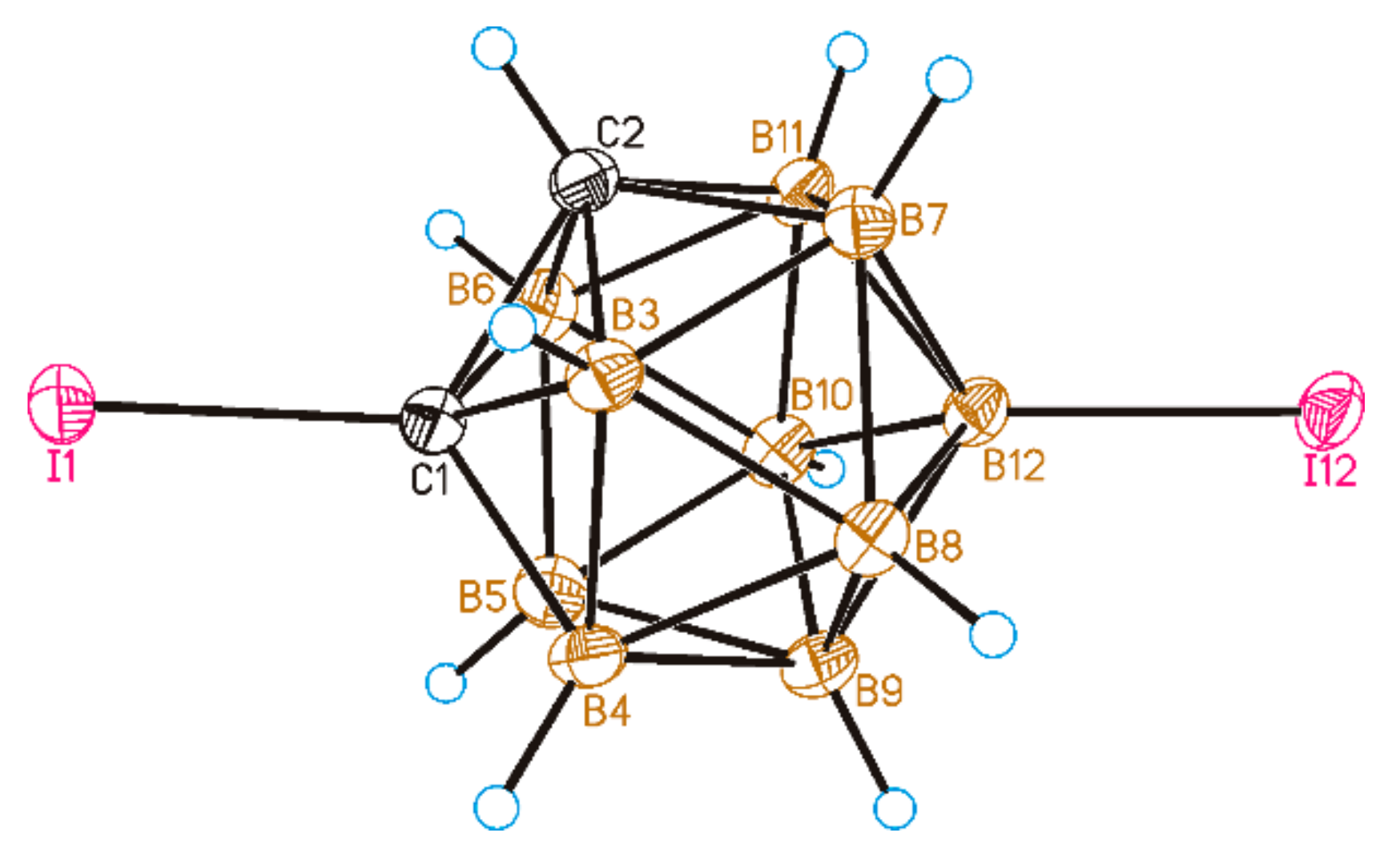
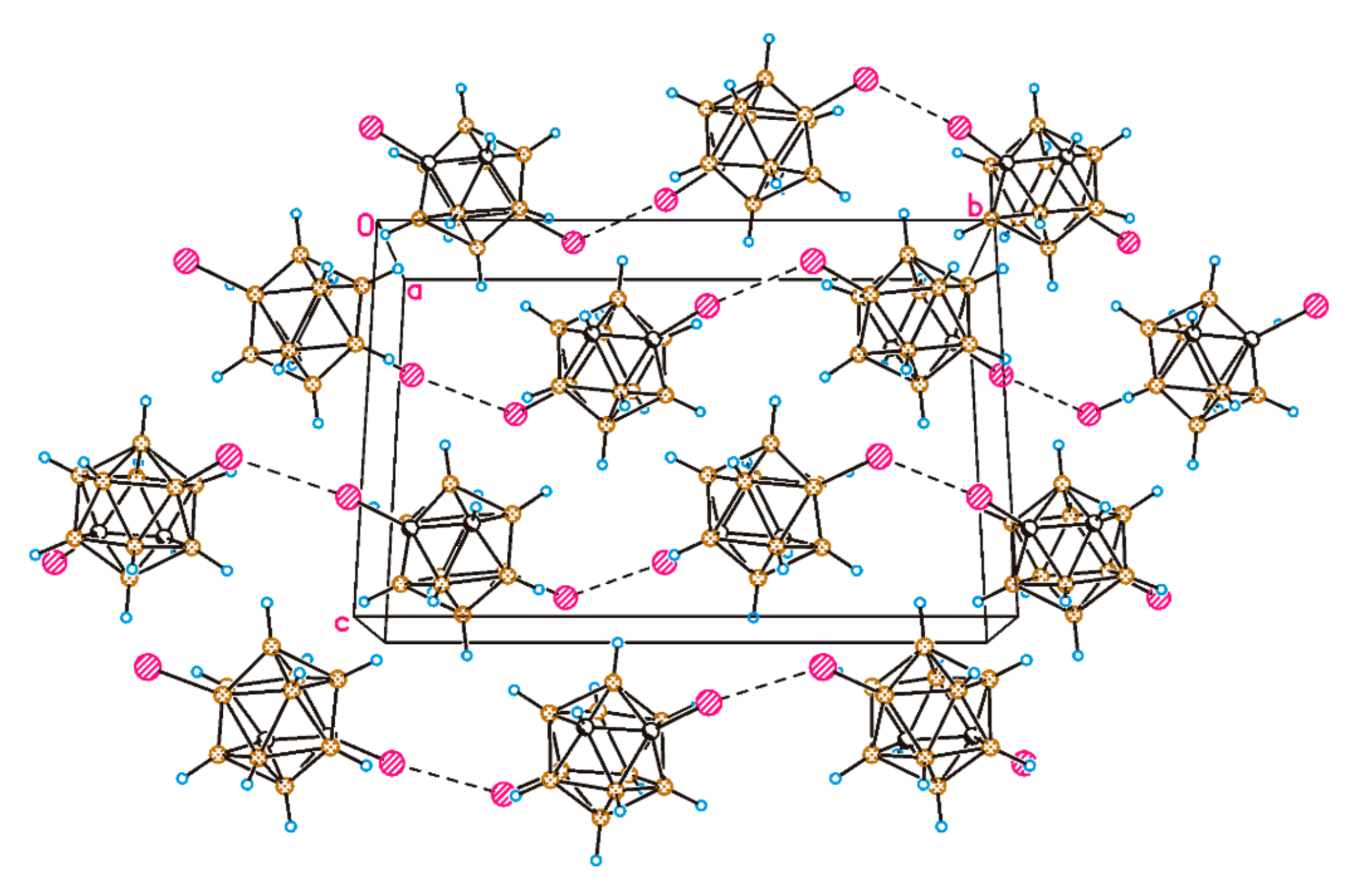
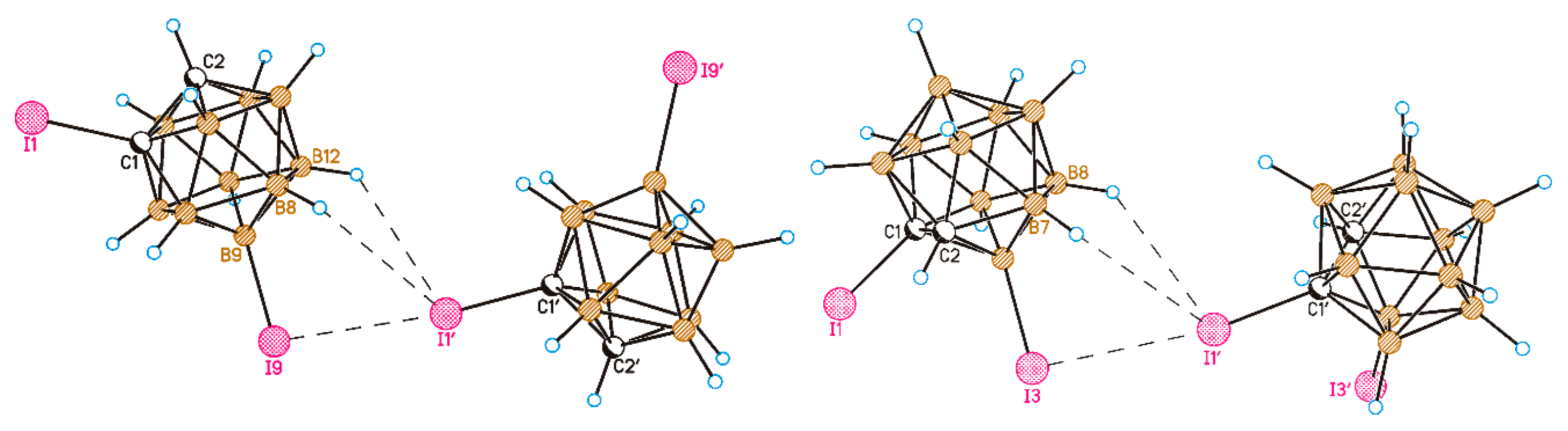
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Cross-Coupling of 9-Iodo-Ortho-Carborane with PhMgBr
3.3. General Synthetic Procedure of C-Iodination of Ortho-Carborane and Its B-I Derivatives
3.4. Synthesis of 1-Iodo-Ortho-Carborane
3.5. X-ray Diffraction Study
3.6. Quantum Chemical Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016; 1042p. [Google Scholar] [CrossRef]
- Kaszynski, P.; Douglass, A.G. Organic derivatives of closo-boranes: A new class of liquid crystal materials. J. Organomet. Chem. 1999, 581, 28–38. [Google Scholar] [CrossRef]
- Pecyna, J.; Pociecha, D.; Kaszynski, P. Zwitterionic pyridinium derivatives of [closo-1- CB9H10]- and [closo-1-CB11H12]- as high Δε additives to a nematic host. J. Mater. Chem. C 2014, 2, 1585–1591. [Google Scholar] [CrossRef]
- Pecyna, J.; Kaszyński, P.; Ringstrand, B.; Pociecha, D.; Pakhomov, S.; Douglass, A.G.; Young, V.G. Synthesis and characterization of quinuclidinium derivatives of the [closo-1-CB11H12]-anion as potential polar components of liquid crystal materials. Inorg. Chem. 2016, 55, 40167–44025. [Google Scholar] [CrossRef]
- Pecyna, J.; Jankowiak, A.; Pociecha, D.; Kaszyński, P. o-Carborane derivatives for probing molecular polarity effects on liquid crystal phase stability and dielectric behavior. J. Mater. Chem. C 2015, 3, 11412–11422. [Google Scholar] [CrossRef]
- Allis, D.G.; Spencer, J.T. Polyhedral-based nonlinear optical materials. 2. Theoretical investigation of some new high non-linear optical response compounds involving polyhedral bridges with charged aromatic donors and acceptors. Inorg. Chem. 2001, 40, 3373–3380. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Wang, L.; Li, R.-R.; Ye, J.-T.; Chen, Z.-Z.; Chen, H.; Qiu, Y.-Q.; Xie, H.-M. Second-order nonlinear optical properties of carboranylated square-planar Pt(II) zwitterionic complexes: One-/two-dimensional difference and substituent effect. J. Phys. Chem. A 2016, 120, 9330–9340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, Z.; Moxey, G.J.; Morshedi, M.; Barlow, A.; Wang, G.; Quintana, C.; Zhang, C.; Cifuentes, M.P.; Humphrey, M.G. Syntheses and quadratic nonlinear optical properties of 2,7-fluorenylene- and 1,4-phenylene-functionalized o-carboranes. Dalton Trans. 2019, 48, 12549–12559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Ye, J.-T.; Zhang, Y.; Zhao, Y.-Y.; Qiu, Y.-Q. A thorough understanding of the nonlinear optical properties of BODIPY/carborane/diketopyrrolopyrrole hybrid chromophores: Module contribution, linear combination, one-/two-dimensional difference and carborane’s arrangement. J. Mater. Chem. C 2019, 7, 7531–7547. [Google Scholar] [CrossRef]
- Wedge, T.J.; Hawthorne, M. Multidentate carborane-containing Lewis acids and their chemistry: Mercuracarborands. Coord. Chem. Rev. 2003, 240, 111–128. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y. Carboranes as hydrophobic pharmacophores: Applications for design of nuclear receptor ligands. In Boron-Based Compounds: Potential and Emerging Applications in Medicine; Hey-Hawkins, E., Viñas Teixidor, C., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; pp. 3–19. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, W.; Knobler, C.B.; Hawthorne, M.F. Rigid-rod molecules: Carborods. Synthesis of tetrameric p-carboranes and the crystal structure of bis(tri-n-butylsilyl)tetra-p-carborane. J. Am. Chem. Soc. 1992, 114, 9719–9721. [Google Scholar] [CrossRef]
- Schöberl, U.; Magnera, T.F.; Harrison, R.M.; Fleischer, F.; Pflug, J.L.; Schwab, P.F.H.; Meng, X.; Lipiak, D.; Noll, B.C.; Allured, V.S.; et al. Toward a Hexagonal Grid Polymer: Synthesis, Coupling, and Chemically Reversible Surface-Pinning of the Star Connectors, 1,3,5-C6H3(CB10H10CX)3. J. Am. Chem. Soc. 1997, 119, 3907–3917. [Google Scholar] [CrossRef]
- Fox, M.A.; Cameron, A.M.; Low, P.J.; Paterson, M.A.J.; Batsanov, A.S.; Goeta, A.E.; Rankin, D.W.H.; Robertson, H.E.; Schirlin, J.T. Synthetic and structural studies on C-ethynyl- and C-bromo-carboranes. Dalton Trans. 2006, 3544–3560. [Google Scholar] [CrossRef] [PubMed]
- Safronov, A.V.; Sevryugina, Y.V.; Pichaandi, K.R.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis of closo- and nido-biscarboranes with rigid unsaturated linkers as precursors to linear metallacarborane-based molecular rods. Dalton Trans. 2014, 43, 4969–4977. [Google Scholar] [CrossRef]
- Himmelspach, A.; Warneke, J.; Schäfer, M.; Hailmann, M.; Finze, M. Salts of the dianions [Hg(12-X-closo-1-CB11H10)2]2- (X = I, C≡CH, C≡CFc, C≡CSiiPr3): Synthesis and spectroscopic and structural characterization. Organometallics 2015, 34, 462–469. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Y.; Liu, J.; Spingler, B.; Duttwyler, S. Crystal structure of a carborane endo/exo-dianion and its use in the synthesis of ditopic ligands for supramolecular frameworks. Chem. Commun. 2017, 54, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Spokoyny, A.M.; Mulfort, K.L.; Hawthorne, M.F.; Mirkin, C.A.; Hupp, J.T. Synthesis and Hydrogen Sorption Properties of Carborane Based Metal−Organic Framework Materials. J. Am. Chem. Soc. 2007, 129, 12680–12681. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Farha, O.K.; Spokoyny, A.M.; Mirkin, C.A.; Hupp, J.T.; Snurr, R.Q. Carborane-based metal–organic frameworks as highly selective sorbents for CO2 over methane. Chem. Commun. 2008, 4135–4137. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Spokoyny, A.M.; Farha, O.K.; Snurr, R.Q.; Hupp, J.T.; Mirkin, C.A. Separation of gas mixtures using Co(II) car-borane-based porous coordination polymers. Chem. Commun. 2010, 46, 3478–3480. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-based metal-organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef]
- Andrews, P.; Hardie, M.J.; Raston, C.L. Supramolecular assemblies of globular main group cage species. Coord. Chem. Rev. 1999, 189, 169–198. [Google Scholar] [CrossRef]
- Hardie, M.J.; Raston, C.L. Crystalline hydrogen bonded complexes of o-carborane. CrystEngComm 2001, 3, 162–164. [Google Scholar] [CrossRef]
- Lo, R.; Fanfrlík, J.; Lepšík, M.; Hobza, P. The properties of substituted 3D-aromatic neutral carboranes: The potential for σ-hole bonding. Phys. Chem. Chem. Phys. 2015, 17, 20814–20821. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Oliva-Enrich, J.M. Hydrogen vs. Halogen Bonds in 1-Halo-Closo-Carboranes. Materials 2020, 13, 2163. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, I.V.; Lyssenko, K.A.; Antipin, M.Y. Crystal packing of 8,9,10,12-tetrafluoro-o-carborane: H. F versus H. H contacts. Struct. Chem. 2007, 18, 465–469. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Holub, J.; Růžičková, Z.; Řezáč, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Růžička, A.; Hobza, P. Competition between Halogen, Hydrogen and Dihydrogen Bonding in Brominated Carboranes. ChemPhysChem 2016, 17, 3373–3376. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Fox, M.A.; Howard, J.A.K.; Hughes, A.K.; Johnson, A.L.; Martindale, S.J. 9,12-diiodo-1,2-dicarba-closo-dodecaborane(12). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, o74–o76. [Google Scholar] [CrossRef] [PubMed]
- Barberà, G.; Viñas, C.; Teixidor, F.; Rosair, G.M.; Welch, A.J. Self-assembly of carborane molecules via C-H⋯I hydrogen bonding: The molecular and crystal structures of 3-I-1,2-closo-C2B10H11. J. Chem. Soc. Dalton Trans. 2002, 19, 3647–3648. [Google Scholar] [CrossRef]
- Vaca, A.; Teixidor, F.; Kivekäs, R.; Sillanpää, R.; Viñas, C. A solvent-free regioselective iodination route of ortho-carboranes. Dalton Trans. 2006, 41, 4884–4885. [Google Scholar] [CrossRef]
- Ramachandran, B.M.; Knobler, C.B.; Hawthorne, M.F. Synthesis and structural characterization of symmetrical clo-so-4,7-I2-1,2- C2B10H10 and [(CH3)3NH][nido-2,4-I2-7,8-C2B9H10]. Inorg. Chem. 2006, 45, 336–340. [Google Scholar] [CrossRef]
- Barberà, G.; Vaca, A.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Designed synthesis of new ortho-carborane derivatives: From mono- to polysubstituted frameworks. Inorg. Chem. 2008, 47, 7309–7316. [Google Scholar] [CrossRef]
- Puga, A.V.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Iodinated ortho-carboranes as versatile building blocks to design intermolecular interactions in crystal lattices. Chem. Eur. J. 2009, 15, 9764–9772. [Google Scholar] [CrossRef]
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992, 92, 209–223. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and Other σ-Hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Kolář, M.; Hostaš, J.; Hobza, P. The strength and directionality of a halogen bond are co-determined by the magnitude and size of the σ-hole. Phys. Chem. Chem. Phys. 2014, 16, 9987–9996. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H2C(X)–X···X– (X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Burakov, N.; Kanibolotsky, A.L.; Mikhailov, V.A. Multiple Noncovalent Bonding in Halogen Complexes with Oxygen Organics. I. Tertiary Amides. J. Phys. Chem. A 2016, 120, 4179–4190. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Zhigareva, G.G.; Kazantsev, A.V. Some reactions of barene Gringard reagents. Zh. Obshch. Khim. 1968, 38, 89–92. [Google Scholar]
- Zakharkin, L.I.; Podvisotskaya, L.S. Cleavage of 1,2-dihalobarenes by alcohols to C,C?-dihalodicarbaundecaboranes (13). Russ. Chem. Bull. 1966, 15, 742. [Google Scholar] [CrossRef]
- Tupchauskas, A.P.; Stanko, V.I.; Ustynyuk, Y.A.; Khrapov, V.V. 1H-{11B} heteronuclear double resonance spectra of ortho-, meta-, and para-carboranes and some of their organotin derivatives. J. Struct. Chem. 1973, 13, 772–776. [Google Scholar] [CrossRef]
- Zakharkin, L.; Kovredov, A.; Ol’Shevskaya, V.; Shaugumbekova, Z. Synthesis of B-organo-substituted 1,2-, 1,7-, and 1,12-dicarbaclosododecarboranes(12). J. Organomet. Chem. 1982, 226, 217–222. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Ol’Shevskaya, V.A.; Nesmeyanov’S, A.N. Synthesis of 9-Organyl-1,2 and 1,7-Dicarba-closo-dodecaboranss(12) via the Cross-Coupling Reactions between Organozinc Compounds and 9-Iodo-1,2- or 1,7-Dicarba-closo-dodecaboranes. Synth. React. Inorg. Met. Chem. 1991, 21, 1041–1046. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, W.; Zinn, A.A.; Knobler, C.B.; Hawthorne, M.F. Facile Electrophilic Iodination of Icosahedral Carboranes. Synthesis of Carborane Derivatives with Boron-Carbon Bonds via the Palladium-Catalyzed Reaction of Diiodocarboranes with Grignard Reagents. Inorg. Chem. 1995, 34, 2095–2100. [Google Scholar] [CrossRef]
- Viñas, C.; Barbera, G.; Oliva, J.M.; Teixidor, F.; Welch, A.J.; Rosair, G.M. Are halocarboranes suitable for substitution reactions? The case for 3-I-1,2-closo-C2B10H11: Molecular orbital calculations, aryldehalogenation reactions, 11B NMR interpretation of closo-carboranes, and molecular structures of 1-Ph-3-Br-1,2-closo-C2B10H10 and 3-Ph-1,2-closo-C2B10H11. Inorg. Chem. 2001, 40, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Aizawa, K.; Ohta, K. Synthesis of 3-Aryl-1,2-dicarba-closo-dodecaboranes by Suzuki-Miyaura Coupling Reaction. Heterocycles 2010, 80, 369–377. [Google Scholar] [CrossRef]
- Anderson, K.P.; Mills, H.A.; Mao, C.; Kirlikovali, K.O.; Axtell, J.C.; Rheingold, A.L.; Spokoyny, A.M. Improved synthesis of icosahedral carboranes containing exopolyhedral B C and C C bonds. Tetrahedron 2019, 75, 187–191. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Shmal’ko, A.V.; Suponitsky, K.Y.; Sivaev, I.B. One-pot synthesis of B-aryl carboranes with sensitive functional groups using sequential cobalt- and palladium-catalyzed reactions. Catalysts 2020, 10, 1348. [Google Scholar] [CrossRef]
- Janoušek, Z.; Hilton, C.L.; Schreiber, P.J.; Michl, J. C-Halogenation of the closo-[CB11H12]- Anion. Collect. Czechoslov. Chem. Commun. 2002, 67, 1025–1034. [Google Scholar] [CrossRef]
- Šembera, F.; Plutnar, J.; Higelin, A.; Janoušek, Z.; Císařova, I.; Michl, J. Metal complexes with very large dipole moments: The anionic carborane nitriles 12-NC-CB11X11- (X = H, F, CH3) as ligands on Pt(II) and Pd(II). Inorg. Chem. 2016, 55, 3797–3806. [Google Scholar] [CrossRef]
- Estrada, J.; Lugo, C.A.; McArthur, S.G.; Lavallo, V. Inductive effects of 10 and 12-vertex closo-carborane anions: Cluster size and charge make a difference. Chem. Commun. 2016, 52, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Plešek, J.; Hanslík, T. Chemistry of boranes. XXIX. The synthesis of isomeric 1,9- and 1,12-dibromo- l,2-dicarba-closo-dodecaboranes. Collect. Czechoslov. Chem. Commun. 1973, 38, 335–337. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of 9,9´,12,12´-substituted cobalt bis(dicarbollide) derivatives. Russ. Chem. Bull. 2015, 64, 712–717. [Google Scholar] [CrossRef]
- Havránek, M.; Samsonov, M.A.; Holub, J.; Ružickova, Z.; Drož, L.; Ružicka, A.; Fanfrlík, J.; Hnyk, D. The influence of halogenated hypercarbon on crystal packing in the series of 1-Ph-2-X-1,2-dicarba-closo-dodecaboranes (X = F, Cl, Br, I). Molecules 2020, 25, 1200. [Google Scholar] [CrossRef]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The influence of halogen type on structural features of compounds containing α-halo-α,α-dinitroethyl moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]
- Wolff, M.; Okrut, A.; Feldmann, C. [(Ph)3PBr][Br7], [(Bz)(Ph)3P]2[Br8], [(n-Bu)3MeN]2[Br20], [C4MPyr]2[Br20], and [(Ph)3PCl]2[Cl2I14]: Extending the Horizon of Polyhalides via Synthesis in Ionic Liquids. Inorg. Chem. 2011, 50, 11683–11694. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Mann, L.; Redeker, F.A.; Schmidt, B.; Riedel, S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem. Int. Ed. 2020, 59, 5464–5493. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen bonding: A halogen-centered noncovalent interaction yet to be understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Tsirelson, V.G.; Feil, D. Electron-density-based calculations of intermolecular energy: Case of urea. Acta Crystallogr. Sect. A Found. Crystallogr. 1999, 55, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Suponitsky, K.Y.; Smol’Yakov, A.F.; Ananyev, I.V.; Khakhalev, A.V.; Gidaspov, A.A.; Sheremetev, A.B. 3,4-Dinitrofurazan: Structural Nonequivalence of ortho -Nitro Groups as a Key Feature of the Crystal Structure and Density. ChemistrySelect 2020, 5, 14543–14548. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Suponitsky, K.Y.; Pivkina, A.N.; Sheremetev, A.B. Novel melt-castable energetic pyrazole: A pyrazol-yl-furazan framework bearing five nitro groups. Prop. Explos. Pyrotech. 2016, 41, 789–792. [Google Scholar] [CrossRef]
- Andrews, J.S.; Zayas, J.; Jones, M. 9-Iodo-o-carborane. Inorg. Chem. 1985, 24, 3715–3716. [Google Scholar] [CrossRef]
- Itatani, H.; Bailar, J.C. Homogenous catalysis in the reactions of olefinic substances. V. Hydrogenation of soybean oil methyl ester with triphenylphosphine and triphenylarsine palladium catalysts. J. Am. Oil Chem. Soc. 1967, 44, 147–151. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Burlington, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H⋯π Interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 38, 4436–4443. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]-. Some evidences of BH···X hydride-halogen bonds in 9- XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Masunov, A.E. Supramolecular step in design of nonlinear optical materials: Effect of π⋯π stacking aggregation on hyperpolarizability. J. Chem. Phys. 2013, 139, 094310. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll; Version 15.05.18; TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Lyssenko, K.A.; Antipin, M.Y.; Aleksandrova, N.S.; Sheremetev, A.B.; Novikova, T.S. 4,4′-Bis(nitramino)azofurazan and its salts. Study of molecular and crystal structure based on X-ray and quantum chemical data. Russ. Chem. Bull. 2009, 58, 2129–2136. [Google Scholar] [CrossRef]
- Lyssenko, K.A. Analysis of supramolecular architectures: Beyond molecular packing diagrams. Mendeleev Commun. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Lyssenko, K.A.; Ananyev, I.V.; Kozeev, A.M.; Sheremetev, A.B. Role of weak intermolecular interactions in the crystal structure of tetrakis-furazano[3,4-c:3′,4′-g:3″,4″-k:3"‘,4"‘-o][1,2,5,6,9,10,13,14]octaazacyclohexadecine and its solvates. Cryst. Growth Des. 2014, 14, 4439–4449. [Google Scholar] [CrossRef]

| Distance in Å or Angle in Deg. | Energy in kcal/mol | ||||
|---|---|---|---|---|---|
| 1,12-I2-1,2- C2B10H10 (X-ray) | 1,12-I2-1,2- C2B10H10 (calc) | 1,12-Br2-1,2- C2B10H10 (calc) | 1,12-I2-1,2- C2B10H10 (calc) | 1,12-Br2-1,2- C2B10H10 (calc) | |
| X12X1’ | 3.5687(9) | 3.455 | 3.704 | −2.9 | −1.0 |
| B12-X12⋯X1’ | 92.98(12) | 94.3 | 91.1 | ||
| X12⋯X1’-C1’H7⋯X1’ | 172.61(11) | 175.9 | 147.6 | ||
| H11⋯X1’ | 3.58(2) | 3.51 | 3.25 | −0.5 | −0.7 |
| H7⋯H5’ | 3.58(2) | 3.52 | 3.37 | −0.5 | −0.5 |
| H11⋯H4’ | - | - | 2.67 | - | −0.5 |
| X12X1’ | - | - | 2.61 | - | −0.6 |
| 1,9-I2-1,2-C2B10H10 | 1,3-I2-1,2-C2B10H10 | ||||
|---|---|---|---|---|---|
| Distance or Angle | Energy | Distance or Angle | Energy | ||
| I9⋯I1’ | 3.461 | −2.7 | I3⋯I1’ | 3.54 | −2.3 |
| B9-I9⋯I1’ | 92.7 | B3-I3⋯I1’ | 90.1 | ||
| I9⋯I1’-C1’ | 174.1 | I3⋯I1’-C1’ | 170.7 | ||
| H12⋯I1’ | 3.47 | −0.6 | H8⋯I1’ | 3.44 | −0.7 |
| H8⋯I1’ | 3.49 | −0.6 | H10⋯I1’ | 3.42 | −0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suponitsky, K.Y.; Anisimov, A.A.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. 1,12-Diiodo-Ortho-Carborane: A Classic Textbook Example of the Dihalogen Bond. Crystals 2021, 11, 396. https://doi.org/10.3390/cryst11040396
Suponitsky KY, Anisimov AA, Anufriev SA, Sivaev IB, Bregadze VI. 1,12-Diiodo-Ortho-Carborane: A Classic Textbook Example of the Dihalogen Bond. Crystals. 2021; 11(4):396. https://doi.org/10.3390/cryst11040396
Chicago/Turabian StyleSuponitsky, Kyrill Yu., Alexei A. Anisimov, Sergey A. Anufriev, Igor B. Sivaev, and Vladimir I. Bregadze. 2021. "1,12-Diiodo-Ortho-Carborane: A Classic Textbook Example of the Dihalogen Bond" Crystals 11, no. 4: 396. https://doi.org/10.3390/cryst11040396
APA StyleSuponitsky, K. Y., Anisimov, A. A., Anufriev, S. A., Sivaev, I. B., & Bregadze, V. I. (2021). 1,12-Diiodo-Ortho-Carborane: A Classic Textbook Example of the Dihalogen Bond. Crystals, 11(4), 396. https://doi.org/10.3390/cryst11040396








