Abstract
Fracture behavior via a flexural test for a newly found CaO–Al2O3–SiO2 (CAS) glass-ceramic (GC) was compared with that of enstatite GC and mica GC, which are well-known GCs with high-fracture toughness and machinability, respectively. By focusing on the nonelastic load–displacement curves, CAS GC was characterized as a less brittle material similar to machinable mica GC, compared with enstatite GC, which showed higher fracture toughness, KIC. The microcrack toughening mechanism in CAS GC was supported by the nondestructive observation of microcracks around the Vickers indentation using the X-ray microcomputed tomography technique. The CAS GC also showed higher transparency than mica GC due to its low crystallinity. Moreover, the precursor glass had easy formability due to its low-liquidus temperature.
1. Introduction
After the discovery of toughened zirconia ceramics in the 1970s [1], studies to increase the toughness of ceramic materials have received a great deal of attention [2]. Many mechanisms of toughening, including phase transformation [2], microcracking [3,4], crack deflection [5,6], and crack bridging [7], were suggested and are well accepted. For characterizing the toughness of ceramics, the fracture toughness (KIC), which describes the critical stress intensity on the crack tip, is mostly used as an index of material toughness. However, the so-called “resistant-curve (R-curve)” behavior, which indicates the increase of fracture resistance as the crack extends, is regarded as another important factor and inherent for understanding the toughness of ceramics [2].
One of the simple and easy methods to evaluate the toughness of ceramic materials is a flexural test using samples introducing various types of notches including chevron notch or a single notch. From load–displacement curves of the flexural test, R-curve behavior can be evaluated [8,9]. Figure 1 illustrates the concept of R-curve behaviors and the related load–displacement curves. Brittle materials break at a critical load in linear elastic behavior. There is no mechanism to increase the resistance against the propagation of cracks; hence, the R-curve is flat (Figure 1A). However, if the resistance increases as the crack extends, the load–displacement curve shows nonelastic behavior and the material gradually breaks (Figure 1B). As a result, one feels the material as “less brittle” or “tough”.
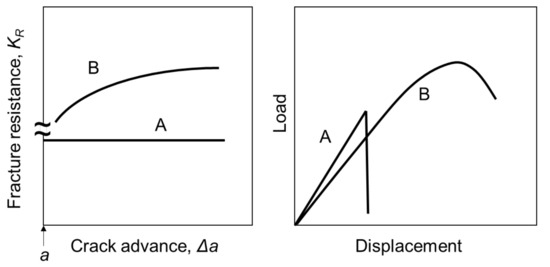
Figure 1.
Schematic drawing of R-curve behaviors and load–displacement curves of ceramic fracture [2,8]. A: brittle ceramics, B: toughened ceramics.
Oxide glass is a typical brittle material with a KIC of 0.6–1.0 MPa m1/2 [10,11], because it does not have secondary phases and grain boundaries that can affect the propagation of cracks. Therefore, many studies have been conducted for glass-ceramic (GC) materials to improve the brittleness of oxide glass. A recent article defined GCs as “inorganic, non-metallic materials prepared by controlled crystallization of glasses via different processing methods” [12]. GCs contain some amounts of crystalline phases; therefore, their fracture toughness can be increased compared with the original glass. GCs also have advantages for formability of its precursor glass as compared with sintered ceramics, as well as less risk of containing pore defects in final products. Table 1 lists typical examples of well-known GCs with high strength and fracture toughness [13,14,15], including silicate, aluminosilicate, and fluorosilicate systems. The silicate family has two main systems, as listed in Table 1. Lithium disilicate GCs are commercially important materials, and their mechanical properties are well characterized [6,16]. A combination of their high strength (150−400 MPa) and high-fracture toughness (2−4 MPa∙m1/2) enables their application to dental materials [15,16]. Enstatite is categorized as a chain silicate, and enstatite GCs are well-known to have a high-fracture toughness of 3.5−4.6 MPa∙m1/2 [15,17]. This high-fracture toughness is attributed to crack deflection and slippage along the interlocking twinned crystals [17]. The combination of nanocrystalline enstatite and spinel is beneficial for smooth surface after polishing and high Young’s modulus, which are essential for magnetic memory disk application [14,15]. For the aluminosilicate family, the Li2O–Al2O3–SiO2 (LAS) system can precipitate a β-quartz solid solution or a β-spodumene solid solution. GCs with good transparency and near-zero thermal expansion can be obtained in this system [15]. LAS GCs are widely used in cooking wares, cooktop plates, and fire-proof glass, among many other applications [15]. Cordierite is known as a low-thermal-expansion crystal. Cordierite GC is used for the radome of aircraft and rockets, thanks to its superior thermal and mechanical properties and good microwave transparency [15,18]. Leucite GC can be used for dental restoration [15]. Although aluminosilicate GCs have found a wide variety of applications, the fracture toughness of these GCs is not as high as that of silicate GCs, such as lithium disilicate and enstatite (Table 1). Some fluorosilicate GCs exhibit superior mechanical properties. Canasite (Ca5Na4K2Si12O30F4) GC is known as one of the strongest GCs [13,17] with a fracture toughness of 5 MPa∙m1/2. Mica-containing GCs are known as machinable GCs that can be drilled and precisely machined with conventional diamond tools [15]. Although many strong and tough GCs have been studied and commercialized, few studies have described the load–displacement curves mentioned above.

Table 1.
Typical silicate, aluminosilicate, and fluorosilicate glass-ceramic (GC) compositions and properties presented in the literature [13,14,15].
However, many attempts are still being made to further enhance the advantages of GCs mentioned above. One of the challenges encountered is the further formability of precursor glass. A higher crystallinity is beneficial for increasing the GC toughness; thus, many studies have focused on the precursor glass composition close to the stoichiometric composition of the relevant crystalline phase. This means that the liquidus temperature of the glass is relatively high and generally increases the risk of devitrification during the forming process of the precursor glass. Obtaining GCs with a high amount of the crystalline phase will be difficult if one selects glass far from the stoichiometric composition. Accordingly, a new toughening mechanism, which is effective, even with a lower crystallinity, must be found to solve this dilemma.
Another issue is GC transparency. The glass–crystal boundary generally generates considerable light scattering, unless the crystal size is small enough compared with visible light [19]. Although the LAS GC in Table 1 shows good transparency, its toughness is not as high as that of the other GCs. The transparency of Li2O–SiO2 (LS) GCs can vary depending on the crystalline size in the GC. Aiming for dental restoration application, a study was recently conducted to improve the GC transparency, while maintaining the good mechanical properties of LS GCs [20]. As a result, a GC with high transparency and high-flexural strength was successfully obtained; however, details relevant to the fracture toughness were not described. The cordierite GC developed for the radome [18], canasite GC for the magnetic memory disk [15], and machinable mica GC [15] are not transparent from the pictures. No pictures for enstatite GC were available in the literature [17]. Therefore, we surveyed the sample appearances of enstatite GC by making samples in our laboratory.
We recently studied the mechanical properties of GCs using metallic molybdenum and tungsten particles as the nucleating agents in the MgO–Al2O3–SiO2 (MAS) [21,22] and CaO–Al2O3–SiO2 (CAS) [23,24,25] systems to develop new GCs with superior mechanical properties. CAS GC precipitating a hexagonal metastable CaAl2Si2O8 crystalline phase was characterized by its easy microcracking. Figure 2 shows optical microscopy images of crack formation around the imprint of Vickers indentation at 98 N load for CAS GC and standard soda-lime–silica (SLS) glass [26]. Many microcracks can be seen in CAS GC with relatively short radial cracks, while long radial cracks, as well as lateral cracks, formed in the case of SLS glass [27]. The tendency of easy microcracking in CAS GC provides this material with unique mechanical properties. Although the microcrack formation and the optimal microstructure were investigated in our previous works [24,25], the characteristic of the CAS GC as compared with other GCs was not clarified. Therefore, the aim of this paper is to review our previous study on CAS GC, compared with some additional experiments for selected GCs in Table 1 (enstatite GC and mica GC), focusing on the fracture behavior, transparency, and formability of GCs. A new observation result of microcracking in CAS GC by the X-ray imaging technique using a synchrotron facility is also presented.

Figure 2.
Optical microscopy image of cracks and around the imprint of the Vickers indentation at 98 N (a): soda-lime–silica (SLS), (b): CaO–Al2O3–SiO2 (CAS) glass-ceramic (GC) [26].
2. Materials and Methods
Figure 3 shows a glass-forming region and an area with low-liquidus temperatures (below 1400 °C) in the CAS ternary system [28,29]. The low-liquidus-temperature area was between the stoichiometric crystalline compositions. As mentioned, we chose the precursor glass composition considering the low-liquidus temperature with an adequate viscosity of 55SiO2–20Al2O3–25CaO wt%. The precursor glass was prepared from related raw materials by melting at 1550 °C through a conventional laboratory-scale melting method. To promote crystallization, 0.05 wt% of MoO3 and 0.4 wt% of carbon powder were added as nucleating and reducing agents, respectively. The glass sample was, then, heat treated at 1050 °C for 2 h (heating and cooling rates: 100 °C/h) for crystallization. The detailed procedure is described in our earlier study [23].
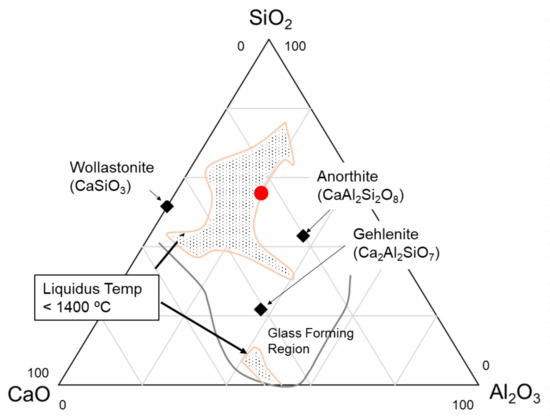
Figure 3.
Glass-forming and low-liquidus-temperature region in the CAS system [28,29].
We also made the GC samples in our laboratory based on the relevant literature for enstatite GC [17] and mica GC [30,31] to evaluate their appearances and mechanical properties. Table 2 lists the precursor glass compositions, as well as precursor glass melting temperatures and heat treatment conditions for crystallization.

Table 2.
Precursor glass compositions used in this study as references.
The three-point flexural test was performed for 3 × 4 × 50 mm3 samples to evaluate the fracture behavior and the fracture toughness through the single-edge v-notched beam (SEVNB) method [32] using the TS Engineering Tensilon UTA-5kN (Tokyo, Japan). The fracture toughness KIC was calculated as follows:
where F is the fracture load, S is the span, B is the sample width, w is the sample height, d is the notch depth, and Y is the stress intensity shape factor [32]. The fractured surface was observed using scanning electron microscopy (SEM; Hitachi High-Technologies TM-3030 PLUS, Tokyo, Japan).
KIC = {3FS/[2(1 − α)3/2Bw2]}d1/2Y,
The Vickers indentation test was conducted to evaluate microcrack formation on the polished surface of GCs. The microcracks in CAS GC were observed using a multiscale X-ray computed tomography (CT) technique in the SPring-8 synchrotron facility (Hyogo, Japan) that was recently developed by Takeuchi and co-workers [33,34]. It is a powerful tool to observe crack-like defects in ceramics nondestructively, consisting of X-ray microtomography and phase-contrast high-energy X-ray nanotomography [35].
3. Results
3.1. Characterization and Response to Vickers Indentation
Figure 4 shows the appearance of the CAS GC after the surface layer removal [24]. Although the sample showed a black coloration, it did not completely lose its transparency; hence, the letters behind were visible. The crystalline phase was identified to be hexagonal metastable CaAl2Si2O8 crystals, although its exact structure was slightly distorted as shown by detailed analysis using the Rietveld method [36]. The mineral with a hexagonal structure is known as “dmisteinbergite” [37]. Its crystal structure and phase diagram have been investigated by several researchers [38,39,40,41,42]. The mineral is also known to have a cleavage behavior [37]. The crystalline phase fraction in CAS GC was estimated as ~21 wt% using Rietveld analysis [25].

Figure 4.
Appearance of a 1.5 mm-thick CAS glass-ceramic doped with 0.05 wt% MoO3 [24].
Figure 5 shows a three-dimensional (3D) image of the CAS GC microstructure [24], in which the hexagonal CaAl2Si2O8 crystals are colored white. The plate-like crystals with a thickness of ~1 μm formed a house-of-cards structure. The thickness direction was identified as the c-axis of the hexagonal crystal using transmission electron microscopy analysis [43]. Most crystals grew to a size probably no longer than 10–20 μm because the crystal growth was terminated when they reached the plane of another crystal. Twins were also formed from the molybdenum particles [24]. Consequently, several plate-shaped crystals intersected, forming a house-of-cards-like structure [24].
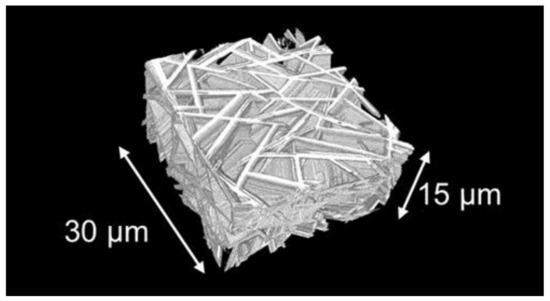
Figure 5.
3D image of the microstructure. The thin plate-like crystals depicted in white color form a house-of-cards structure [24].
Microcracks induced by Vickers indentation were observed nondestructively using a multiscale X-ray CT technique. Figure 6 depicts the microcracks around the imprints of Vickers indentation at the 9.8 N load. The microcracks are indicated in white color. It was observed that the microcracks were formed in the region of ~100 μm in width and ~50 μm in depth from the surface by micromode X-ray CT observation (Figure 6b). Although the resolution was not enough to identify the shape and the distribution of each microcrack, those were observed more clearly when nanomode X-ray CT was applied (Figure 6c,d). It was found that the two microcracks inclined to the surface and traveled in parallel with a gap of 1.0 μm (Figure 6c).
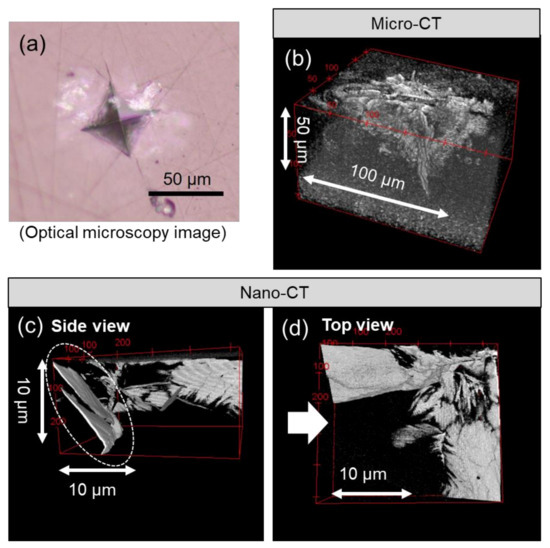
Figure 6.
Microcracks observed using X-ray computed tomography. (a): optical microscopy image, (b): X-ray CT image (micromode), (c): Side view of X-ray CT image (nanomode) from white arrow in image (d), (d): Top view of X-ray CT image (nanomode).
These GAS GC characteristics were then compared with those having high-fracture toughness (enstatite GC) and machinable GC (mica GC). Figure 7 shows pictures of sample appearance and optical microscopy images around the Vickers imprint after a 98 N load indentation for the GCs synthesized in our laboratory. GCs were not transparent with 1-mm thickness. No remarkable microcracking was observed around the Vickers imprints for the enstatite GC. In contrast, microcracking seemed to occur in the mica GC. However, the picture of the mica GC is not like that of CAS GC (Figure 2) due to the absence of light reflection from inside the material.
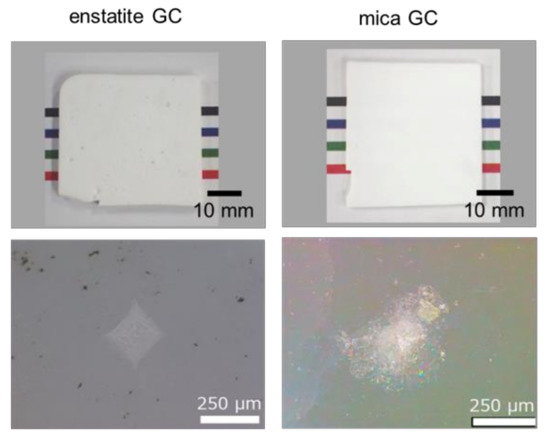
Figure 7.
Appearances (sample thickness: 1 mm) and optical microscopy images of Vickers indentation of the reference GCs.
3.2. Flexural Test
Figure 8 illustrates the typical load–displacement curves of the CAS glasses before and after crystallization obtained by the three-point flexural tests of the samples with a v-notch [23]. The calculated KIC values of each sample are also indicated in the figure. In this study, the average KIC values with their standard deviations were 1.02 ± 0.09 MPa∙m1/2 (before heat treatment) and 2.22 ± 0.06 MPa∙m1/2 (after heat treatment) respectively (number of samples were four in both cases). Although the precursor glass showed a typical brittle behavior, the CAS GC indicated a nonelastic behavior as the load increased and gradually broke. The fractured surface of CAS GC was very rough, and a hexagonal shape attributed to the crystal morphology was observed (Figure 9).
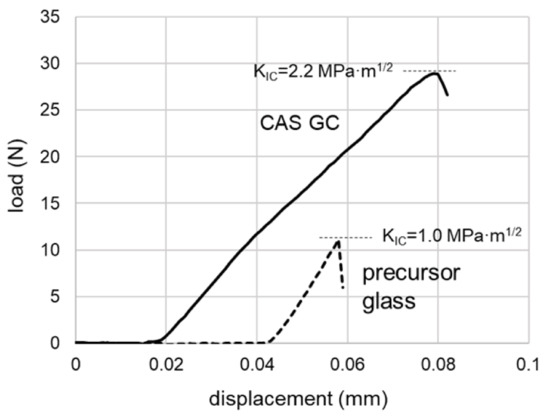
Figure 8.
Load–displacement curves of the glasses during the three-point flexural tests [23].
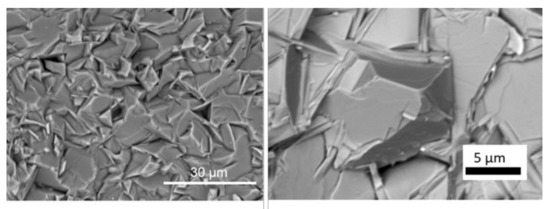
Figure 9.
SEM images of the fractured surface of CAS GC [23].
Figure 10 presents the flexural test results of the enstatite GC and the mica GC using the procedure followed for the CAS GC. The average KIC values with their standard deviations were 2.76 ± 0.156 MPa∙m1/2 for enstatite GC (number of samples was three) and 1.67 ± 0.39 MPa∙m1/2 for mica GC (number of samples was four). A nonelastic behavior was observed just before the sample broke for the enstatite GC, but it was not as much as that of the mica GC. For the mica GC, the load gradually increased until maximum, and the sample slowly broke. Figure 11 shows the fractured surface microstructures for each sample. Fine microstructures were observed in the enstatite GC, whereas rougher microstructures with thin plate-like crystals were seen in the fracture surface of the mica GC.
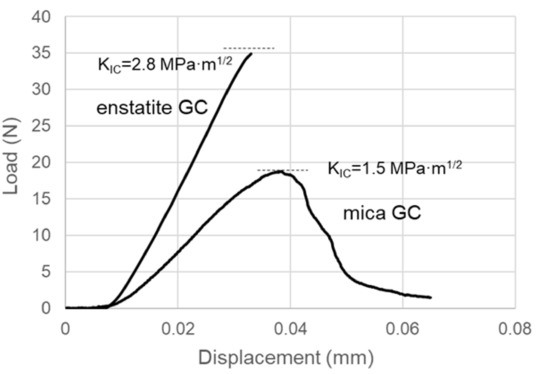
Figure 10.
Load–displacement curves of the reference GCs during the three-point flexural tests.
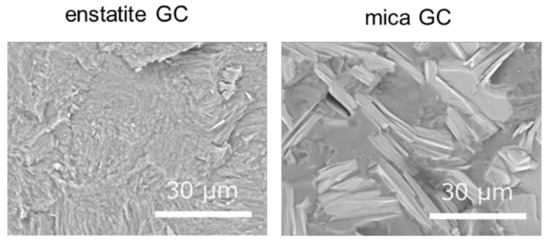
Figure 11.
SEM images of the fractured surface of the reference GCs.
4. Discussion
The differences in the nonlinearity of the load–displacement curves indicate different fracture behaviors of the GC materials investigated in this study (Figure 8 and Figure 10). The materials were “less brittle” when the samples gradually broke like the mica and CAS GC. In contrast, the enstatite GC showed higher fracture toughness (KIC) values than the mica and CAS GC, because KIC was determined by the maximum load of the fracture using Equation (1).
As discussed in our previous papers [23,24], the nonelastic behavior of the CAS GC can be attributed to microcrack formation due to the cleavage characteristics of the hexagonal CaAl2Si2O8 crystal. In this study, the microcracks induced by Vickers indentation were nondestructively observed for the first time (Figure 6). Our previous observation by SEM, as well as a theoretical approach using molecular dynamics simulation, indicated that the microcracks tend to travel in a glass–crystal interface [24,44]. Because the gap between two parallel microcracks and the size of the microcrack planes observed in Figure 6c agreed with the typical thickness and size of the hexagonal crystals, it is suggested that the microcracks in Figure 6c rendered the hexagonal crystal; hence, many microcracks were induced depending on the distribution of the hexagonal crystals around the imprint of the Vickers indentation. These observations strongly support that the toughness of CAS GC is based on the microcrack toughening mechanism. Mica has a layered crystal structure and easily shows cleavage [15]. This similarity of the crystal structure and the cleavage property could have given both GCs similar fracture behaviors for the flexural test and the microcracks by Vickers indentation. Thus, CAS GC can be characterized as close to mica GCs in terms of the toughening mechanism, although its nonelastic tendency is not as high as that of mica GC.
According to the linear elastic fracture mechanics, the strength (S) of ceramic materials is described by fracture toughness KIC and flaw size a, and its geometry parameter Y using the following simple Equation (2);
S = Y KIC/a1/2,
As higher KIC is beneficial for high strength of the materials, the GCs with higher KIC values including enstatite GC can exhibit high flexural strength (Table 1).
Ota et al. investigated microcrack-toughened ceramics for sintered Al2TiO5 [45] and KZr2(PO4)3–KAlSi2O6 composite [46], mimicking natural sandstone itacolumite, which is well-known as a “flexible stone.” Microcracks were introduced in ceramic bodies using thermal expansion anisotropy (Al2TiO5) or a large difference of thermal expansion coefficient (KZr2(PO4)3–KAlSi2O6) during their fabrication process. They demonstrated that those ceramics exhibited nonlinear load–displacement curves and even flexibility when those were conducted in a flexural test [45,46]. The mechanical characteristics of CAS GC and mica GC may have an analogy with those flexible ceramics, although CAS and mica GCs showed microcracking only when they were broken. Although the strength of this type of ceramics is not high (less than 20 MPa in case of sintered Al2TiO5 [45]), it is expected that those are beneficial for damage tolerance [2]. Our previous work revealed that the CAS GC had higher mechanical reliability with average flexural strength of 102.9 MPa after abrasion because of its low susceptibility against mechanical damage [26].
The CAS GC is advantageous in terms of transparency compared with the mica GC; hence, the microcracks induced by Vickers indentation were easily observed (Figure 2). The low crystallinity of the CAS GC can be a cause of higher transparency, because the light scattering induced by the refractive index difference between the crystals and residual glass was minimized.
Another important advantage of CAS GC is the precursor glass formability. The glass composition was selected in a low-liquidus-temperature region (Figure 3); thus, the glass could be formed from the melt with less risk of devitrification. Moreover, obtaining good melt homogeneity was relatively easy, because the CAS GC did not contain any volatile components such as fluorine.
5. Conclusions
Mechanical properties of newly found CAS GC were investigated and compared with other GCs in this study. CAS GC has a nonelastic behavior similar to the conventional mica GC due to the cleavage behavior of the crystal and higher transparency due to its low crystal fraction. The microcrack formation caused by the crystal cleavage is attributed to this unique mechanical property. CAS GC also has the advantages of easy melting and forming characteristics. In the future, a further improvement of toughness and transparency can be expected based on the same concept.
6. Patents
US10487002B2
JP6769476
Author Contributions
Conceptualization, K.M.; methodology, K.M., K.A. and G.O.; writing—original draft preparation, K.M.; writing—review and editing, K.A., A.Y. and G.O.; supervision, A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are deeply grateful to Akihisa Takeuchi and Masayuki Uesugi for establishing the multiscale X-ray CT system and supporting our operation in this study. (The X-ray CT experiments at SPring-8 were performed with the approval of JASRI: Grant No. 2020A1603). The authors thank Kenichiro Iwasaki for obtaining the 3D image of material by SEM observation. The authors also appreciate Fumihiro Wakai, professor at Tokyo Institute of Technology, Koichi Yasuda, assistant professor at Tokyo Institute of Technology, Setsuro Ito, invited professor at Tokyo University of Science, and Shingo Urata in AGC Inc. for their useful discussions on the fracture behavior of glass and ceramic materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Evans, A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Hoagland, R.G.; Embury, J.D. A treatment of Inelastic deformation around a crack tip due to microcracking. J. Am. Ceram. Soc. 1980, 63, 404–410. [Google Scholar] [CrossRef]
- Evans, A.G.; Faber, K.T. Crack-growth resistance of microcracking brittle materials. J. Am. Ceram. Soc. 1984, 67, 255–260. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack deflection processes-I. Theory. Acta Metall. 1983, 31, 565–576. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack deflection processes-II. Experiment. Acta Metall. 1983, 31, 577–584. [Google Scholar] [CrossRef]
- Becher, P.F. Microstructural design of toughened ceramics. J. Am. Ceram. Soc. 1991, 74, 255–269. [Google Scholar] [CrossRef]
- Mazzei, A.C.; Rodrigues, J.A.; Pandolfelli, V.C. Alumina-mullite-zirconia composites obtained by reaction sintering Part II R-curve behavior. J. Mat. Sci. 2000, 35, 2815–2824. [Google Scholar] [CrossRef]
- Dai, Y.; Harmuth, H.; Jin, S.; Gruber, D.; Li, Y. R-curves determination of ordinary refractory ceramics assisted by digital image correlation method. J. Eur. Ceram. Soc. 2020, 40, 4655–4663. [Google Scholar] [CrossRef]
- Wiederhorn, S.M. Fracture surface energy of glass. J. Am. Ceram. Soc. 1969, 52, 99–105. [Google Scholar] [CrossRef]
- Vullo, P.; Davis, M.J. Comparative study of micro-indentation and Chevron notch fracture toughness measurements of silicate and phosphate glasses. J. Non-Cryst. Solids 2004, 349, 180–184. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Fu, Q.; Beall, G.H.; Smith, C.M. Nature-inspired design of strong, tough glass-ceramics. MRS Bull. 2017, 42, 220–225. [Google Scholar] [CrossRef]
- Pinckney, L.R.; Beall, G.H. Nanocrystalline non-alkali glass-ceramics. J. Non Cryst. Solids 1997, 219, 219–227. [Google Scholar] [CrossRef]
- Holland, H.; Beall, G.H. (Eds.) Glass-Ceramic Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Serbena, F.C.; Mathias, I.; Foerster, C.E.; Zanotto, E.D. Crystallization toughening of a model glass-ceramic. Act. Mat. 2015, 86, 216–228. [Google Scholar] [CrossRef]
- Beall, G.H. Chain silicate glass-ceramics. J. Non-Cryst. Solids 1991, 129, 163–173. [Google Scholar] [CrossRef]
- Pyroceram. In Glass-Ceramic Materials; Strnad, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 190–195. [Google Scholar]
- Beall, G.H.; Duke, D.A. Transparent glass-ceramics. J. Mater. Sci. 1969, 4, 340–352. [Google Scholar] [CrossRef]
- Soares, V.O.; Soares, F.C.; Oliveira, G.S.; Cruz, C.; Muniz, R.F.; Zanotto, E.D. Highly translucent nanostructured glass-ceramic. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Effect of molybdenum and tungsten oxides on nucleation and crystallization behaviors of MgO–Al2O3–SiO2 glasses. J. Non-Cryst. Solids 2015, 427, 152–159. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Effect of molybdenum and titanium oxides on mechanical and thermal properties of cordierite-enstatite glass-ceramics. J. Non-Cryst. Solids 2016, 434, 13–22. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Toughening of CaO-Al2O3-SiO2 glass by dmisteinbergite precipitation. Mater. Lett. 2016, 180, 231–234. [Google Scholar] [CrossRef]
- Maeda, K.; Iwasaki, K.; Urata, S.; Akatsuka, K.; Yasumori, A. 3D microstructure and crack pathways of toughened CaO–Al2O3–SiO2 glass by precipitation of hexagonal CaAl2Si2O8 crystal. J. Am. Ceram. Soc. 2019, 102, 5535–5544. [Google Scholar] [CrossRef]
- Inage, K.; Akatsuka, K.; Iwasaki, K.; Nakanishi, T.; Maeda, K.; Yasumori, A. Effect of crystallinity and microstructure on mechanical properties of CaO–Al2O3–SiO2 glass toughened by precipitation of hexagonal CaAl2Si2O8 crystals. J. Non-Cryst. Solids. 2020, 534, 119948. [Google Scholar] [CrossRef]
- Maeda, K.; Akatsuka, K.; Yasumori, A. Practical strength of damage resistant CaO-Al2O3-SiO2 glass-ceramic. Ceram. Int. 2021, 47, 8728–8731. [Google Scholar] [CrossRef]
- Cook, R.F.; Pharr, G.M. Direct observation and analysis of indentation cracking in glasses and ceramics. J. Am. Ceram. Soc. 1990, 73, 787–817. [Google Scholar] [CrossRef]
- Sakka, S.; Sakaino, T.; Takahashi, K. (Eds.) Glass Handbook; Asakura Shoten: Tokyo, Japan, 1975. [Google Scholar]
- Osborn, E.F.; Muan, A. revised and redrawn “Phase Equilibrium Diagrams of Oxide Systems,” Plate 1; Published by the American Ceramic Society and the Edward Orton, Jr., Ceramic Foundation, 1960. Phase Equilibria Diagram Online, American Ceramic Society. Available online: https://phaseonline.ceramics.org/ (accessed on 8 April 2021).
- Henry, J.; Hill, R.G. Influence of alumina content on the nucleation crystallization and microstructure of barium fluorphlogopite glass-ceramics based on 8SiO2•yAl2O3•4MgO•2MgF2•BaO part I Nucleation and crystallization behaviour. J. Mater. Sci. 2004, 39, 2499–2507. [Google Scholar] [CrossRef]
- Henry, J.; Hill, R.G. Influence of alumina content on the nucleation crystallization and microstructure of barium fluorphlogopite glass-ceramics based on 8SiO2•yAl2O3•4MgO•2MgF2•BaO part II Microstructure, microhardness and machinability. J. Mater. Sci. 2004, 39, 2509–2515. [Google Scholar] [CrossRef]
- Kübler, J. Fracture Toughness of Ceramics Using the SEVNB Method; round robin; VAMAS Report, No. 37; ESIS Document D2-99; EMPA: Dübendorf, Switzerland, 1999. [Google Scholar] [CrossRef]
- Takeuchi, A.; Uesugi, K.; Uesugi, M.; Yoshinaka, F.; Nakamura, T. Nondestructive multiscale X-ray tomography by combining microtomography and high-energy phase-contrast nanotomography. Microsc. Microanal. 2018, 24, 106–107. [Google Scholar] [CrossRef]
- Takeuchi, A.; Uesugi, K.; Uesugi, M.; Toda, H.; Hirayama, K.; Shimizu, K.; Matsuo, K.; Nakamura, T. High-energy X-ray nanotomography introducing an apodization Fresnel zone plate objective lens. Rev. Sci. Instrum. 2021, 92, 023701. [Google Scholar] [CrossRef]
- Okuma, G.; Watanabe, S.; Shinobe, K.; Nishiyama, N.; Takeuchi, A.; Uesugi, K.; Tanaka, S.; Wakai, F. 3D multiscale-imaging of processing-induced defects formed during sintering of hierarchical powder packings. Sci. Rep. 2019, 9, 11595. [Google Scholar] [CrossRef]
- Akatsuka, K.; Yasumori, A.; Maeda, K. Structure of crystalline CaAl2Si2O8 precipitated in a CaO–Al2O3–SiO2 glass-ceramic. Mater. Lett. 2019, 242, 163–165. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. (Eds.) Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA; Available online: http://www.handbookofmineralogy.org/ (accessed on 8 April 2021).
- Takeuchi, Y.; Donnay, G. The crystal structure of hexagonal CaAl2Si2O8. Acta Cryst. 1959, 12, 465–470. [Google Scholar] [CrossRef]
- Ito, J. High temperature solvent growth of anorthite on the join CaAl2Si2O8–SiO2. Contrib. Mineral. Petrol. 1976, 59, 187–194. [Google Scholar] [CrossRef]
- Abe, T.; Tsukamoto, K.; Sunagawa, I. Nucleation, growth and stability of CaAl2Si2O8 polymorphs. Phys. Chem. Miner. 1991, 17, 473–484. [Google Scholar] [CrossRef]
- Abe, T.; Sunagawa, I. Hexagonal CaAl2Si2O8 in a high temperature solution; metastable crystallization and transformation to anorthite. Miner. J. 1995, 17, 257–281. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Panikorovskii, T.L.; Gurzhiy, V.V.; Bocharov, V.N.; Rassomakhin, M.A. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals 2019, 9, 570. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Nucleation and growth of hexagonal CaAl2Si2O8 crystals in CaO–Al2O3–SiO2 glass. Mater. Lett. 2017, 206, 241–244. [Google Scholar] [CrossRef]
- Urata, S.; Takato, Y.; Maeda, K. Molecular dynamics investigation of the fracture mechanism of a glass-ceramic containing cleavable crystals. J. Am. Ceram. Soc. 2019, 102, 5138–5148. [Google Scholar] [CrossRef]
- Babelot, C.; Guignard, A.; Huger, M.; Gault, C.; Chotard, T.; Ota, T.; Adachi, N. Preparation and thermomechanical characterisation of aluminum titanate flexible ceramics. J. Mater. Sci. 2010, 46, 1211–1219. [Google Scholar] [CrossRef]
- Sato, I.; Ichikawa, Y.; Sakanoue, J.; Mizutani, M.; Adachi, N.; Ota, T. Flexible Ceramics in the System KZr2(PO4)3–KAlSi2O6 Prepared by Mimicking the Microstructure of Itacolumite. J. Am. Ceram. Soc. 2008, 91, 607–610. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).