Impact of CdCl2 Treatment in CdTe Thin Film Grown on Ultra-Thin Glass Substrate via Close Spaced Sublimation
Abstract
1. Introduction
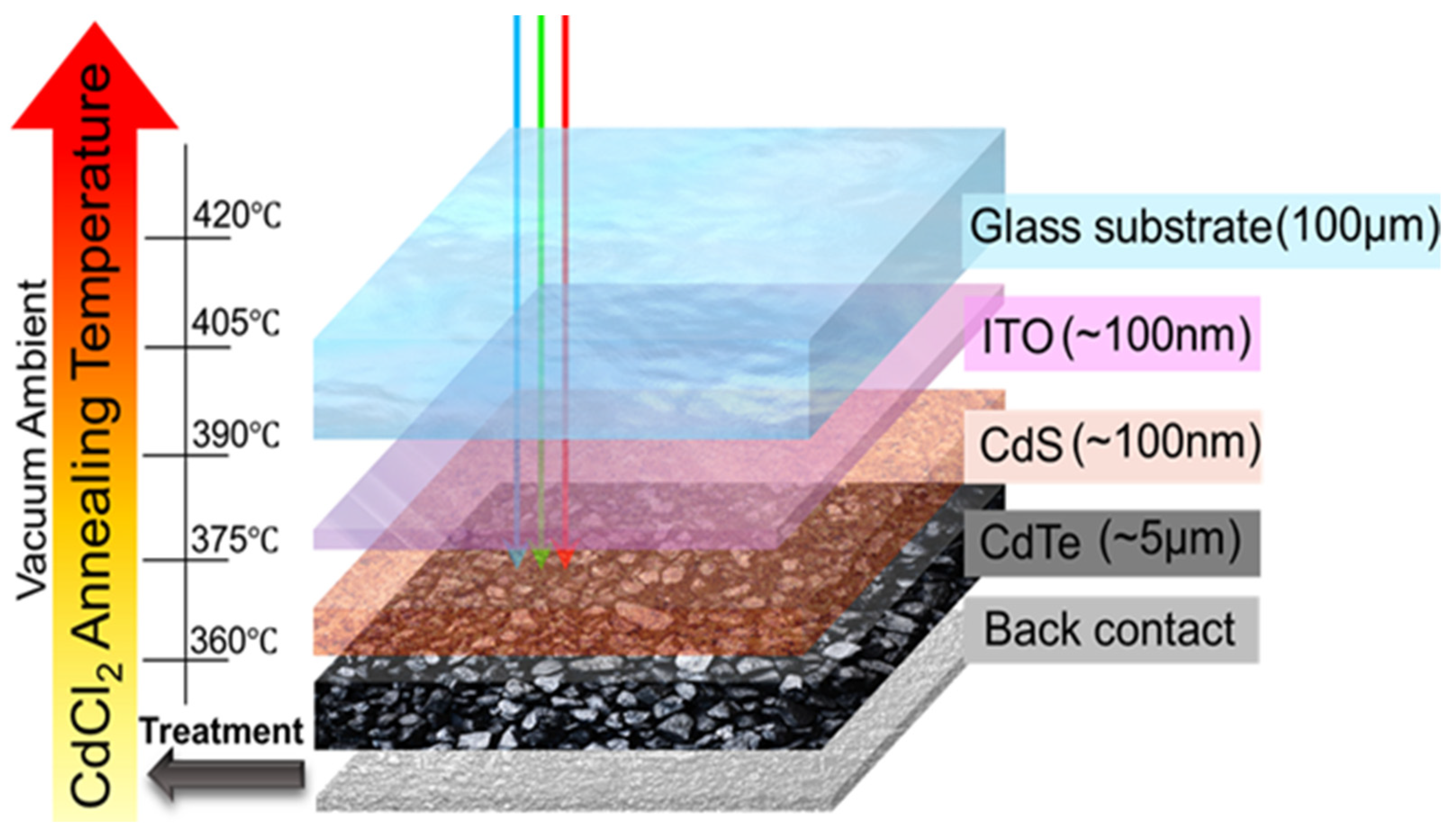
2. Experimental Procedure
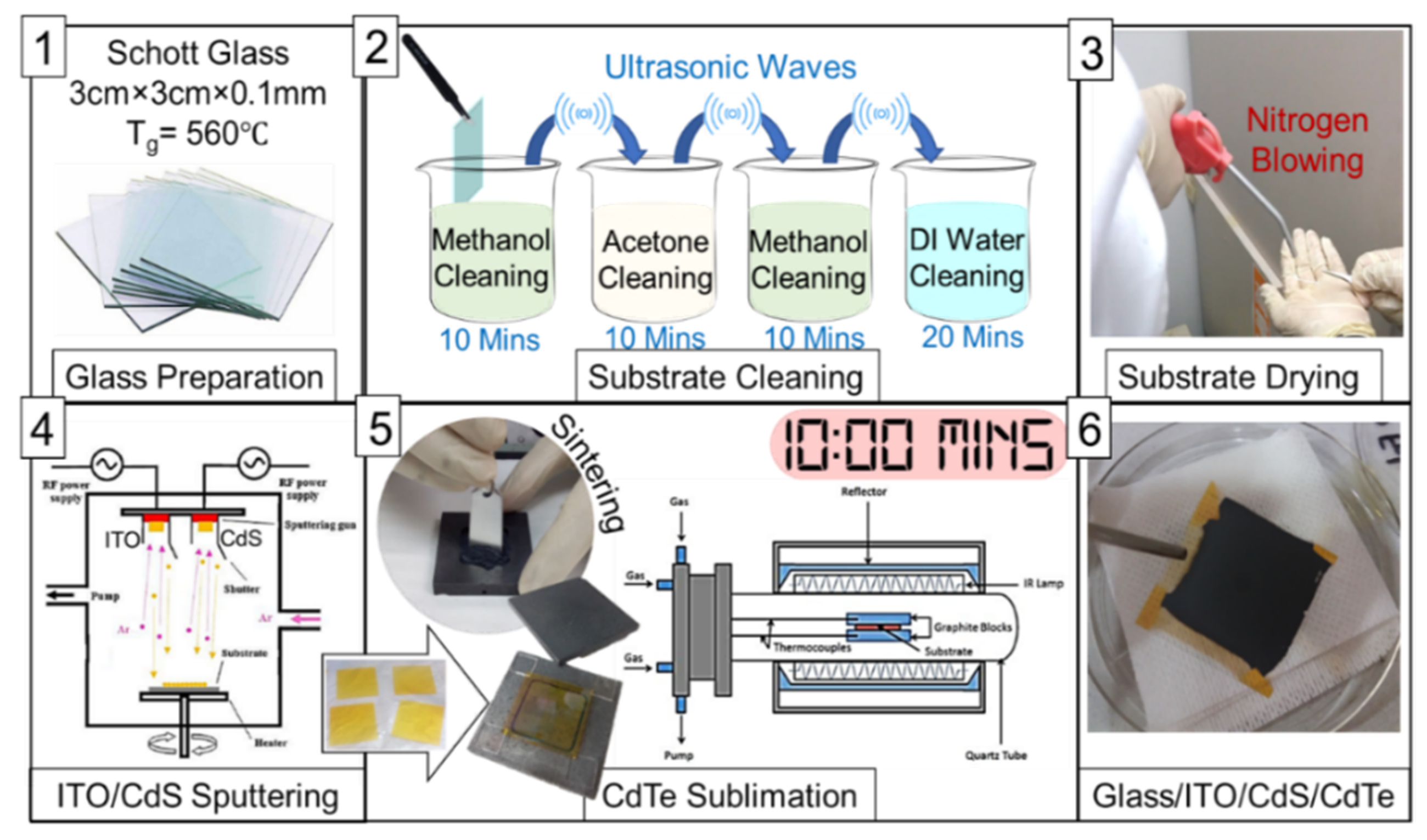
2.1. Thin Film Growth
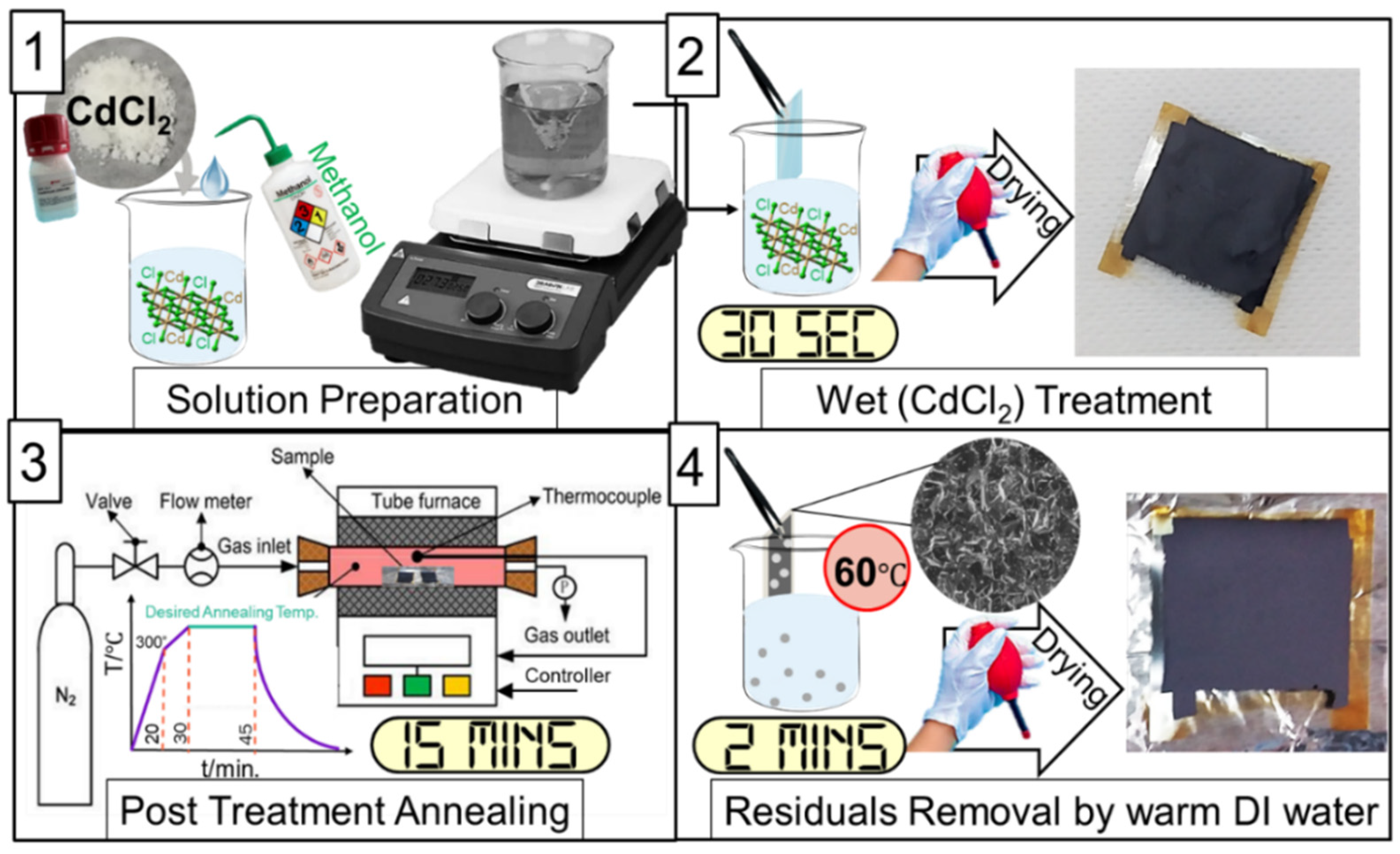
2.2. CdCl2 Treatment Process
2.3. CdTe Thin Film Characterization
3. Results and Discussion
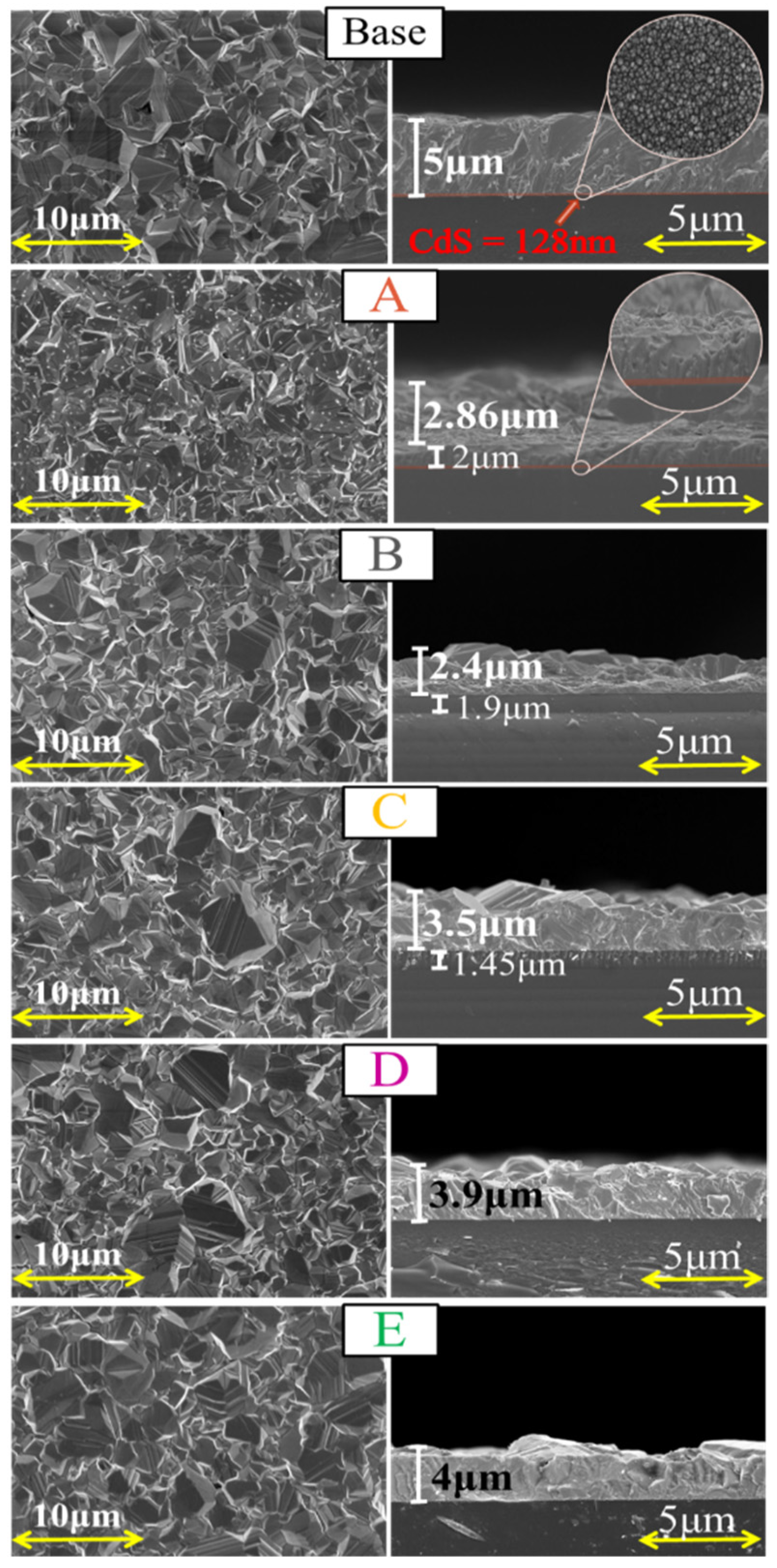
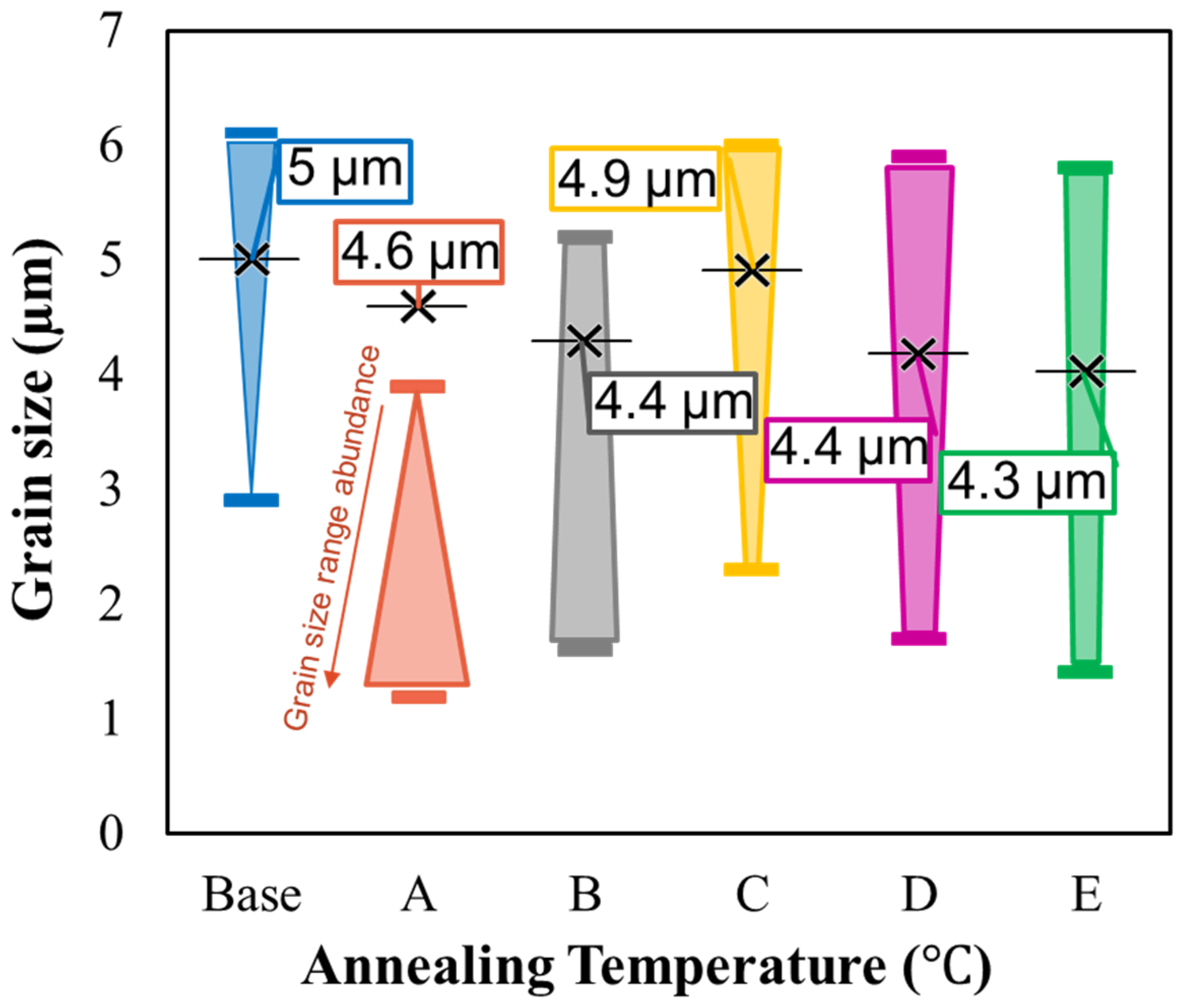
3.1. Surface Morphology by FESEM
3.2. Compositional Properties by EDX
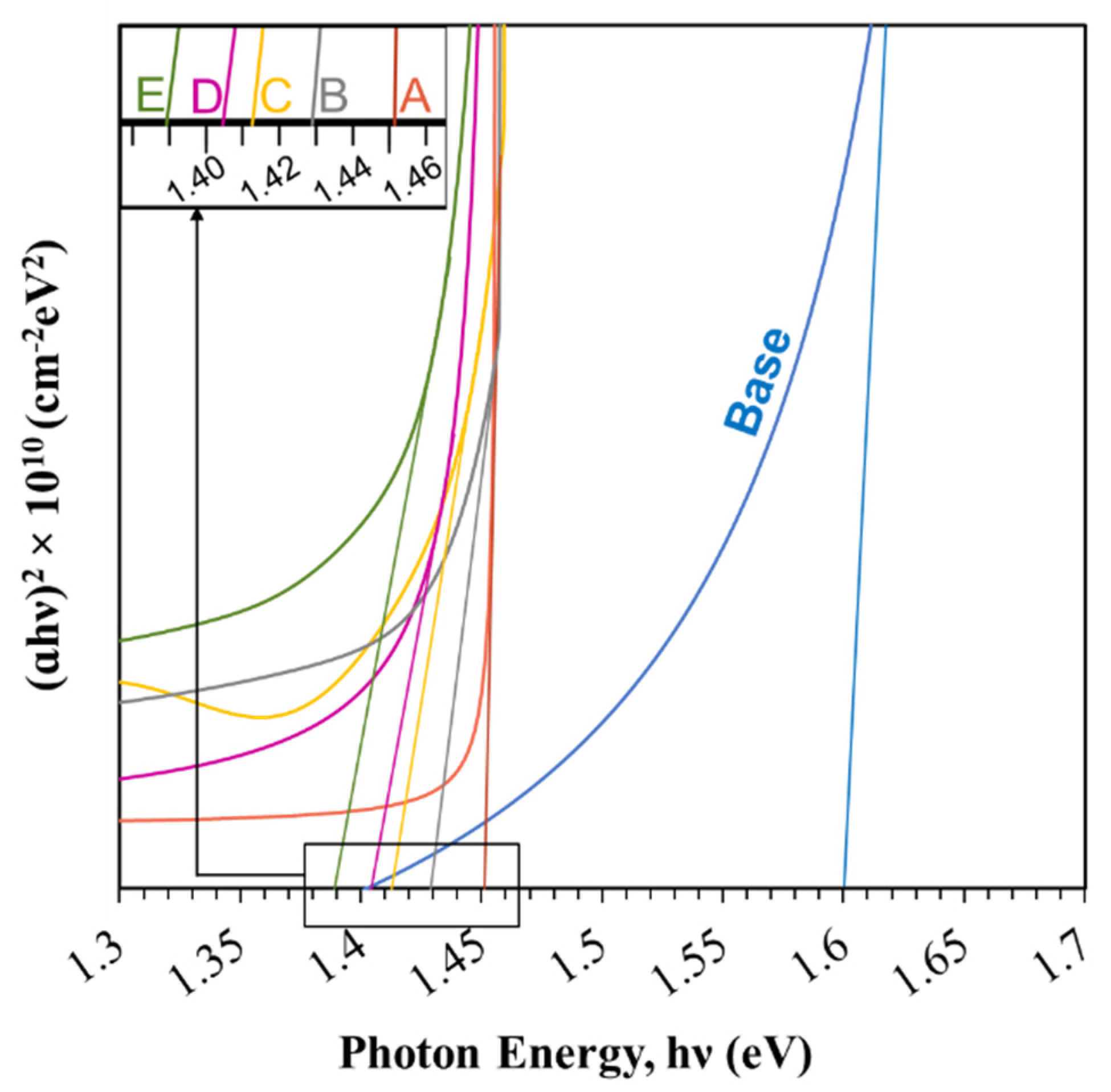
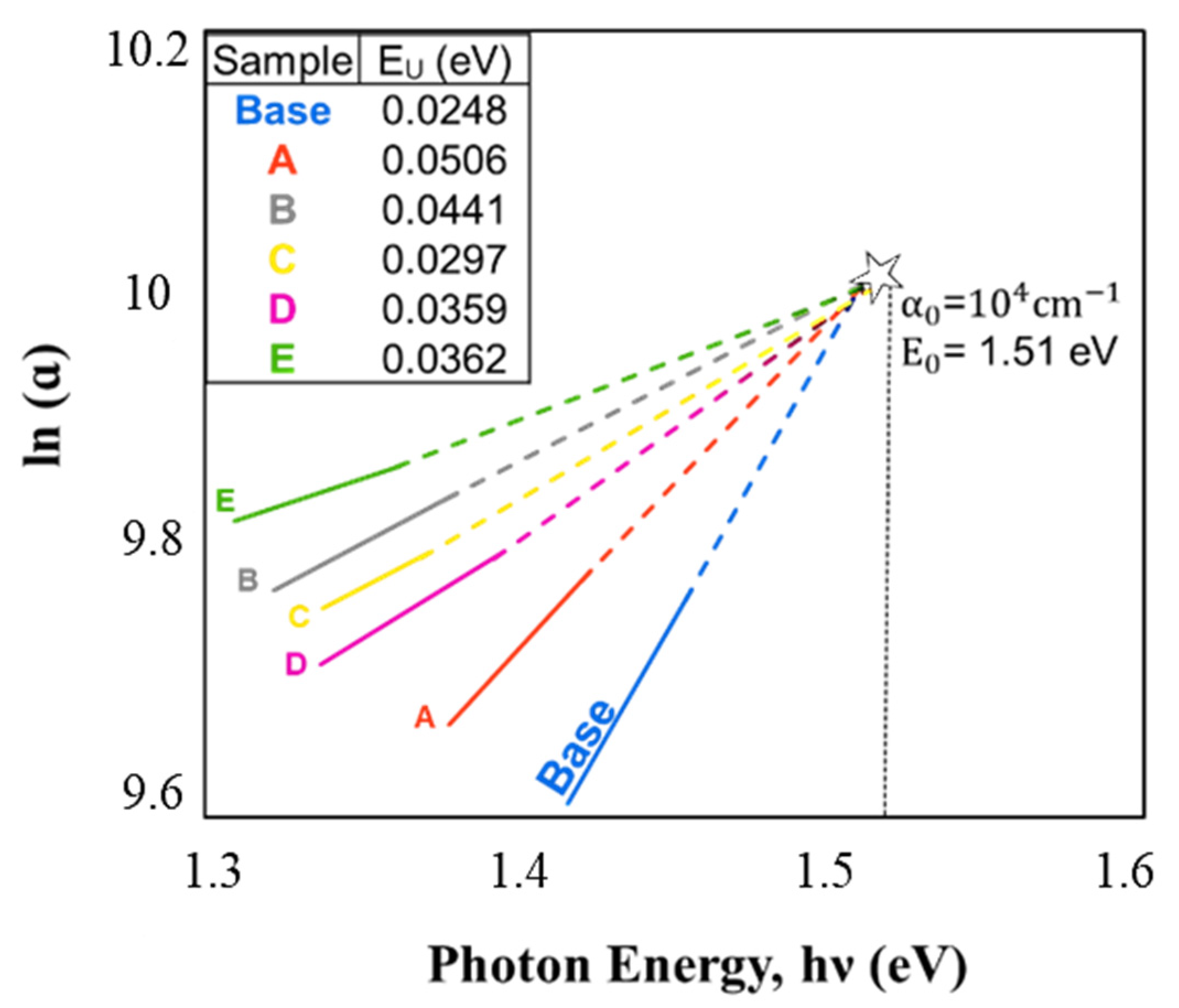
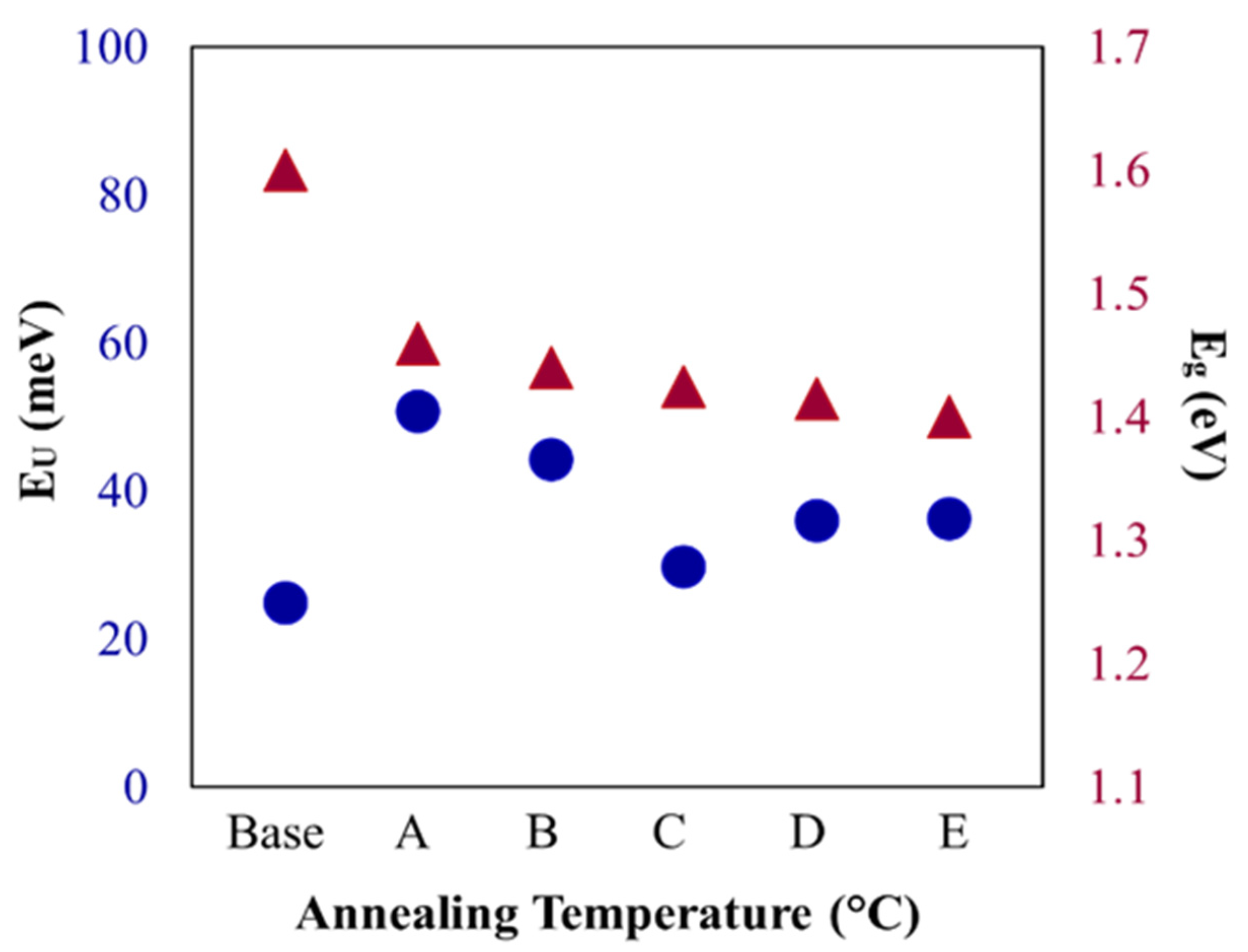
3.3. Optical Analysis
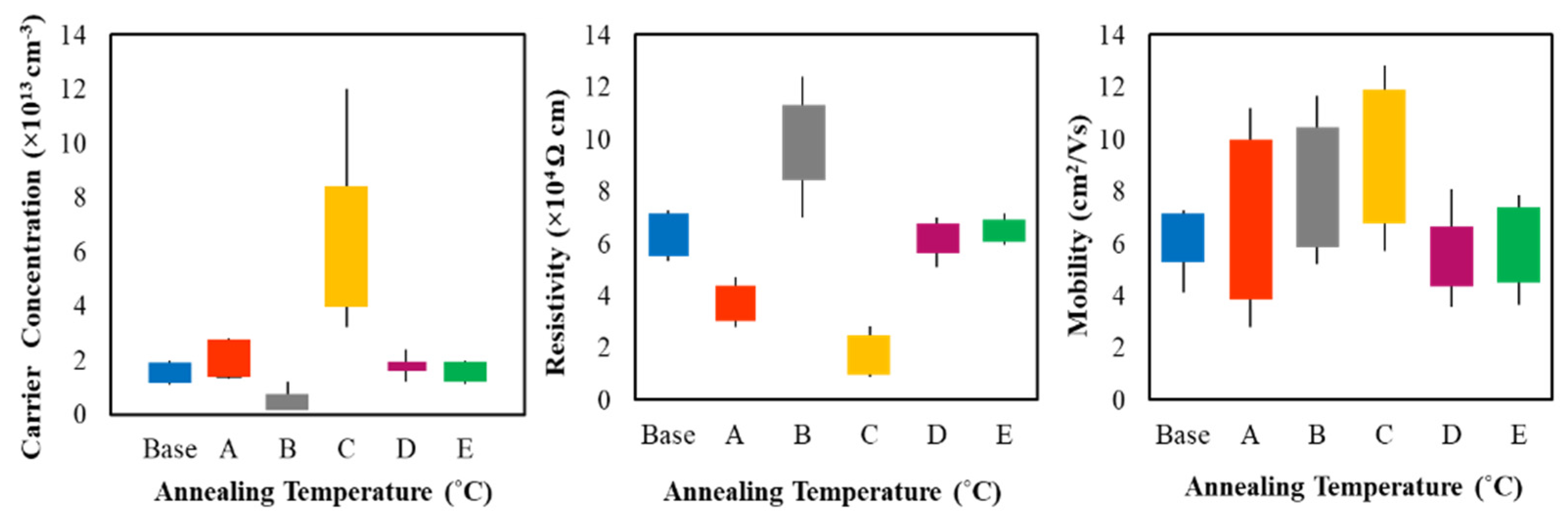
3.4. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Ren, S.; Li, C.; Li, C.; Chen, G.; Lai, H.; Zhang, J.; Wu, L. Investigation of recombination mechanisms of CdTe solar cells with different buffer layers. Sol. Energy Mater. Sol. Cells 2018, 188, 93–98. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, K.S.; Sobayel, K.; Enam, T.; Ali, A.M.; Zaman, M.; Akhtaruzzaman, M.; Amin, N. Fabrication of high efficiency sputtered CdS: O/CdTe thin film solar cells from window/absorber layer growth optimization in magnetron sput-tering. Sol. Energy Mater. Sol. Cells 2017, 172, 384–393. [Google Scholar] [CrossRef]
- Oluyamo, S.S.; Akinboyewa, L.O.; Fuwape, I.A.; Olusola, O.I.O.; Adekoya, M.A. Influence of nanocellulose concentration on the tunability of energy bandgap of cadmium telluride thin films. Cellulose 2020, 27, 8147–8153. [Google Scholar] [CrossRef]
- Doroody, C.; Rahman, K.; Abdullah, S.; Harif, M.; Rosly, H.; Tiong, S.; Amin, N. Temperature difference in close-spaced sub-limation (CSS) growth of CdTe thin film on ultra-thin glass substrate. Results Phys. 2020, 18, 103213. [Google Scholar] [CrossRef]
- Le Bourhis, E. Glass: Mechanics and Technology; John Wiley & Sons: Weinheim, Germany, 2014. [Google Scholar]
- Ho, S.M. Synthesis of Thin Films on Flexible Substrates: A Review. Middle-East. J. Sci. Res. 2016, 24, 2235–2238. [Google Scholar]
- Paul, S.; Swartz, C.; Sohal, S.; Grice, C.; Bista, S.S.; Li, D.-B.; Yan, Y.; Holtz, M.; Li, J.V. Buffer/absorber interface recombination reduction and improvement of back-contact barrier height in CdTe solar cells. Thin Solid Film. 2019, 685, 385–392. [Google Scholar] [CrossRef]
- Song, T.; Kanevce, A.; Sites, J.R. Emitter/absorber interface of CdTe solar cells. J. Appl. Phys. 2016, 119, 233104. [Google Scholar] [CrossRef]
- Amarasinghe, M.; Colegrove, E.; Moseley, J.; Moutinho, H.; Albin, D.; Duenow, J.; Jensen, S.; Kephart, J.; Sampath, W.; Si-vananthan, S.; et al. Obtaining large columnar CdTe grains and long lifetime on nanocrystalline CdSe, MgZnO, or CdS layers. Adv. Energy Mater. 2018, 8, 1702666. [Google Scholar] [CrossRef]
- Abbas, A.; West, G.D.; Bowers, J.W.; Kaminski, P.M.; Maniscalco, B.; Walls, J.M.; Barth, K.L.; Sampath, W.S. Cadmium chloride assisted re-crystallization of CdTe: The effect of varying the annealing time. Mrs Online Proc. Libr. Arch. 2014, 1638, 1–6. [Google Scholar] [CrossRef]
- McCandless, B.; Metzger, W.K.; Buchanan, W.; Sriramagiri, G.; Thompson, C.; Duenow, J.; Albin, D.; Jensen, S.A.; Moseley, J.; Al-Jassim, M. Enhanced p-Type Doping in Polycrystalline CdTe Films: Deposition and Activation. IEEE J. Photovolt. 2019, 9, 912–917. [Google Scholar] [CrossRef]
- Awni, R.A.; Li, D.B.; Song, Z.; Bista, S.S.; Razooqi, M.A.; Grice, C.R.; Chen, L.; Liyanage, G.K.; Li, C.; Phillips, A.B.; et al. In-fluences of buffer material and fabrication atmosphere on the electrical properties of CdTe solar cells. Prog. Photovolt. Res. Appl. 2019, 27, 1115–1123. [Google Scholar] [CrossRef]
- Schuler, G.P.; Maniscalco, B.; Bowers, J.W.; Claudio, G.; M, J. Walls Optimisation of Cadmium Chlo-Ride Solution Processing of Close Space Sublimated Thin Film CdTe Solar Cells. 2013. pp. 175–178. Available online: https://repository.lboro.ac.uk/articles/conference_contribution/Optimisation_of_cadmium_chloride_solution_processing_of_close_space_sublimated_thin_film_CdTe_solar_cells/9556787/1 (accessed on 22 February 2021).
- Bai, Z.; Wan, L.; Hou, Z.; Wang, D. Effect of CdCl2 annealing treatment on CdS thin films and CdTe/CdS thin film solar cells. Phys. Status Solidi 2010, 8, 628–630. [Google Scholar] [CrossRef]
- Chander, S.; Dhaka, M.S. CdCl2 treatment concentration evolution of physical properties correlation with surface morphology of CdTe thin films for solar cells. Mater. Res. Bull. 2018, 97, 128–135. [Google Scholar] [CrossRef]
- Abbas, A.; West, G.D.; Bowers, J.W.; Isherwood, P.; Kaminski, P.M.; Maniscalco, B.; Rowley, P.; Walls, J.M.; Barricklow, K.; Sampath, W.S.; et al. The effect of cadmium chloride treatment on close spaced sublimated cadmium telluride thin film solar cells. In Proceedings of the 2012 IEEE 38th Photovoltaic Specialists Conference (PVSC) PART 2, Austin, TX, USA, 3–8 June 2012; pp. 1–6. [Google Scholar]
- Kim, M.; Sohn, S.; Lee, S. Reaction kinetics study of CdTe thin films during CdCl2 heat treatment. Sol. Energy Mater. Sol. Cells 2011, 95, 2295–2301. [Google Scholar] [CrossRef]
- Moutinho, H.R.; Al-Jassim, M.M.; Levi, D.H.; Dippo, P.C.; Kazmerski, L.L. Effects of CdCl2 treatment on the recrystallization and electro-optical properties of CdTe thin films. J. Vac. Sci. Technol. A: Vac. Surf. Film. 1998, 16, 1251–1257. [Google Scholar] [CrossRef]
- Li, C.; Poplawsky, J.; Wu, Y.; Lupini, A.R.; Mouti, A.; Leonard, D.N.; Paudel, N.; Jones, K.; Yin, W.; Al-Jassim, M.; et al. From atomic structure to photovoltaic properties in CdTe solar cells. Ultramicroscopy 2013, 134, 113–125. [Google Scholar] [CrossRef]
- Chander, S.; Dhaka, M. Time evolution to CdCl2 treatment on Cd-based solar cell devices fabricated by vapor evaporation. Sol. Energy 2017, 150, 577–583. [Google Scholar] [CrossRef]
- Samoilenko, Y.; Yeung, G.; Munshi, A.H.; Abbas, A.; Reich, C.L.; Walker, M.; Reese, M.O.; Zakutayev, A.; Walls, J.M.; Sampath, W.S.; et al. Stable magnesium zinc oxide by reactive Co-Sputtering for CdTe-based solar cells. Sol. Energy Mater. Sol. Cells 2020, 210, 110521. [Google Scholar] [CrossRef]
- Jain, G.; Ahnood, A.; Chanaewa, A.; Fox, K.; Mulvaney, P. The role of CdCl2 treatments and annealing in the formation of sintered CdTe nanocrystal solar cells. Phys. Lett. A 2019, 383, 1199–1202. [Google Scholar] [CrossRef]
- Wang, L.; Luo, M.; Qin, S.; Liu, X.; Chen, J.; Yang, B.; Leng, M.; Xue, D.-J.; Zhou, Y.; Gao, L.; et al. Ambient CdCl2 treatment on CdS buffer layer for improved performance of Sb2Se3 thin film photovoltaics. Appl. Phys. Lett. 2015, 107, 143902. [Google Scholar] [CrossRef]
- Willeke, G.P.; Weber, E.R. Advances in Photovoltaics: Part 2; Newnes: Oxford, UK, 17 October 2013. [Google Scholar]
- Harvey, S.P.; Teeter, G.; Moutinho, H.; Al-Jassim, M.M. Direct evidence of enhanced chlorine segregation at grain boundaries in polycrystalline CdTe thin films via three-dimensional TOF-SIMS imaging. Prog. Photovolt. Res. Appl. 2015, 23, 838–846. [Google Scholar] [CrossRef]
- AbuEl-Rub, K.M.; Hahn, S.R.; Tari, S.; Dissanayake, M.A.K.L. Effects of CdCl2 heat treatment on the morphological and chemical properties of CdTe/CdS thin films solar cells. Appl. Surf. Sci. 2012, 258, 6142–6147. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Ojo, A.A. Unravelling complex nature of CdS/CdTe based thin film solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 16598–16617. [Google Scholar] [CrossRef]
- Dharmadasa, I.M. Review of the CdCl2 Treatment Used in CdS/CdTe Thin Film Solar Cell Development and New Evidence towards Improved Understanding. Coatings 2014, 4, 282–307. [Google Scholar] [CrossRef]
- Romeo, N.; Bosio, A.; Tedeschi, R.; Romeo, A.; Canevari, V. A highly efficient and stable CdTe/CdS thin film solar cell. Sol. Energy Mater. Sol. Cells 1999, 58, 209–218. [Google Scholar] [CrossRef]
- Hajimammadov, R.; Fathi, N.; Bayramov, A.; Khrypunov, G.; Klochko, N.; Li, T. Effect of ‘CdCl2 Treatment’ on properties of CdTe-based solar cells prepared by physical vapor deposition and close-spaced sublimation methods. Jpn. J. Appl. Phys. 2011, 50, 05FH01. [Google Scholar] [CrossRef]
- Salavei, A.; Rimmaudo, I.; Piccinelli, F.; Menossi, D.; Romeo, N.; Bosio, A.; Dharmadasa, R.; Romeo, A. Flexible CdTe solar cells by a low temperature process on ITO/ZnO coated polymers. In Proceedings of the 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, Germany, 24 September 2012; pp. 24–28. [Google Scholar]
- Major, J.D.; Al Turkestani, M.; Bowen, L.; Brossard, M.; Li, C.; Lagoudakis, P.; Pennycook, S.J.; Phillips, L.J.; Treharne, R.E.; DuRose, K. In-depth analysis of chloride treatments for thin-film CdTe solar cells. Nat. Commun. 2016, 7, 13231. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Echendu, O.K.; Fauzi, F.; Abdul-Manaf, N.A.; Olusola, O.I.; Salim, H.I.; Madugu, M.L.; Ojo, A.A. Im-provement of composition of CdTe thin films during heat treatment in the presence of CdCl2. J. Mater. Sci. Mater. Electron. 2017, 28, 2343–2352. [Google Scholar] [CrossRef]
- Ulmer, M.P.; Graham, M.E.; Vaynman, S.; Cao, J.; Takacs, P.Z. Magnetic smart material application to adaptive x-ray optics. In Adaptive X-ray Optics; International Society for Optics and Photonics: San Diego, CA, USA, 2010; Volume 7803, p. 780309. [Google Scholar]
- Ottermann, C.; Sparschuh, G. Process for Producing Organic Light-Emitting Devices. U.S. Patent Application 10/556,752, 29 November 2007. [Google Scholar]
- Osedach, T.P.; Geyer, S.M.; Ho, J.C.; Arango, A.C.; Bawendi, M.G.; Bulović, V. Lateral heterojunction photodetector consisting of molecular organic and colloidal quantum dot thin films. Appl. Phys. Lett. 2009, 94, 26. [Google Scholar] [CrossRef]
- Rahman, K.S.; Harif, M.N.; Rosly, H.N.; Kamaruzzaman, M.I.B.; Akhtaruzzaman, M.; Alghoul, M.; Misran, H.; Amin, N. Influence of deposition time in CdTe thin film properties grown by Close-Spaced Sublimation (CSS) for photovoltaic applica-tion. Results Phys. 2019, 14, 102371. [Google Scholar] [CrossRef]
- Romeo, A.; Arnold, M.; Bätzner, D.L.; Zogg, H.; Tiwari, A. Development of high efficiency flexible CdTe solar cells. In Proceedings of the PV in Europe from PV Technology to Energy Solutions Conference and Exhibition, Rome, Italy, 7 October 2002; pp. 377–381. [Google Scholar]
- Harif, M.N.; Rahman, K.S.; Rosly, H.N.; Chelvanathan, P.; Doroody, C.; Misran, H.; Amin, N. An approach to alternative post-deposition treatment in CdTe thin films for solar cell application. Superlattices Microstruct. 2020, 147, 106687. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Bingham, P.A.; Echendu, O.K.; Salim, H.I.; Druffel, T.; Dharmadasa, R.; Sumanasekera, G.U.; Dharmasena, R.R.; Dergacheva, M.B.; Mit, K.A.; et al. Fabrication of CdS/CdTe-based thin film solar cells using an electrochemical technique. Coatings 2014, 4, 380–415. [Google Scholar] [CrossRef]
- Munshi, A.H.; Kephart, J.M.; Abbas, A.; Danielson, A.; Gḗlinas, G.; Beaudry, J.-N.; Barth, K.L.; Walls, J.M.; Sampath, W.S. Effect of CdCl2 passivation treatment on microstructure and performance of CdSeTe/CdTe thin-film photovoltaic devices. Sol. Energy Mater. Sol. Cells 2018, 186, 259–265. [Google Scholar] [CrossRef]
- Kajikawa, Y. Analysing surface roughness evolution in thin films. In Thin Film Growth; Woodhead Publishing: Cambridge, UK, 2011; pp. 60–82. [Google Scholar]
- Berg, M.; Kephart, J.M.; Sampath, W.S.; Ohta, T.; Chan, C. Effects of CdCl2 treatment on the local electronic properties of polycrystalline CdTe measured with photoemission electron microscopy. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; pp. 3417–3421. [Google Scholar]
- Major, J.D. Grain boundaries in CdTe thin film solar cells: A review. Semicond. Sci. Technol. 2016, 31, 093001. [Google Scholar] [CrossRef]
- Amin, N.; Rahman, K.S. Close-Spaced Sublimation (CSS): A Low-Cost, High-Yield Deposition System for Cadmium Telluride (CdTe) Thin Film Solar Cells. Mod. Technol. Creat. Thin-Film Syst. Coat. 2017, 361, 361–380. [Google Scholar]
- Li, C.; Wu, Y.; Poplawsky, J.; Pennycook, T.J.; Paudel, N.; Yin, W.; Haigh, S.J.; Oxley, M.P.; Lupini, A.R.; Al-Jassim, M.; et al. Grain-Boundary-Enhanced Carrier Collection in CdTe Solar Cells. Phys. Rev. Lett. 2014, 112, 156103. [Google Scholar] [CrossRef]
- Berg, M.; Kephart, J.M.; Munshi, A.; Sampath, W.S.; Ohta, T.; Chan, C. Local Electronic Structure Changes in Polycrystalline CdTe with CdCl2 Treatment and Air Exposure. ACS Appl. Mater. Interfaces 2018, 10, 9817–9822. [Google Scholar] [CrossRef]
- Ayoub, M.; Hage-Ali, M.; Koebel, J.M.; Zumbiehl, A.; Klotz, F.; Rit, C.; Regal, R.; Fougeres, P.; Siffert, P. Annealing effects on defect levels of CdTe: Cl materials and the uniformity of the electrical properties. IEEE Trans. Nucl. Sci. 2003, 50, 229–237. [Google Scholar] [CrossRef]
- Shaaban, E.; Afify, N.; El-Taher, A. Effect of film thickness on microstructure parameters and optical constants of CdTe thin films. J. Alloy. Compd. 2009, 482, 400–404. [Google Scholar] [CrossRef]
- Gorji, N.E. Deposition and doping of CdS/CdTe thin film solar cells. J. Semicond. 2015, 36, 54001. [Google Scholar] [CrossRef]
- Maxwell, G.L. Characterization and Modeling of CdCl2 Treated CdTe/CdS Thin-Film Solar Cell. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2010. [Google Scholar]
- Khairnar, U.P.; Bhavsar, D.S.; Vaidya, R.U.; Bhavsar, G.P. Optical properties of thermally evaporated cadmium telluride thin films. Mater. Chem. Phys. 2003, 80, 421–427. [Google Scholar] [CrossRef]
- Hasaneen, M.; Alrowaili, Z.A.; Mohamed, W. Structure and optical properties of polycrystalline ZnSe thin films: Validity of Swanepol’s approach for calculating the optical parameters. Mater. Res. Express 2020, 7, 016422. [Google Scholar] [CrossRef]
- Chandramohan, S.; Sathyamoorthy, R.; Lalitha, S.; Senthilarasu, S. Structural properties of CdTe thin films on different sub-strates. Sol. Energy Mater. Sol. Cells 2006, 90, 686–693. [Google Scholar] [CrossRef]
- Cho, E.; Kang, Y.; Kim, D. Post-growth process for flexible CdS/CdTe thin film solar cells with high specific power. Opt. Express 2016, 24, A791–A796. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ren, A.; Zhang, J.; Li, K.; Li, C.; Wang, W.; Xu, H. The study of oxygen concentration in the CdTe thin film prepared by vapor transport deposition for CdTe photovoltaic devices. J. Mater. Sci. Mater. Electron. 2017, 28, 9442–9449. [Google Scholar] [CrossRef]
- Regalado-Perez, E.; Reyes-Banda, M.G.; Mathew, X. Influence of oxygen concentration in the CdCl2 treatment process on the photovoltaic properties of CdTe/CdS solar cells. Thin Solid Film. 2015, 582, 134–138. [Google Scholar] [CrossRef]
- McCandless, B.E.; Buchanan, W.A.; Thompson, C.P.; Sriramagiri, G.; Lovelett, R.J.; Duenow, J.; Albin, D.; Jensen, S.; Colegrove, E.; Moseley, J.; et al. Overcoming Carrier Concentration Limits in Polycrystalline CdTe Thin Films with In Situ Doping. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Rahman, K.S.; Haque, F.; Khan, N.A.; Islam, M.A.; Alam, M.M.; Alothman, Z.A.; Sopian, K.; Amin, N. Effect of CdCl2 treatment on thermally evaporated CdTe thin films. Chalcogenide Lett. 2014, 11, 129–139. [Google Scholar]


| Parameter | ITO Layer | CdS Layer |
|---|---|---|
| Mode | DC | RF |
| Substrate Temperature | Ambient | Ambient |
| Deposition Time (mins) | 40 | 10 |
| Power (W) | 50 | 40 |
| Argon Flow Rate (SCCM) | 4 | 5 |
| Base Pressure (Torr) | 10−5 | 10−5 |
| Operating Pressure (mTorr) | 25 | 18 |
| Parameter | Condition |
|---|---|
| Substrate Temperature | 500 °C |
| Source Temperature | 600 °C |
| Argon Flow Rate | 1–1.5 Torr |
| Source-Substrate Distance | 1 mm |
| Deposition Time | 10 min |
| Ambient Gas | Argon |
| Sample ID# | Annealing Temperature (°C) |
|---|---|
| Base | - |
| A | 360 |
| B | 375 |
| C | 390 |
| D | 405 |
| E | 420 |
| Sample | Band Gap, Eg (eV) | Urbach Energy, EU (meV) | Refractive Index (n) |
|---|---|---|---|
| Base | 1.6 | 24.80 | 2.90 |
| A | 1.46 | 50.68 | 2.97 |
| B | 1.44 | 44.17 | 2.98 |
| C | 1.425 | 29.75 | 2.99 |
| D | 1.415 | 35.95 | 3.00 |
| E | 1.39 | 36.29 | 3.00 |
| Sample | Carrier Concentration [×1013] (/cm3) | Mobility (cm2/Vs) | Resistivity [×104] (Ω-cm) |
|---|---|---|---|
| Base | 1.4092 | 6.5753 | 6.7451 |
| A | 2.3245 | 7.1571 | 3.7567 |
| B | 0.7098 | 7.1811 | 11.2617 |
| C | 6.9849 | 8.7650 | 1.0208 |
| D | 1.8141 | 5.3388 | 6.453 |
| E | 1.8114 | 5.4954 | 6.2786 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, N.; Karim, M.R.; ALOthman, Z.A. Impact of CdCl2 Treatment in CdTe Thin Film Grown on Ultra-Thin Glass Substrate via Close Spaced Sublimation. Crystals 2021, 11, 390. https://doi.org/10.3390/cryst11040390
Amin N, Karim MR, ALOthman ZA. Impact of CdCl2 Treatment in CdTe Thin Film Grown on Ultra-Thin Glass Substrate via Close Spaced Sublimation. Crystals. 2021; 11(4):390. https://doi.org/10.3390/cryst11040390
Chicago/Turabian StyleAmin, Nowshad, Mohammad Rezaul Karim, and Zeid Abdullah ALOthman. 2021. "Impact of CdCl2 Treatment in CdTe Thin Film Grown on Ultra-Thin Glass Substrate via Close Spaced Sublimation" Crystals 11, no. 4: 390. https://doi.org/10.3390/cryst11040390
APA StyleAmin, N., Karim, M. R., & ALOthman, Z. A. (2021). Impact of CdCl2 Treatment in CdTe Thin Film Grown on Ultra-Thin Glass Substrate via Close Spaced Sublimation. Crystals, 11(4), 390. https://doi.org/10.3390/cryst11040390








