Cocrystals Based on 4,4’-bipyridine: Influence of Crystal Packing on Melting Point
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and General Methods
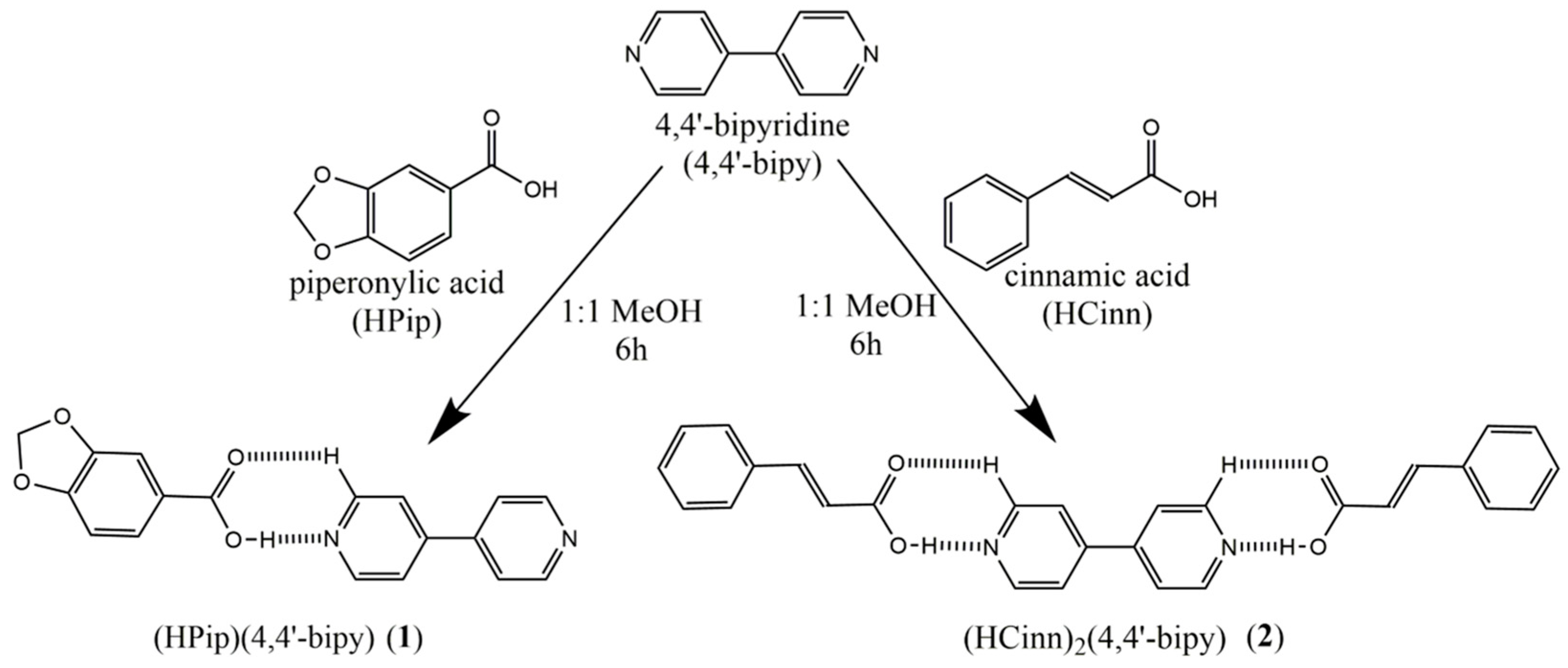
2.2. Synthesis of Cocrystals 1 and 2
2.3. X-ray Crystallographic Data
2.4. Computational Details of Hirshfeld Surface Analysis, Energy Frameworks, and Lattice Energy
3. Results and Discussion
3.1. Synthesis and Characterization of Cocrystals 1 and 2
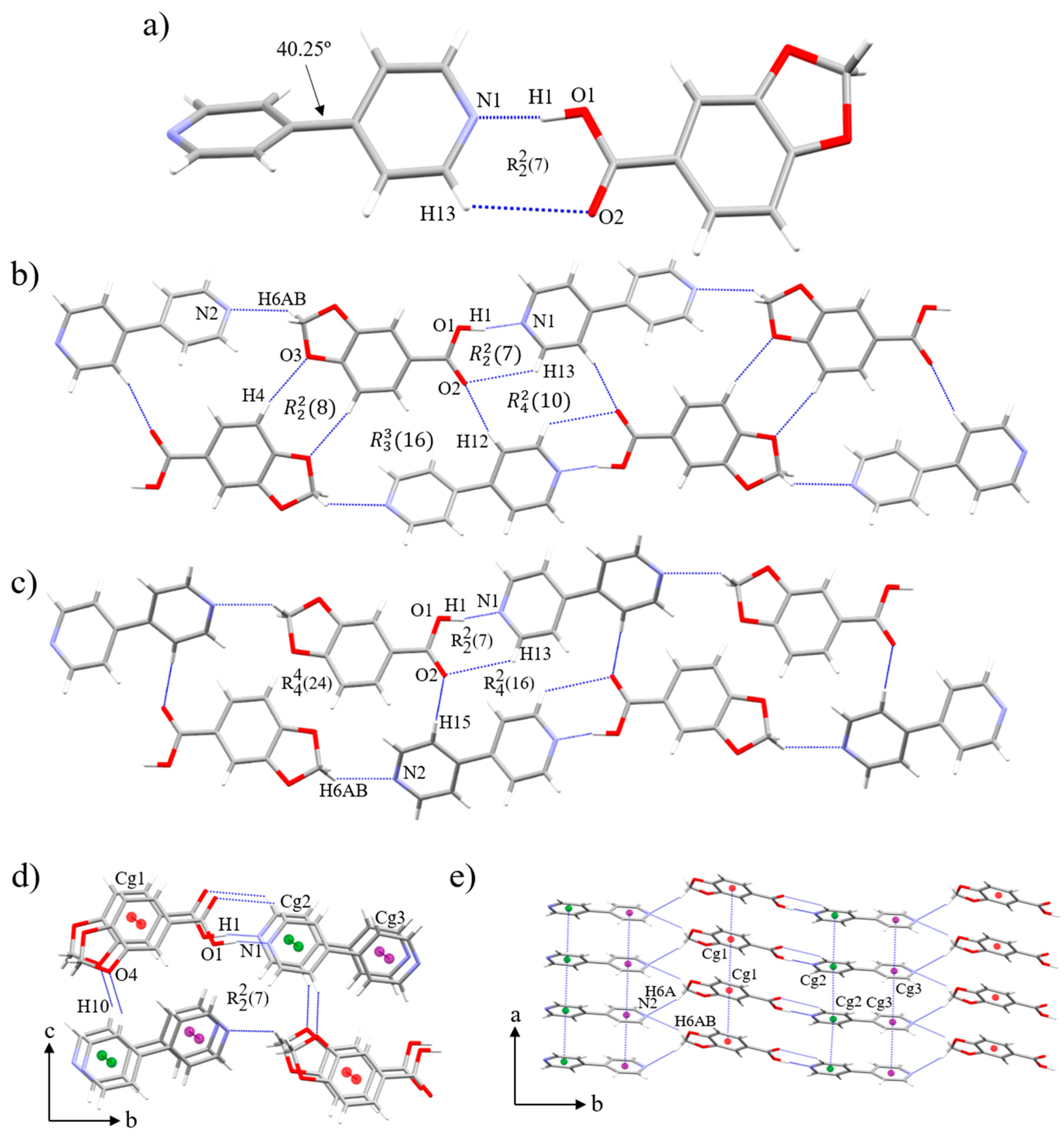
3.2. Crystal and Extended Structure of (HPip)(4,4’-bipy) (1)
3.3. Crystal and Extended Structure of (HCinn)2(4,4’-bipy) (2)
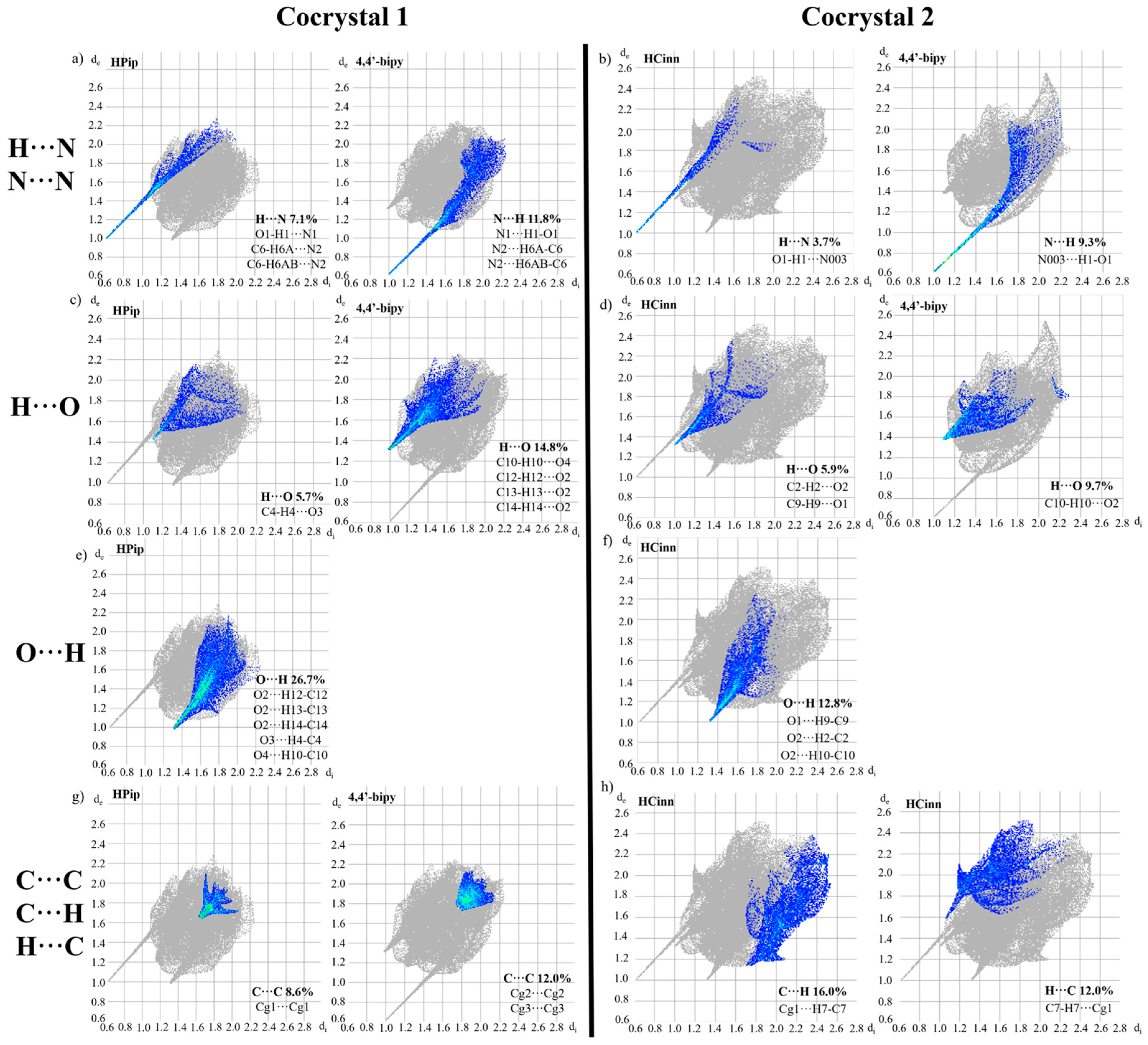
3.4. Hirshfeld Surface Analysis and Energy Frameworks Calculations of 1 and 2
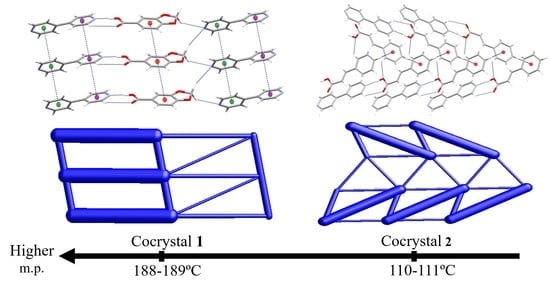
3.5. Relationship of Melting Point Values with Packing Effects of Cocrystals 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Braga, D.; Grepioni, F. Reactions between or within molecular crystals. Angew. Chem. Int. Ed. 2004, 43, 4002–4011. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering-A New Organic Synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Desiraju, G.R. The Supramolecular Synthon in Crystal Engineering. In Stimulating Concepts in Chemistry; Vögtle, F., Stoddart, J.F., Shibasaki, M., Eds.; Wiley-VCH: Weinheim, Germany, 2000; pp. 293–306. [Google Scholar]
- Nayak, A.; Pedireddi, V.R. Rational Analysis of Melting Point Behavior of Co-Crystals of 4-Nitrophenol with Some Aza-Compounds. Cryst. Growth Des. 2016, 16, 5966–5975. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef]
- Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Characterization, physicochemical and photo-stability of a co-crystal involving an antibiotic drug, nitrofurantoin, and 4-hydroxybenzoic acid. CrystEngComm 2011, 13, 759–762. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.P.; Zode, S.S.; Bansal, A.K. Challenges in Translational Development of Pharmaceutical Cocrystals. J. Pharm. Sci. 2017, 106, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal Engineering: A Collaborative Strategy toward Functional Materials. Adv. Mater. 2019, 31, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, R.; Sarma, B. Drug-Drug and Drug-Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef]
- Feiler, T.; Bhattacharya, B.A.L.; Michalchuk, A.; Schröder, V.; List-Kratochvil, E.; Emmerling, F. Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers. Crystals 2020, 10, 889. [Google Scholar] [CrossRef]
- Arkhipov, S.G.; Losev, E.A.; Nguyen, T.T.; Rychkov, D.A.; Boldyreva, E.V. A large anisotropic plasticity of L-leucinium hydrogen maleate preserved at cryogenic temperatures. Acta Cryst. 2019, B75, 143–151. [Google Scholar] [CrossRef]
- Ahmed, E.; Karothu, D.P.; Naumov, P. Crystal Adaptronics: Mechanically Reconfigurable Elastic and Superelastic Molecular Crystals. Angew. Chem. Int. Ed. 2018, 57, 8837–8846. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Kumar, S.; Nanda, A. Approaches to Design of Pharmaceutical Cocrystals: A Review. Mol. Cryst. Liq. Cryst. 2018, 667, 54–77. [Google Scholar] [CrossRef]
- Rajput, L.; Banik, M.; Yarava, J.R.; Joseph, S.; Pandey, M.K.; Nishiyama, Y.; Desiraju, G.R. Exploring the salt-cocrystal continuum with solid-state NMR using natural-abundance samples: Implications for crystal engineering. IUCrJ 2017, 4, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Tothadi, S.; Shaikh, T.R.; Gupta, S.; Dandela, R.; Vinod, C.P.; Nangia, A.K. Can We Identify the Salt–Cocrystal Continuum State Using XPS? Cryst. Growth Des. 2021, 21, 735–747. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Pharmaceutical cocrystal and salts of norfloxacin. Cryst. Growth Des. 2006, 6, 2699–2708. [Google Scholar] [CrossRef]
- Ferretti, V.; Dalpiaz, A.; Bertolasi, V.; Ferraro, L.; Beggiato, S.; Spizzo, F.; Spisni, E.; Pavan, B. Indomethacin co-crystals and their parent mixtures: Does the intestinal barrier recognize them differently? Mol. Pharm. 2015, 12, 1501–1511. [Google Scholar] [CrossRef]
- Banik, M.; Gopi, S.P.; Ganguly, S.; Desiraju, G.R. Cocrystal and salt forms of furosemide: Solubility and diffusion variations. Cryst. Growth Des. 2016, 16, 5418–5428. [Google Scholar] [CrossRef]
- Steiner, T. Competition of hydrogen-bond acceptors for the strong carboxyl donor. Acta Cryst. 2001, B57, 103–106. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Z.; Parkin, S.; Zhou, P.; Cheng, K.; Li, C.; Yu, F.; Long, S. Preferred formation of the carboxylic acid-pyridine heterosynthon in 2-anilinonicotinic acids. RSC Adv. 2016, 6, 81101–81109. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Isonicotinamide-Based Compounds: From Cocrystal to Polymer. Molecules 2019, 24, 4169. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Persistence of Vision Pty. Ltd. Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2004; Available online: http://www.povray.org/ (accessed on 28 January 2021).
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 (2017). University of Western Australia. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 28 January 2021).
- Jayatilaka, D.; Grimwood, D.J. Tonto: A Fortran Based Object-Oriented System for Quantum Chemistry and Crystallography. Comput. Sci. ICCS 2003, 4, 142–151. [Google Scholar]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Thomas, S.P.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Accurate Lattice Energies for Molecular Crystals from Experimental Crystal Structures. J. Chem. Theory Comput. 2018, 14, 1614–1623. [Google Scholar] [CrossRef]
- Spackman, M.A. Towards the use of experimental electron densities to estimate reliable lattice energies. CrystEngComm 2018, 20, 5340–5347. [Google Scholar] [CrossRef]
- Perlovich, G.L. Thermodynamic characteristics of cocrystal formation and melting points for rational design of pharmaceutical two-component systems. CrystEngComm 2015, 17, 7019–7028. [Google Scholar] [CrossRef]
- Nanubolu, J.B.; Ravikumar, K. Correlating the melting point alteration with the supramolecular structure in aripiprazole drug cocrystals. CrystEngComm 2016, 18, 1024–1038. [Google Scholar] [CrossRef]
- Roy, P.; Ghosh, A. Mechanochemical cocrystallization to improve the physicochemical properties of chlorzoxazone. CrystEngComm 2020, 22, 4611–4620. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Wicker, J.G.P.; Crowley, L.M.; Robshaw, O.; Little, E.J.; Stokes, S.P.; Cooper, R.I.; Lawrence, S.E. Will they co-crystallize? CrystEngComm 2017, 19, 5336–5340. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Synthon polymorphism and pseudopolymorphism in co-crystals. The 4,4’-bipyridine–4-hydroxybenzoic acid structural landscape. Chem. Commun. 2011, 47, 4090–4092. [Google Scholar] [CrossRef]
- Khavasi, H.R.; Mir Mohammad Sadegh, B. Effect of robust π–π stacking synthon on the formation of mercury coordination compounds; an unusual pseudo-square planar geometry. Dalton Trans. 2014, 43, 5564–5573. [Google Scholar] [CrossRef] [PubMed]
- Tothadi, S. Polymorphism in cocrystals of urea:4,4′-bipyridine and salicylic acid:4,4′-bipyridine. CrystEngComm 2014, 16, 7587–7597. [Google Scholar] [CrossRef]
- Surov, A.O.; Simagina, A.A.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Fenamate cocrystals with 4,4′-bipyridine: Structural and thermodynamic aspects. Cryst. Growth Des. 2015, 15, 228–238. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Dey, D.; Bhandary, S.; Thomas, S.P.; Spackman, M.A.; Chopra, D. Energy frameworks and a topological analysis of the supramolecular features in in situ cryocrystallized liquids: Tuning the weak interaction landscape via fluorination. Phys. Chem. Chem. Phys. 2016, 18, 31811–31820. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Kendrick, J.; Neumann, M.A.; Leusen, F.J.J. Towards ab initio screening of co-crystal formation through lattice energy calculations and crystal structure prediction of nicotinamide, isonicotinamide, picolinamide and paracetamol multi-component crystals. CrystEngComm 2013, 15, 3799–3807. [Google Scholar] [CrossRef]
- Taylor, C.R.; Day, G.M. Evaluating the Energetic Driving Force for Cocrystal Formation. Cryst. Growth Des. 2018, 18, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Karamertzanis, P.G.; Welch, G.W.A.; Price, S.L. Can the formation of pharmaceutical cocrystals be computationally predicted? I. Comparison of lattice energies. Cryst. Growth Des. 2009, 9, 442–453. [Google Scholar] [CrossRef]
- Du, J.J.; Lai, F.; Váradi, L.; Williams, P.A.; Groundwater, P.W.; Platts, J.A.; Hibbs, D.E.; Overgaard, J. Monoclinic paracetamol vs. Paracetamol-4,4′ -bipyridine co-crystal; what is the difference? a charge density study. Crystals 2018, 8, 46. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Vasilev, N.A.; Churakov, A.V.; Perlovich, G.L. Cocrystals of Fluconazole with Aromatic Carboxylic Acids: Competition between Anhydrous and Hydrated Solid Forms. Cryst. Growth Des. 2020, 20, 1218–1228. [Google Scholar] [CrossRef]
- Boag, N.M.; Coward, K.M.; Jones, A.C.; Pemble, M.E.; Thompson, J.R. 4,4′-Bipyridyl at 203 K. Acta Cryst. 1999, C55, 672–674. [Google Scholar] [CrossRef]
- Li, J.-T.; Wang, Y.-L.; Zhou, L.-N.; Wang, J.-K. 3,4-Methylenedioxybenzoic acid. Acta Cryst. 2006, E62, 1893–1894. [Google Scholar] [CrossRef]
- Wang, Z.; Miller, B.; Mabin, M.; Shahni, R.; Wang, Z.D.; Ugrinov, A.; Chu, Q.R. Cyclobutane-1,3-Diacid (CBDA): A Semi-Rigid Building Block Prepared by [2+2] Photocyclization for Polymeric Materials. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Ghiassi, K.B.; Guenthner, A.J.; Redeker, N.D.; Boatz, J.A.; Harvey, B.G.; Davis, M.C.; Chafin, A.P.; Groshens, T.J. Insights into Melting Behavior of Propyl-Bridged Di(cyanate ester) Monomers through Crystal Packing, Thermal Characterization, and Computational Analysis. Cryst. Growth Des. 2018, 18, 1030–1040. [Google Scholar] [CrossRef]
- Monika; Verma, A.; Tiwari, M.K.; Show, B.; Saha, S. Modulation of Weak Interactions in Structural Isomers: Positional Isomeric Effects on Crystal Packing and Physical Properties and Solid-State Thin-Film Fabrication. ACS Omega 2020, 5, 448–459. [Google Scholar] [CrossRef]


| 1 | 2 | |
|---|---|---|
| CCDC | 2058460 | 2058461 |
| Empirical Formula | C18H14N2O4 | C28H24N2O4 |
| Formula weight | 322.31 | 452.49 |
| T (K) | 100(2) | 100(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| System, space group | Monoclinic, P21/c | Monoclinic, P21/n |
| a (Å) | 3.7524(6) | 7.8058(10) |
| b (Å) | 18.427(3) | 7.1639(8) |
| c (Å) | 21.139(3) | 20.786(3) |
| α (°) | 90 | 90 |
| β (°) | 92.646(6) | 93.147(5) |
| γ (°) | 90 | 90 |
| V (Å3) | 1460.1(4) | 1160.6(3) |
| Z | 4 | 2 |
| Dcalc (mg/m3) | 1.466 | 1.295 |
| µ (mm−1) | 0.105 | 0.087 |
| F (000) | 672 | 476 |
| Crystal size (mm3) | ||
| hkl ranges | −4<=h<=4, −23<=k<=23, −26<=l<=26 | −11<=h<=11, −10<=k<=10, −29<=l<=29 |
| θ range (°) | 2.210 to 26.402 | 2.741 to 30.909 |
| Reflections collected/unique/[Rint] | 20300 / 2999 / [Rint] = 0.0640 | 18015 / 3609 / [Rint] = 0.0778 |
| Completeness to θ (%) | 99.8 | 99.9 |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.7474 and 0.6133 | 0.7461 and 0.6585 |
| Refinement method | Full-matrix least-squares on 2 | Full-matrix least-squares on 2 |
| Data/Restrains/Parameters | 2999 / 0 / 221 | 3609 / 0 / 157 |
| Goodness-on-fit (GOF) on 2 | 1.107 | 1.063 |
| Final R indices [I>2σ(I)] | R1 = 0.0539 wR2 = 0.1113 | R1 = 0.0553 wR2 = 0.1108 |
| R indices (all data) | R1 = 0.0692 wR2 = 0.1174 | R1 = 0.1229 wR2 = 0.1507 |
| Extinction coefficient | n/a | 0.019(3) |
| Largest diff-peak and hole (e. Å−3) | 0.232 and −0.290 | 0.301 and −0.297 |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | > D-H···A (°) | |
| O(1)-H(1)···N(1) | 0.99(3) | 1.62(4) | 2.603(2) | 172(3) | |
| C(13)-H(13)···O(2) | 0.95 | 2.95 | 3.540(3) | 121 | |
| C(4)-H(4)···O(3) | 0.95 | 2.69 | 3.612(2) | 164 | |
| C(6)-H(6AB)···N(2) | 0.99 | 2.58 | 3.348(3) | 134 | |
| C(12)-H(12)···O(2) | 0.95 | 2.47 | 3.369(3) | 159 | |
| C(15)-H(15)···O(2) | 0.95 | 2.45 | 3.395(3) | 173 | |
| C(10)-H(10)···O(4) | 0.95 | 2.47 | 3.326(3) | 150 | |
| C(6)-H(6A)···N(2) | 0.99 | 2.73 | 3.589(3) | 146 | |
| Cg(I)···Cg(J) | dCg-Cga (Å) | αb (°) | β, γc (°) | dplane-planed | doffsete |
| Cg(1)···Cg(1) | 3.7523(13) | 0 | 28.4 | 3.3021(8) | 1.782 |
| Cg(2)···Cg(2) | 3.7524(14) | 0 | 20.3 | 3.5187(9) | 1.304 |
| Cg(3)···Cg(3) | 3.7523(13) | 0 | 20.6 | 3.5113(9) | 1.323 |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | > D-H···A (°) |
|---|---|---|---|---|
| O(1)-H(f··N(003) | 1.03(2) | 1.61(2) | 2.636(2) | 178.4(14) |
| C(10)-H(10)···O2 | 0.95 | 2.57 | 3.227(2) | 126 |
| C(2)-H(2)···O(2) | 0.95 | 2.47 | 3.381(2) | 160 |
| C(9)-H(9)···O(1) | 0.95 | 2.68 | 3.401(2) | 133 |
| C(7)-H(7)···Cg(1) | 0.95 | 2.95 | 3.770(2) | 146 |
| Structure | CCDC Number | Elat (KJ/mol) |
|---|---|---|
| 4,4’-bipy | 131130 [56] | −93.8 |
| HPip | 608427 [57] | −121.2 |
| HCinn | 1547787 [58] | −115.6 |
| 1 | 2058460 | −225.4 |
| 2 | 2058461 | −333.7 |
| Structure | CCDC Number | Melting Point (°C) | Crystal Density (mg/m3) | Packing Efficiency (%) |
|---|---|---|---|---|
| 4,4’-bipy | 131130 [56] | 112–113 | 1.255 | 68.00 |
| HPip | 608427 [57] | 231–232 | 1.555 | 72.63 |
| HCinn | 1547787 [58] | 135–136 | 1.321 | 70.96 |
| 1 | 2058460 | 188–189 | 1.466 | 73.79 |
| 2 | 2058461 | 110–111 | 1.295 | 69.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Cocrystals Based on 4,4’-bipyridine: Influence of Crystal Packing on Melting Point. Crystals 2021, 11, 191. https://doi.org/10.3390/cryst11020191
Ejarque D, Calvet T, Font-Bardia M, Pons J. Cocrystals Based on 4,4’-bipyridine: Influence of Crystal Packing on Melting Point. Crystals. 2021; 11(2):191. https://doi.org/10.3390/cryst11020191
Chicago/Turabian StyleEjarque, Daniel, Teresa Calvet, Mercè Font-Bardia, and Josefina Pons. 2021. "Cocrystals Based on 4,4’-bipyridine: Influence of Crystal Packing on Melting Point" Crystals 11, no. 2: 191. https://doi.org/10.3390/cryst11020191
APA StyleEjarque, D., Calvet, T., Font-Bardia, M., & Pons, J. (2021). Cocrystals Based on 4,4’-bipyridine: Influence of Crystal Packing on Melting Point. Crystals, 11(2), 191. https://doi.org/10.3390/cryst11020191