On the Coordination Role of Pyridyl-Nitrogen in the Structural Chemistry of Pyridyl-Substituted Dithiocarbamate Ligands
Abstract
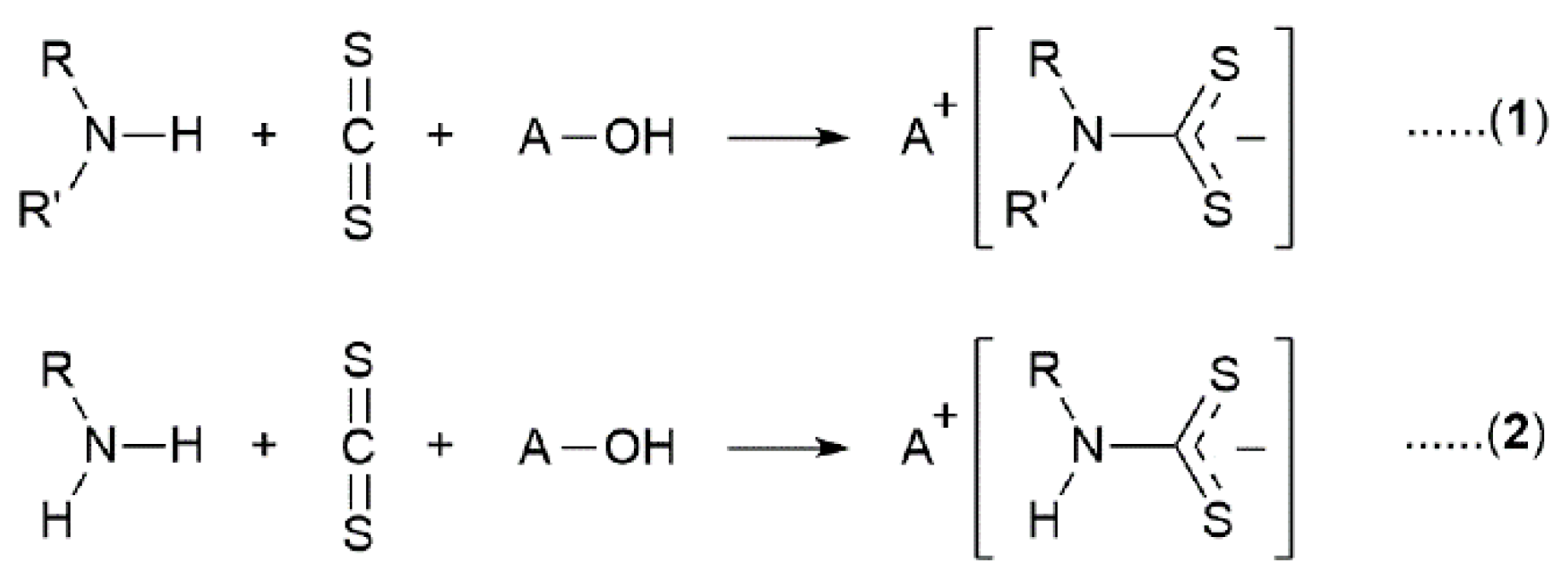
1. Introduction
2. Methods
3. Results
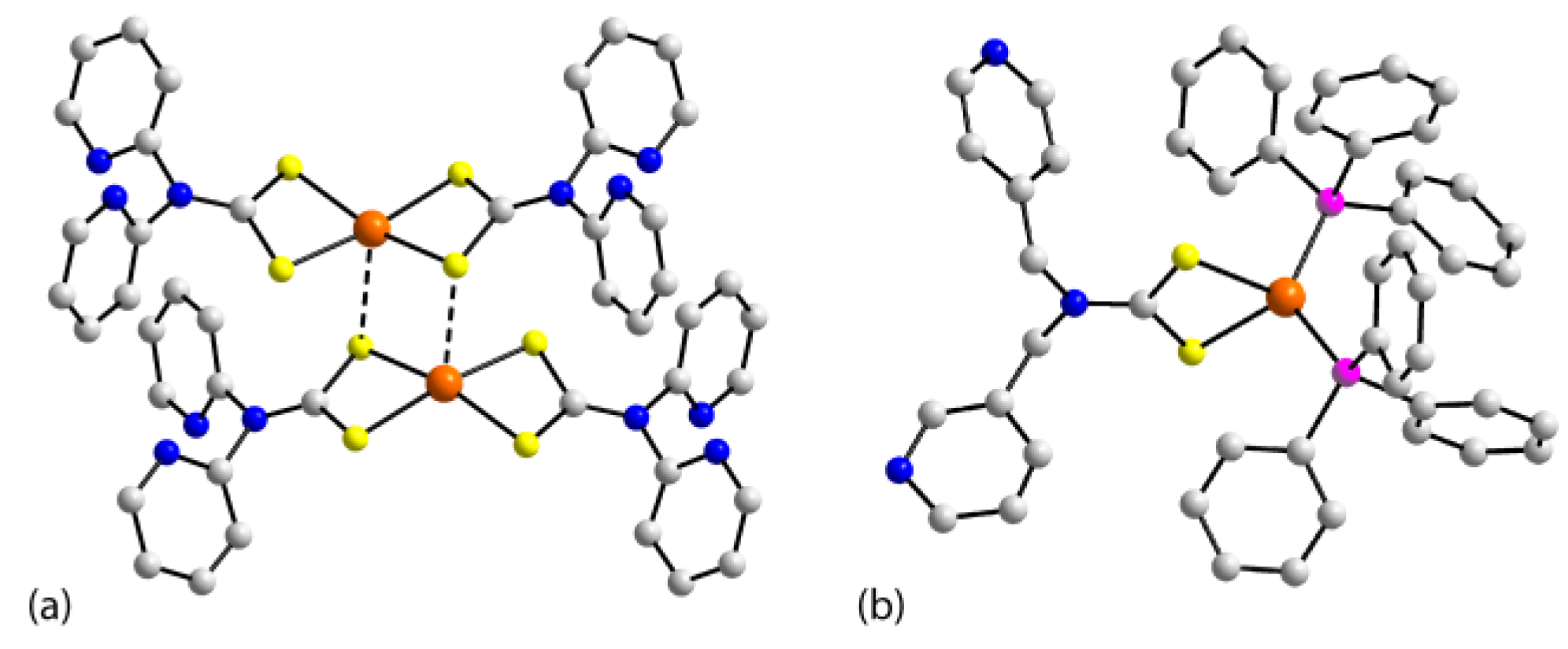
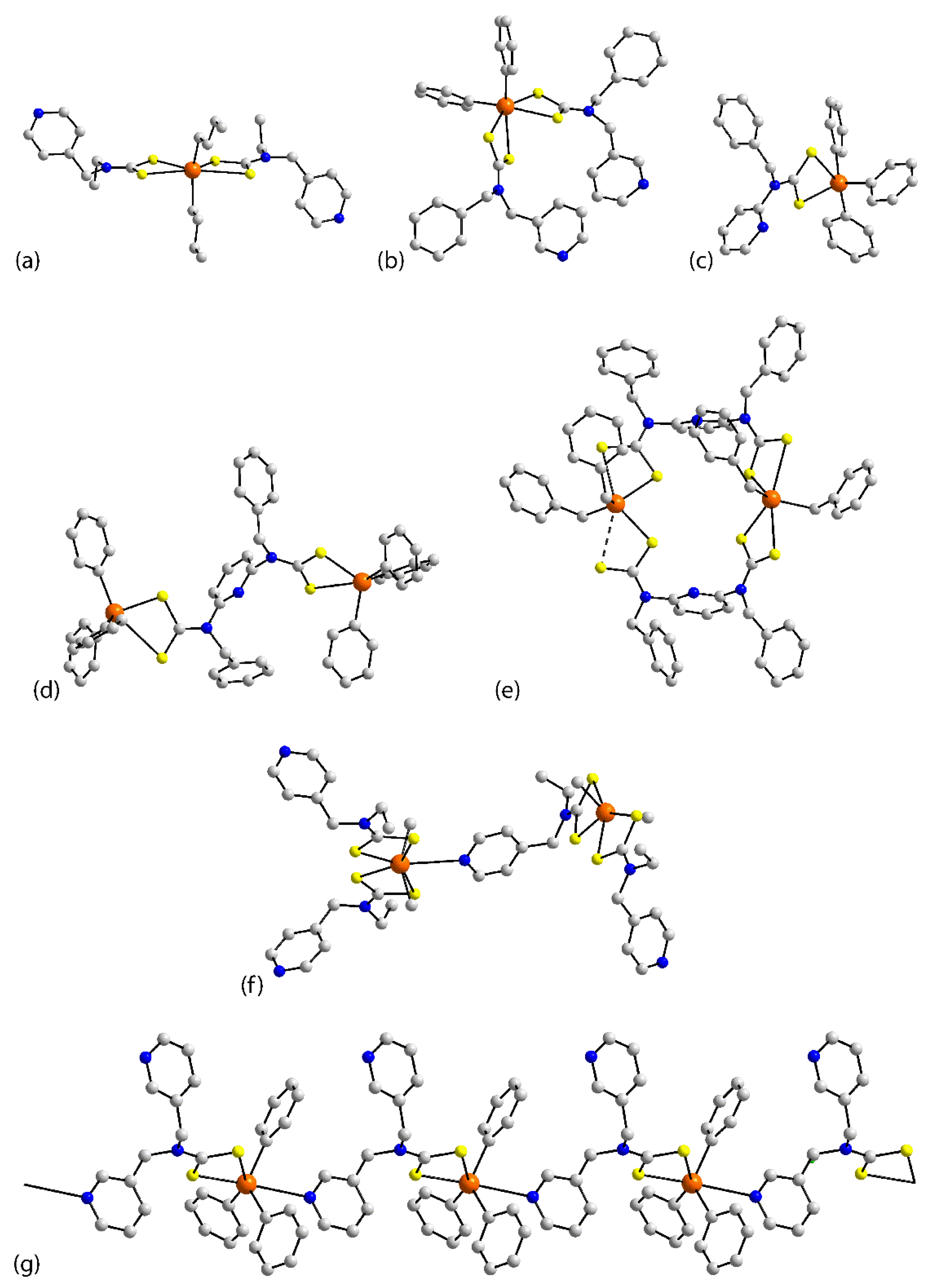
3.1. Structures of the Nickel-Triad Elements
3.2. Structures of the Copper-Triad Elements
3.3. Structures of the Zinc-Triad Elements
3.4. Structures of Thallium(I)
3.5. Structures of Organotin(IV)
3.6. Structures of Bismuth(III)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debus, H. Ueber die Verbindungen der Sulfocarbaminsäure. Justus Liebigs Ann. Chem. 1850, 73, 26–34. [Google Scholar] [CrossRef]
- Delépine, M. Metallic salts of dithiocarbamic acids; preparation of isothiocyanates in the aliphatic series. Compt. Rend. 1907, 144, 1125–1127. [Google Scholar]
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1970; Volume 11, pp. 234–371. [Google Scholar] [CrossRef]
- Eisenberg, R. Structural systematics of 1,1- and 1,2-dithiolato chelates. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1970; Volume 12, pp. 295–369. [Google Scholar] [CrossRef]
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes, 1968–1977. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1979; Volume 26, pp. 301–469. [Google Scholar] [CrossRef]
- Hogarth, G. Transition metal dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 71–561. [Google Scholar] [CrossRef]
- Heard, P.J. Main group dithiocarbamate complexes. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 1–69. [Google Scholar] [CrossRef]
- Lee, S.M.; Heard, P.J.; Tiekink, E.R.T. Molecular and supramolecular chemistry of mono- and di-selenium analogues of metal dithiocarbamates. Coord. Chem. Rev. 2018, 375, 410–423. [Google Scholar] [CrossRef]
- Poplaukhin, P.; Tiekink, E.R.T. (μ-Piperazine-1,4-dicarbodithioato-κ4S,S′:S″,S‴)bis[triphenyltin(IV)] dichloromethane solvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2008, 64, m1177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Tiekink, E.R.T. A structural survey of poly-functional dithiocarbamate ligands and the aggregation patterns they sustain. Inorganics 2021, 9, 7. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Sun, Q.-F.; Lee, T.K.-M.; Cheng, E.C.-C.; Li, Y.-Z.; Yam, V.W.-W. Au36 crown: A macrocyclization directed by metal-metal bonding interactions. Angew. Chem. Int. Ed. 2008, 47, 4551–4554. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Wang, J.; Wang, K.-Y.; Dang, S.; Song, S.; Liu, D.; Wang, C. Luminescence of samarium(III) bis-dithiocarbamate frameworks: Codoped lanthanide emitters that cover visible and near-infrared domains. J. Mater. Chem. C 2017, 5, 6620–6628. [Google Scholar] [CrossRef]
- Castillo, M.; Criado, J.J.; Macias, B.; Vaquero, M.V. Chemistry of dithiocarbamate derivatives of amino acids. I. Study of some dithiocarbamate derivatives of linear α-amino acids and their nickel(II) complexes. Inorg. Chim. Acta 1986, 124, 127–132. [Google Scholar] [CrossRef]
- Liebing, P.; Witzorke, J.; Oehler, F.; Schmeide, M. Dithiocarbamatocarboxylate (DTCC) ligands—building blocks for hard/soft-heterobimetallic coordination polymers. Inorg. Chem. 2020, 59, 2825–2832. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A million crystal structures: The whole is greater than the sum of its parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brandenburg, K.; Berndt, M. DIAMOND, Version 3.2k; GbR: Bonn, Germany, 2006. [Google Scholar]
- Rajput, G.; Singh, V.; Gupta, A.N.; Yadav, M.K.; Kumar, V.; Singh, S.K.; Prasad, A.; Drew, M.G.B.; Singh, N. Unusual C–H⋯Ni anagostic interactions in new homoleptic Ni(II) dithio complexes. CrystEngComm 2013, 15, 4676–4683. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Torabi, S.; Amani, V.; Notash, B.; Saidi, M.R. Synthesis and characterization of metal dithiocarbamate derivatives of 3-((pyridin-2-yl)methylamino)propanenitrile: Crystal structure of [3-((pyridin-2-yl)methylamino)propanenitrile dithiocarbamato] nickel(II). Polyhedron 2015, 102, 643–648. [Google Scholar] [CrossRef]
- Yadav, M.K.; Rajput, G.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Rare intermolecular M⋯H–C anagostic interactions in homoleptic Ni(II)–Pd(II) dithiocarbamate complexes. Polyhedron 2015, 39, 5493–5499. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Gupta, A.N.; Singh, S.K.; Drew, M.G.B.; Singh, N. Cooperative influence of ligand frameworks in sustaining supramolecular architectures of Ni(II)/Pd(II) heteroleptic dithio-dipyrrin complexes via non-covalent interactions. Polyhedron 2015, 89, 304–312. [Google Scholar] [CrossRef]
- Gupta, A.N.; Kumar, V.; Singh, V.; Manar, K.K.; Drew, M.G.B.; Singh, N. Intermolecular anagostic interactions in group 10 metal dithiocarbamates. CrystEngComm 2014, 16, 9299–9307. [Google Scholar] [CrossRef]
- Khan, S.Z.; Zia-ur-Rehman; Butler, I.S.; Bélanger-Gariepy, F. New ternary palladium(II) complexes: Synthesis, characterization, in vitro anticancer and antioxidant activities. Inorg. Chem. Commun. 2019, 105, 140–146. [Google Scholar] [CrossRef]
- Poirier, S.; Rahmani, F.; Reber, C. Large d–d luminescence energy variations in square-planar bis(dithiocarbamate) platinum(II) and palladium(II) complexes with near-identical MS4 motifs: A variable-pressure study. Dalton Trans. 2017, 46, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Roberts, R.J.; Le, D.; Leznoff, D.B.; Reber, C. Interpreting effects of structure variations induced by temperature and pressure on luminescence spectra of platinum(II) bis(dithiocarbamate) compounds. Inorg. Chem. 2015, 54, 3728–3735. [Google Scholar] [CrossRef]
- Imran, M.; Zia-ur-Rehman; Kondratyuk, T.; Bélanger-Gariepy, F. New ternary platinum(II) dithiocarbamates: Synthesis, characterization, anticancer, DNA binding and DNA denaturing studies. Inorg. Chem. Commun. 2019, 103, 12–20. [Google Scholar] [CrossRef]
- Gupta, A.N.; Singh, V.; Kumar, V.; Rajput, A.; Singh, L.; Drew, M.G.B.; Singh, N. Syntheses, crystal structures and conducting properties of new homoleptic copper(II) dithiocarbamate complexes. Inorg. Chim. Acta 2013, 408, 145–151. [Google Scholar] [CrossRef]
- Lanfredi, A.M.M.; Ugozzoli, F.; Camus, A. X-ray crystal structure of the copper(II)bis(2,2′-dipyridyl)dithiocarbamate, produced by an unusual reaction of CS2 with Cu−N bonds. J. Chem. Crystallogr. 1996, 26, 141–145. [Google Scholar] [CrossRef]
- Rajput, G.; Singh, V.; Singh, S.K.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Cooperative metal–ligand-induced properties of heteroleptic copper(I) xanthate/dithiocarbamate PPh3 complexes. Eur. J. Inorg. Chem. 2012, 3885–3891. [Google Scholar] [CrossRef]
- Gupta, A.N.; Singh, V.; Kumar, V.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Syntheses, crystal structures and optical properties of heteroleptic copper(I) dithio/PPh3 complexes. Polyhedron 2014, 79, 324–329. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, C.L.; Mishra, K.B.; Singh, S.K.; Gupta, A.N.; Tiwari, V.K.; Drew, M.G.B.; Singh, N. Highly efficient and recyclable pre-catalysts based on mono- and dinuclear heteroleptic Cu(I) dithio-PPh3 complexes to produce variety of glycoconjugate triazoles. Mol. Catal. 2019, 470, 152–163. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Gupta, A.N.; Manar, K.K.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Influence of ligand environment on the structure and properties of silver(I) dithiocarbamate cluster-based coordination polymers and dimers. New J. Chem. 2014, 38, 4478–4485. [Google Scholar] [CrossRef]
- Roberts, R.J.; Bélanger-Desmarais, N.; Reber, C.; Leznoff, D.B. The luminescence properties of linear vs. kinked aurophilic 1-D chains of bis(dithiocarbamato)gold(I) dimers. Chem. Commun. 2014, 50, 3148–3150. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jung, O.-S.; Lee, Y.-A. Adduct effects on structure and luminescence in a series of new dithiocarbamatogold(I) complexes. Transit. Met. Chem. 2011, 36, 691–697. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, V.; Gupta, A.N.; Drew, M.G.B.; Singh, N. Effect of pyridyl substituents leading to the formation of green luminescent mercury(II) coordination polymers, zinc(II) dimers and a monomer. New J. Chem. 2014, 38, 3737–3748. [Google Scholar] [CrossRef]
- Kumar, V.; Manar, K.K.; Gupta, A.N.; Singh, V.; Drew, M.G.B.; Singh, N. Impact of ferrocenyl and pyridyl groups attached to dithiocarbamate moieties on crystal structures and luminescent characteristics of group 12 metal complexes. J. Organomet. Chem. 2016, 820, 62–69. [Google Scholar] [CrossRef]
- Poplaukhin, P.; Arman, H.D.; Tiekink, E.R.T. A one-dimensional coordination polymer, catena-poly[[[[N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ2S,S′]zinc(II)]-μ2-N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ3S,S′:N] 4-methylpyridine hemisolvate]. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 72, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Manar, K.K.; Neetu; Anamika; Srivastava, P.; Drew, M.G.B.; Singh, N. A new series of heteroleptic Cd(II) diimine-ferrocenyl dithiocarbamate complexes which successfully co-sensitizes TiO2 photoanode with Ru N719 dye in DSSC. Chem. Sel. 2017, 2, 8301–8311. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, V.; Gupta, A.N.; Drew, M.G.B.; Singh, N. Influence of ligand environments on the structures and luminescence properties of homoleptic cadmium(II) pyridyl functionalized dithiocarbamates. CrystEngComm 2014, 16, 6765–6774. [Google Scholar] [CrossRef]
- Arman, H.D.; Poplaukhin, P.; Tiekink, E.R.T. A two-dimensional coordination polymer: Poly[[bis[μ2-N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ3N:S,S′]cadmium(II)] 3-methylpyridine monosolvate]. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 73, 488–492. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, A.; Prasad, R.; Rajput, G.; Drew, M.G.B.; Singh, N. The interplay of secondary Hg⋯S, Hg⋯N and Hg⋯π bonding interactions in supramolecular structures of phenylmercury(II) dithiocarbamates. CrystEngComm 2011, 13, 6817–6826. [Google Scholar] [CrossRef]
- Rajput, G.; Yadav, M.K.; Thakur, T.S.; Drew, M.G.B.; Singh, N. Versatile coordination environment and interplay of metal assisted secondary interactions in the organization of supramolecular motifs in new Hg(II)/PhHg(II) dithiolates. Polyhedron 2014, 69, 225–233. [Google Scholar] [CrossRef]
- Yadav, M.K.; Rajput, G.; Gupta, A.N.; Kumar, V.; Drew, M.G.B.; Singh, N. Exploring the coordinative behaviour and molecular architecture of new PhHg(II)/Hg(II) dithiocarbamate complexes. Inorg. Chim. Acta 2014, 241, 210–217. [Google Scholar] [CrossRef]
- Singh, V.; Chauhan, R.; Kumar, A.; Bahadur, L.; Singh, N. Efficient phenylmercury(II) methylferrocenyldithiocarbamate functionalized dye-sensitized solar cells. Dalton Trans. 2010, 39, 9779–9788. [Google Scholar] [CrossRef] [PubMed]
- Manar, K.K.; Rajput, G.; Yadav, M.K.; Yadav, C.L.; Kumari, M.; Drew, M.G.B.; Singh, N. Potential impact of substituents on the crystal structures and properties of Tl(I) ferrocenyl/picolyl-functionalized dithiocarbamates; Tl⋯H-C anagostic interactions. Chem. Sel. 2016, 1, 5733–5742. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Gupta, A.N.; Drew, M.G.B.; Singh, N. Intermolecular Tl⋯H–C anagostic interactions in luminescent pyridyl functionalized thallium(I) dithiocarbamates. Daltons Trans. 2015, 44, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.N.; Kumar, V.; Singh, V.; Rajput, A.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Influence of functionalities on the structure and luminescent properties of organotin(IV) dithiocarbamate complexes. J. Organomet. Chem. 2015, 787, 65–72. [Google Scholar] [CrossRef]
- Barba, V.; Arenaza, B.; Guerrero, J.; Reyes, R. Synthesis and structural characterization of diorganotin dithiocarbamates from 4-(ethylaminomethyl)pyridine. Heteroat. Chem. 2012, 23, 422–428. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, H.; Wei, Z.-W.; Tang, L.-F. Synthesis, structure, and fungicidal activity of organotin dithiocarbamates derived from pyridinamines and aryl diamines. Heteroat. Chem. 2014, 25, 274–281. [Google Scholar] [CrossRef]
- Guo, Y.-C.; Ma, Q.-G.; Chen, S.-Y.; Feng, Y.-Q.; Sun, R.-Z. Syntheses, crystal structures and anticancer activities of dithiocarbamate bismuth(Ⅲ) complexes. Chin. J. Struct. Chem. 2015, 34, 1028–1034. [Google Scholar] [CrossRef]
- Anamika; Singh, R.; Manar, K.K.; Yadav, C.L.; Kumar, A.; Singh, R.K.; Drew, M.G.B.; Singh, N. Impact of substituents on the crystal structures and anti-leishmanial activity of new homoleptic Bi(III) dithiocarbamates. New J. Chem. 2019, 43, 16921–16931. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Perplexing coordination behaviour of potentially bridging bipyridyl-type ligands in the coordination chemistry of zinc and cadmium 1,1-dithiolate compounds. Crystals 2018, 8, 18. [Google Scholar] [CrossRef]
- Poirier, S.; Lynn, H.; Reber, C.; Tailleur, E.; Marchivie, M.; Guionneau, P.; Probert, M.R. Variation of M···H-C interactions in square-planar complexes of nickel(II), palladium(II), and platinum(II) probed by luminescence spectroscopy and X-ray diffraction at variable pressure. Inorg. Chem. 2018, 57, 7713–7723. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. The remarkable propensity for the formation of C–H⋯π(chelate ring) interactions in the crystals of the first-row transition metal dithiocarbamates and the supramolecular architectures they sustain. CrystEngComm 2020, 22, 7308–7333. [Google Scholar] [CrossRef]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E Cryst. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Jamaludin, N.S.; Halim, S.N.A.; Khoo, C.-H.; Chen, B.-J.; See, T.-H.; Sim, J.-H.; Cheah, Y.-K.; Seng, H.-L.; Tiekink, E.R.T. Bis(phosphane)copper(I) and silver(I) dithiocarbamates: Crystallography and anti-microbial assay. Z. Kristallogr. 2016, 231, 341–349. [Google Scholar] [CrossRef]
- Tan, Y.J.; Tan, Y.S.; Yeo, C.I.; Chew, J.; Tiekink, E.R.T. In Vitro anti-bacterial and time kill evaluation of binuclear tricyclohexylphosphanesilver(I) dithiocarbamates, {Cy3PAg(S2CNRR′)}2. J. Inorg. Biochem. 2019, 192, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T.; Kang, J.-G. Luminescence properties of phosphinegold(I) halides and thiolates. Coord. Chem. Rev. 2009, 253, 1627–1648. [Google Scholar] [CrossRef]
- Shattock, R.T.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of supramolecular synthons: Persistent carboxylic acid···pyridine hydrogen bonds in cocrystals that also contain a hydroxyl moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly of molecular gold(I) compounds: An evaluation of the competition and complementarity between aurophilic (Au⋯Au) and conventional hydrogen bonding interactions. Coord. Chem. Rev. 2014, 275, 130–153. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Exploring the topological landscape exhibited by binary zinc-triad 1,1-dithiolates. Crystals 2018, 8, 292. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Tin dithiocarbamates: Applications and structures. Appl. Organomet. Chem. 2008, 22, 533–550. [Google Scholar] [CrossRef]
- Haezam, F.N.; Awang, N.; Kamaludin, N.F.; Jotani, M.M.; Tiekink, E.R.T. (N,N-Diallyldithiocarbamato-κ2S,S′)triphenyltin(IV) and bis(N,N-diallyldithiocarbamato-κ2S,S′)diphenyltin(IV): Crystal structure, Hirshfeld surface analysis and computational study. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 167–176. [Google Scholar] [CrossRef]
- Li, H.; Lai, C.S.; Wu, J.; Ho, P.C.; de Vos, D.; Tiekink, E.R.T. Cytotoxicity, qualitative structure-activity relationship (QSAR), and anti-tumor activity of bismuth dithiocarbamate complexes. J. Inorg. Biochem. 2007, 101, 809–816. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Prevalence of intermolecular Bi…S interactions in bismuth dithiocarbamate compounds: Bi(S2CNR2)3. Z. Kristallogr. 2007, 222, 532–538. [Google Scholar] [CrossRef]
- Alcock, N.W. Secondary bonding to nonmetallic elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1–58. [Google Scholar] [CrossRef]
- Liu, Y.; Tiekink, E.R.T. Supramolecular associations in binary antimony(III) dithiocarbamates: Influence of ligand steric bulk, influence on coordination geometry, and competition with hydrogen bonding. CrystEngComm 2005, 7, 20–27. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Aggregation patterns in the crystal structures of organometallic Group XV 1,1-dithiolates: The influence of the Lewis acidity of the central atom, metal- and ligand-bound steric bulk, and coordination potential of the 1,1-dithiolate ligands upon supramolecular architecture. CrystEngComm 2006, 8, 104–118. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Stereochemical activity of lone pairs of electrons and supramolecular aggregation patterns based on secondary interactions involving tellurium in its 1,1-dithiolate structures. Coord. Chem. Rev. 2010, 254, 46–76. [Google Scholar] [CrossRef]
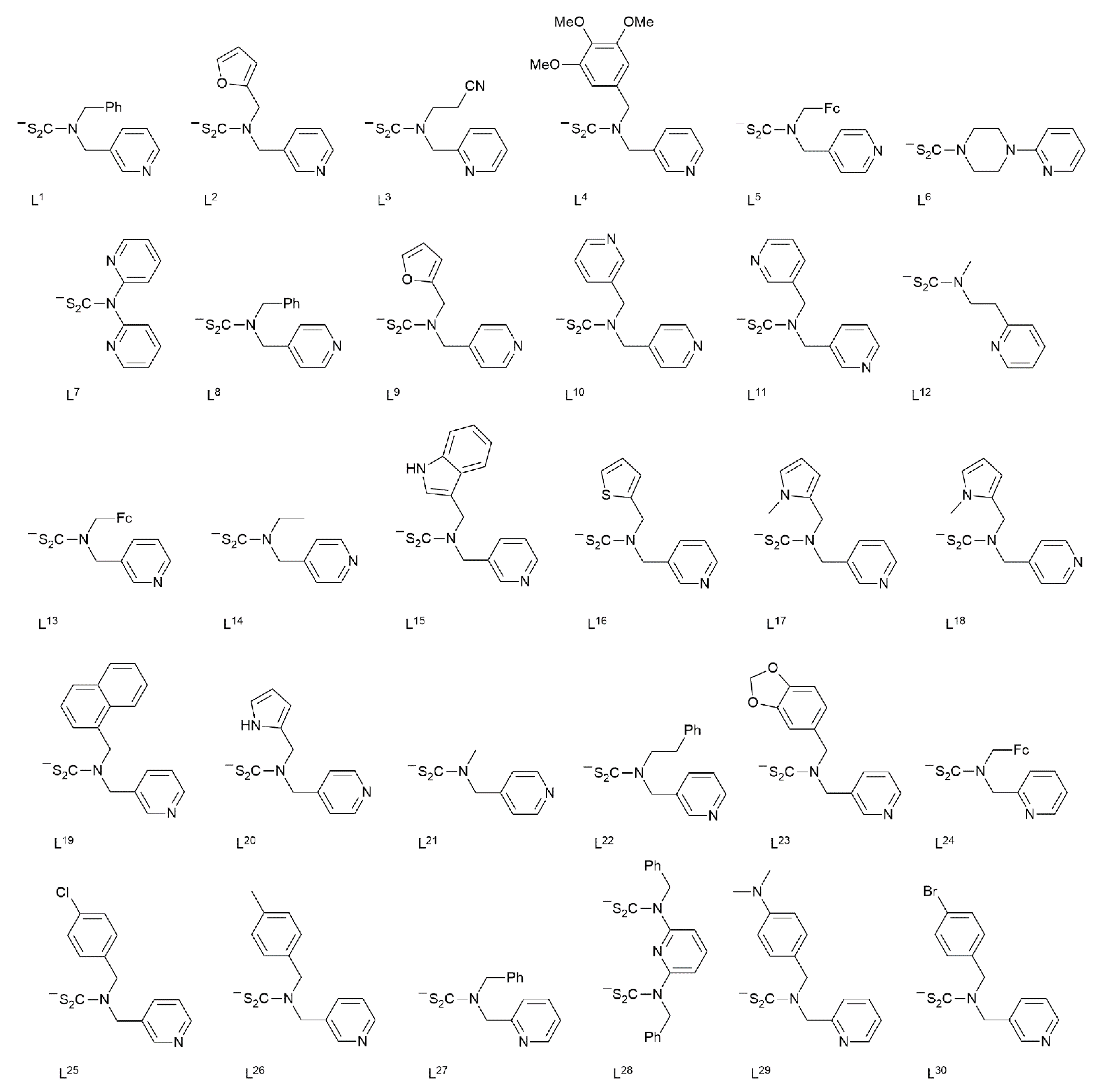

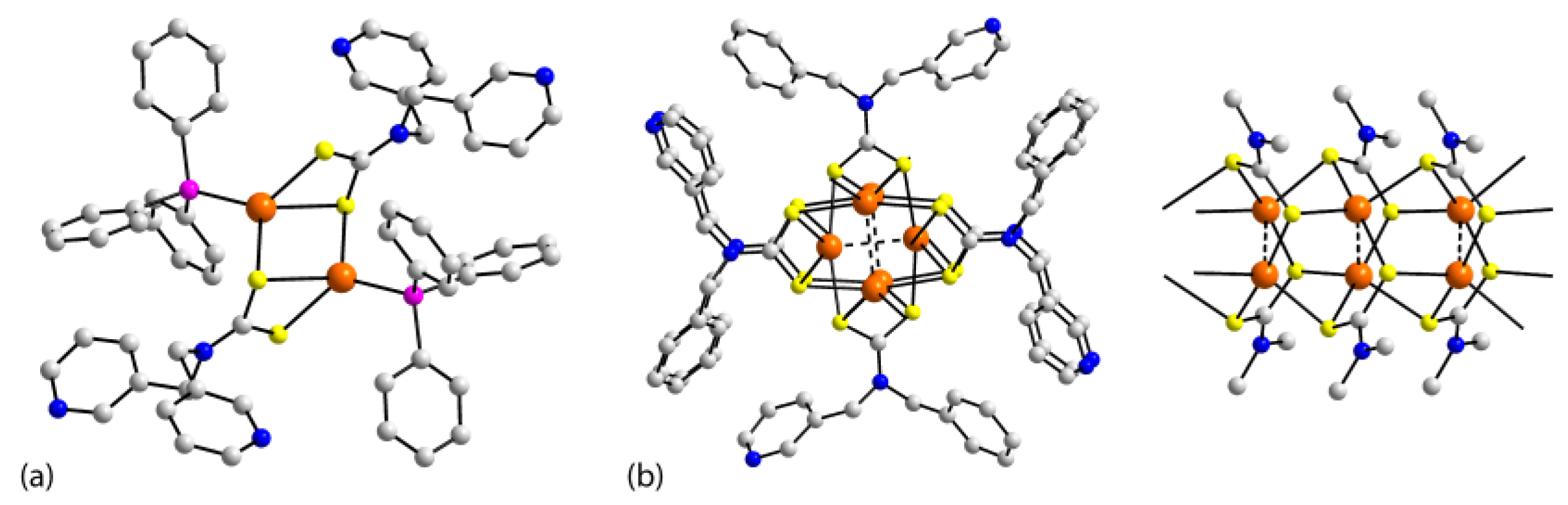
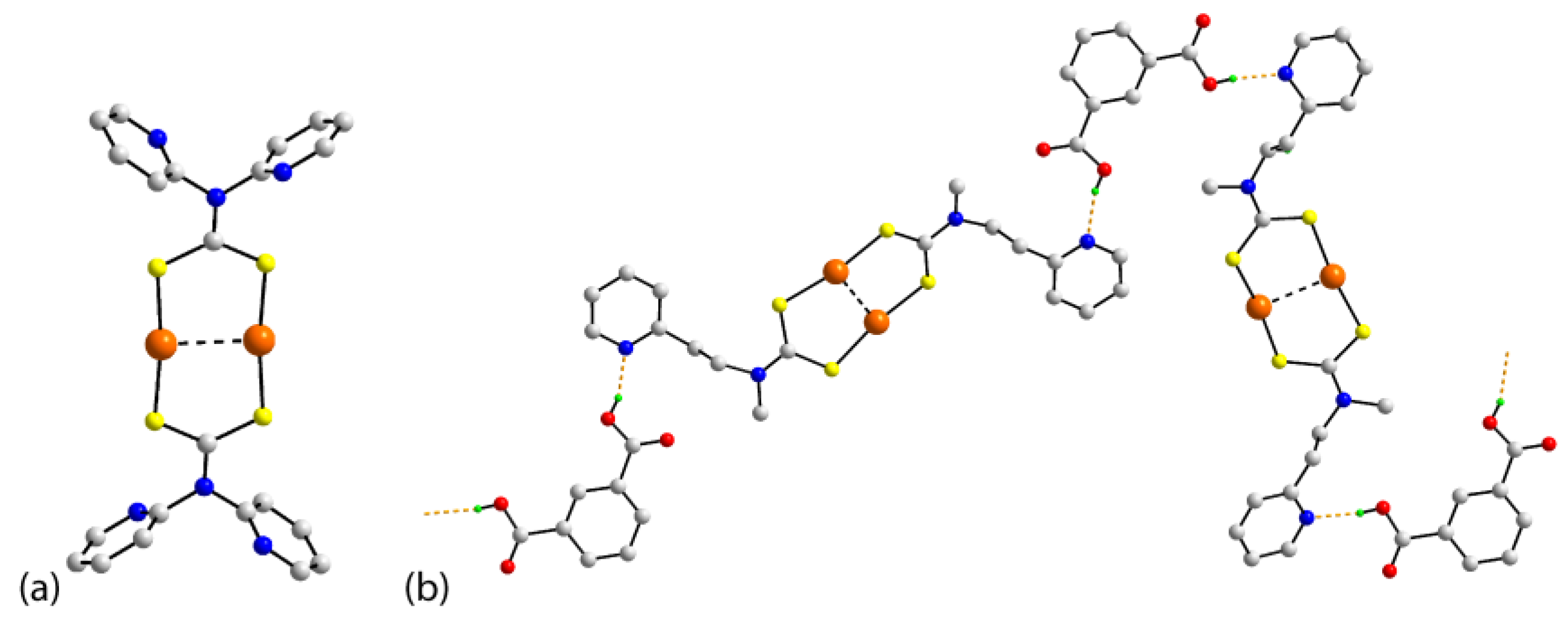

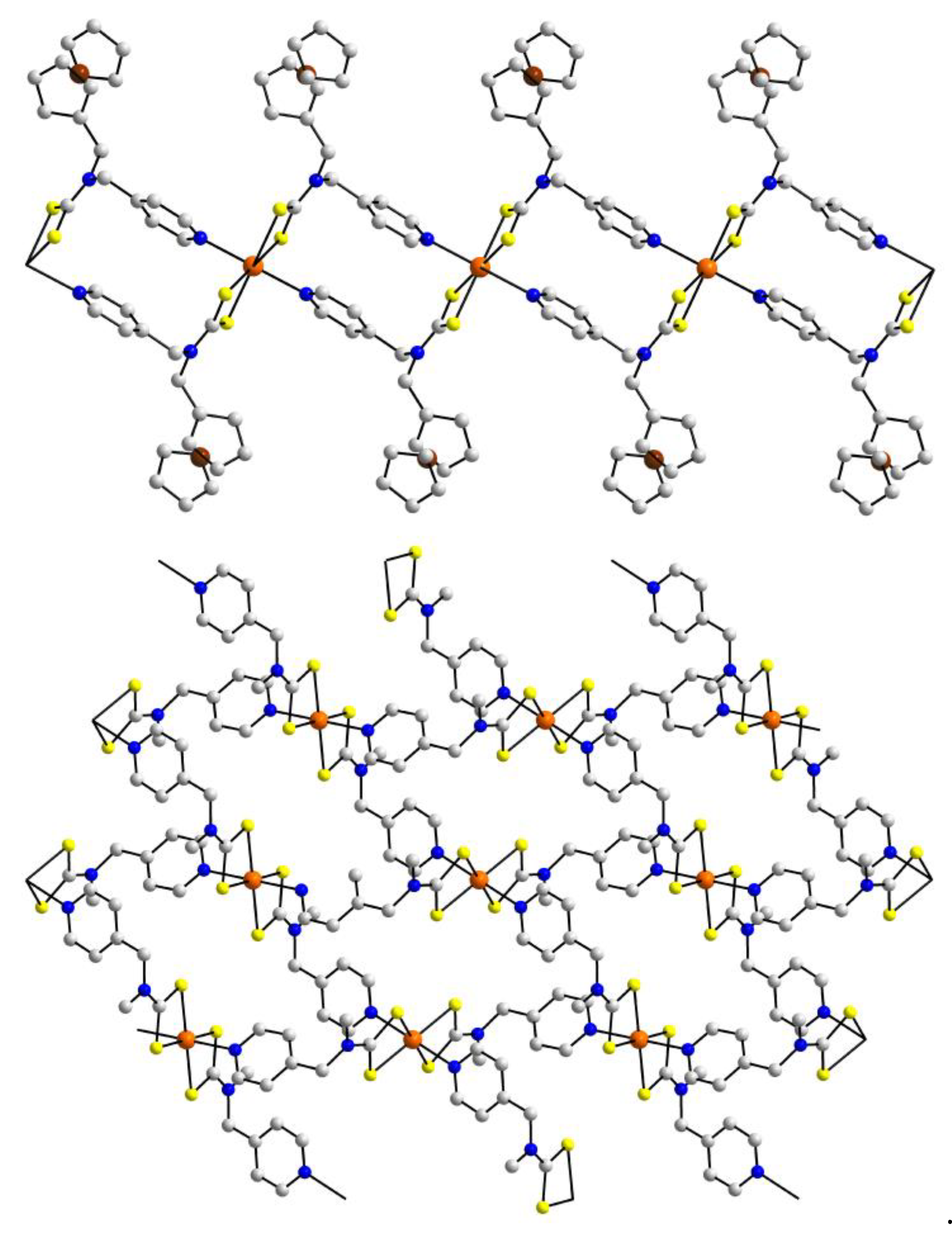
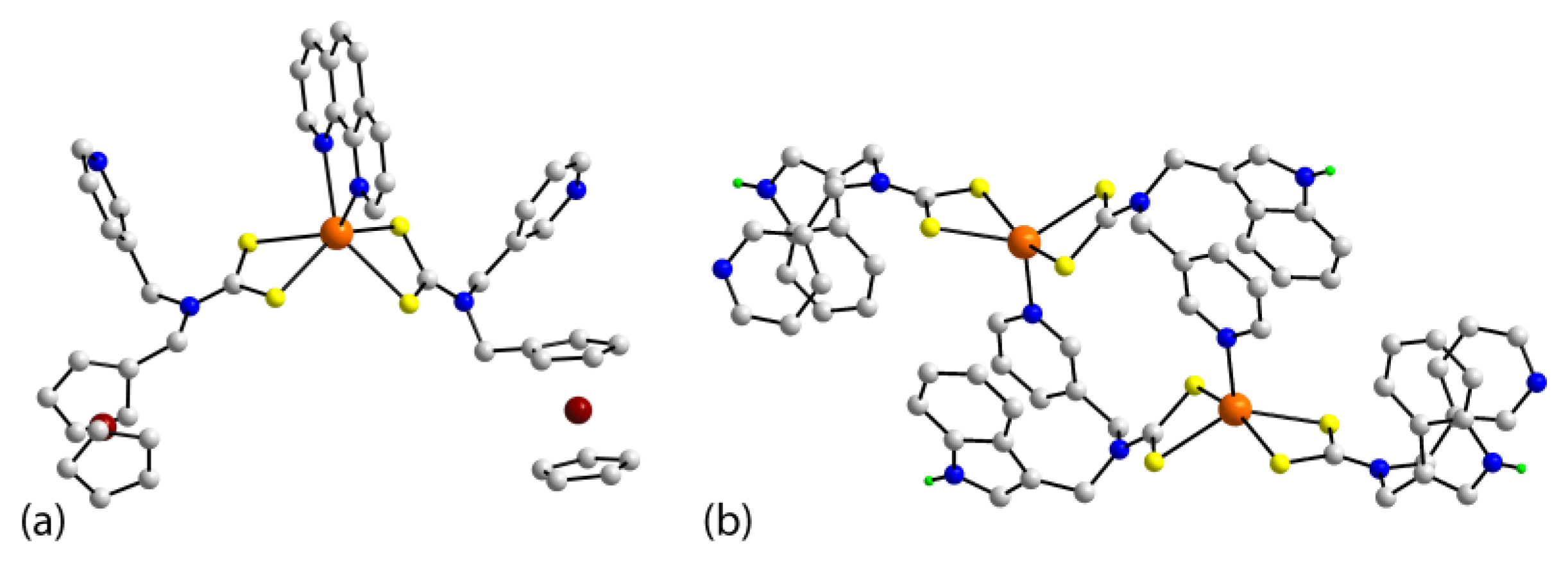
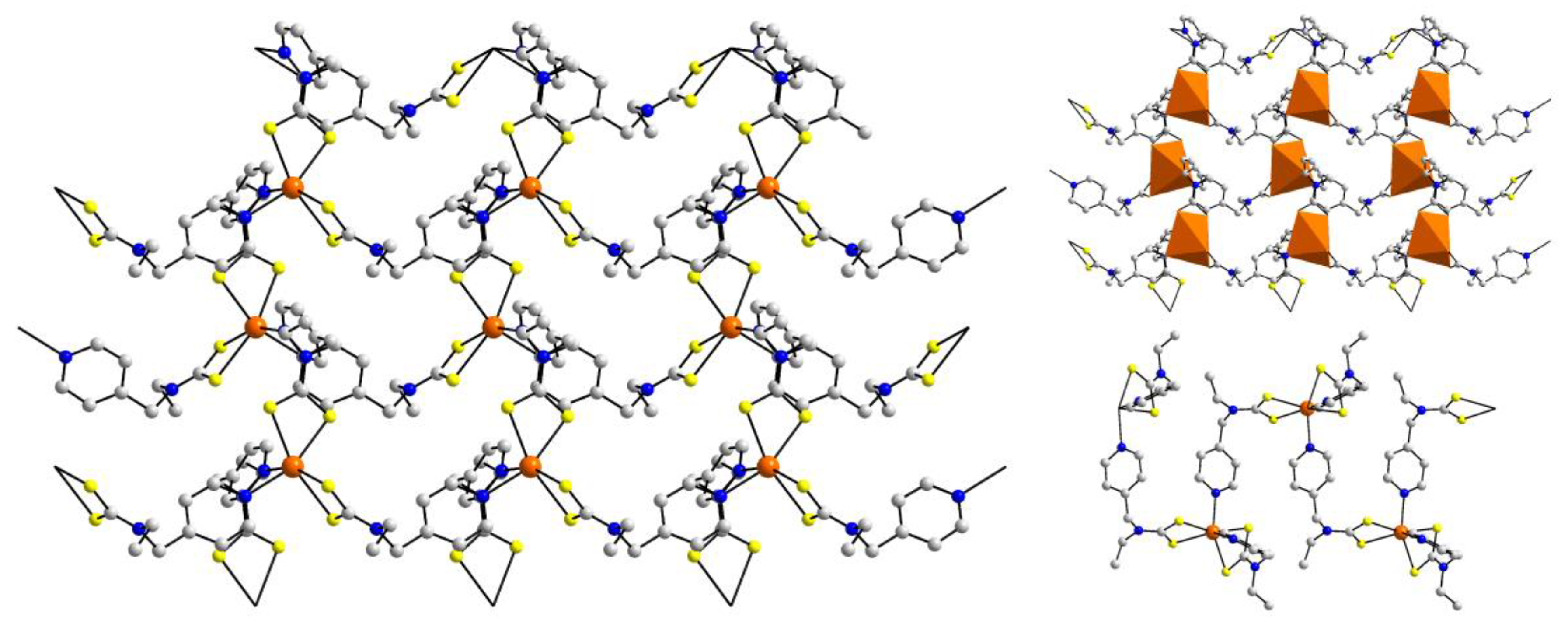
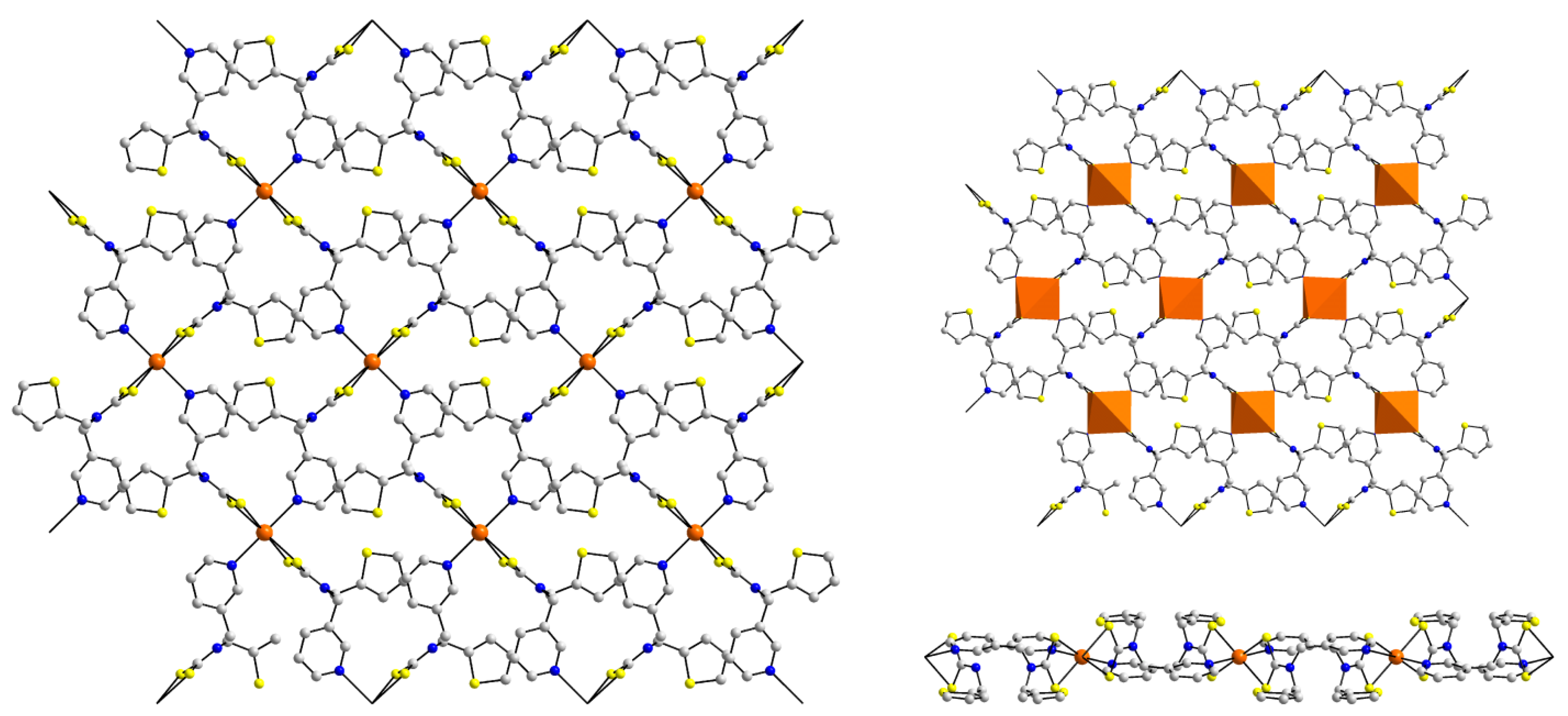
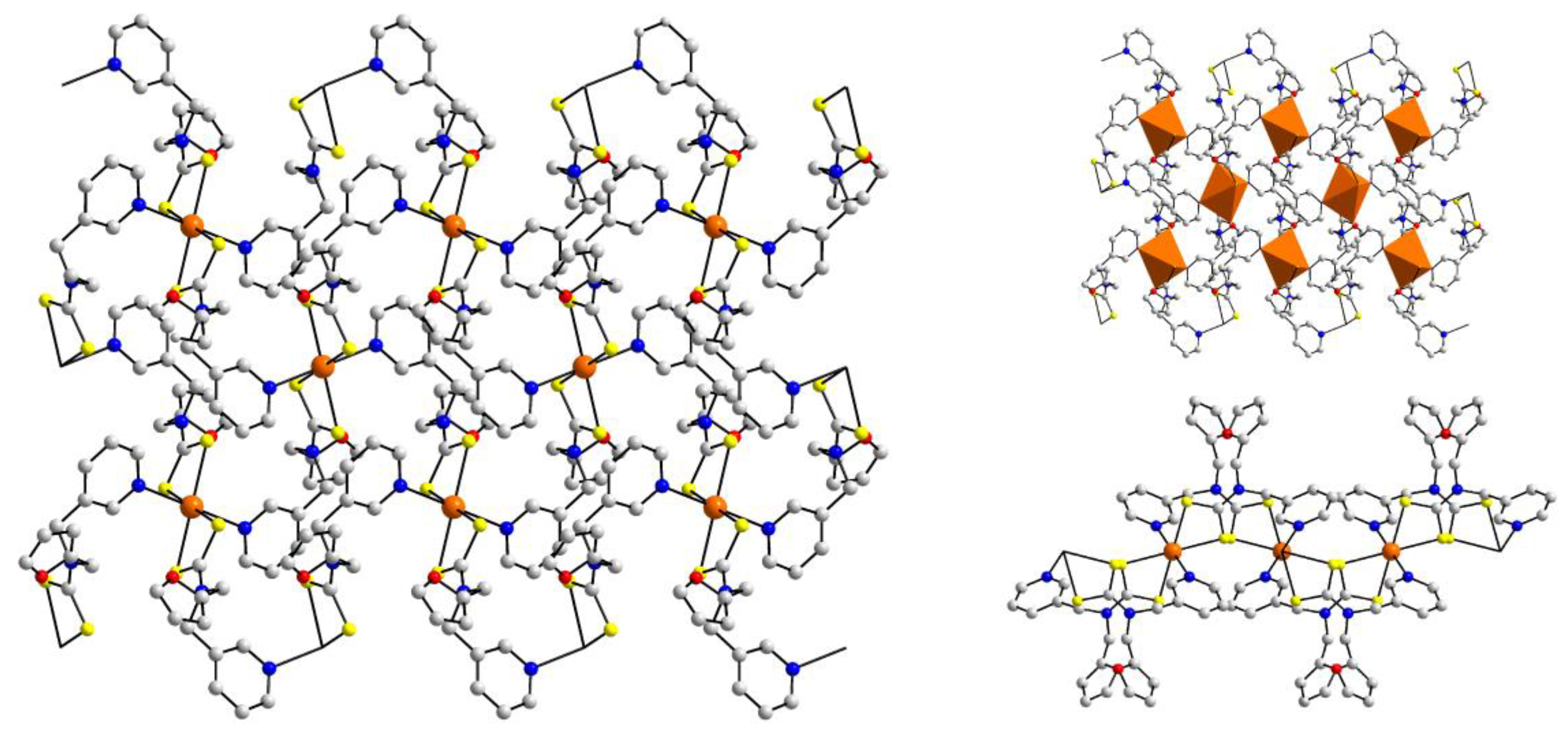
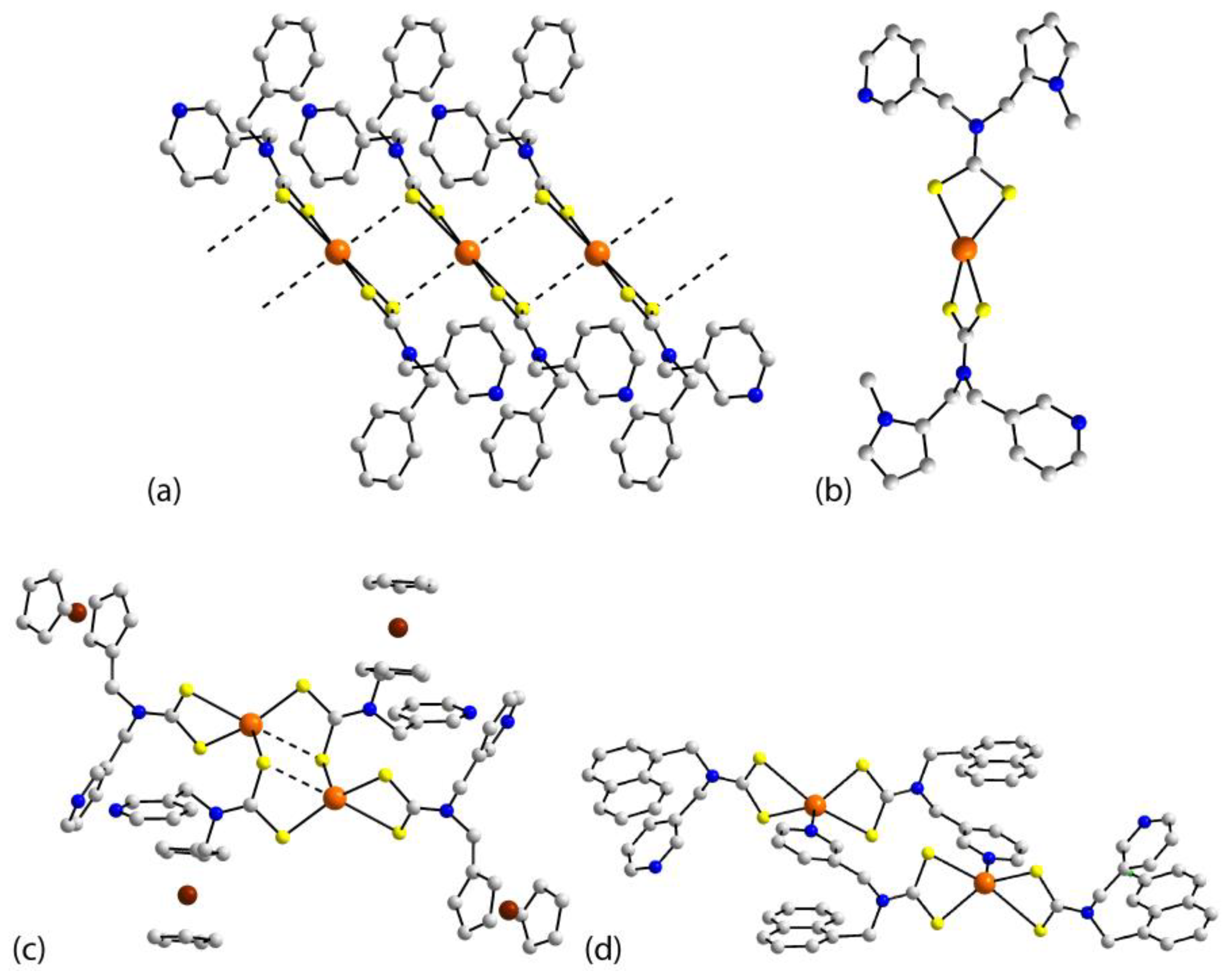
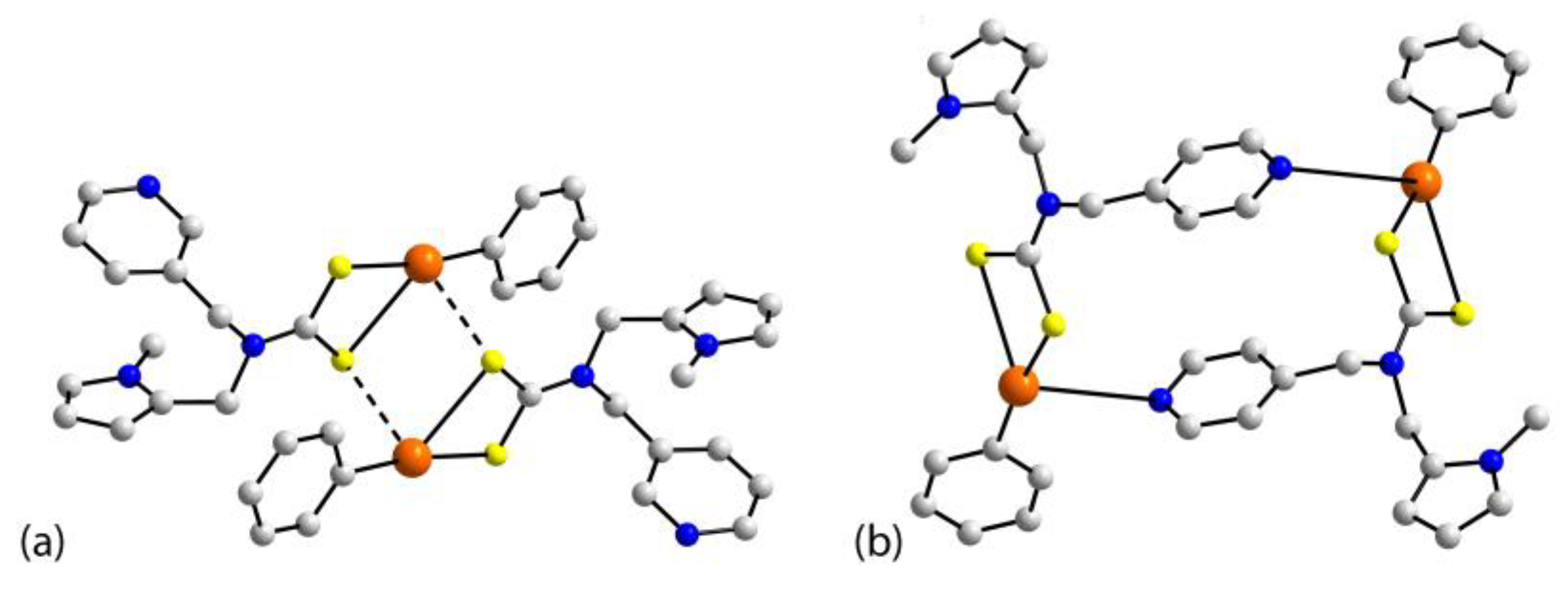
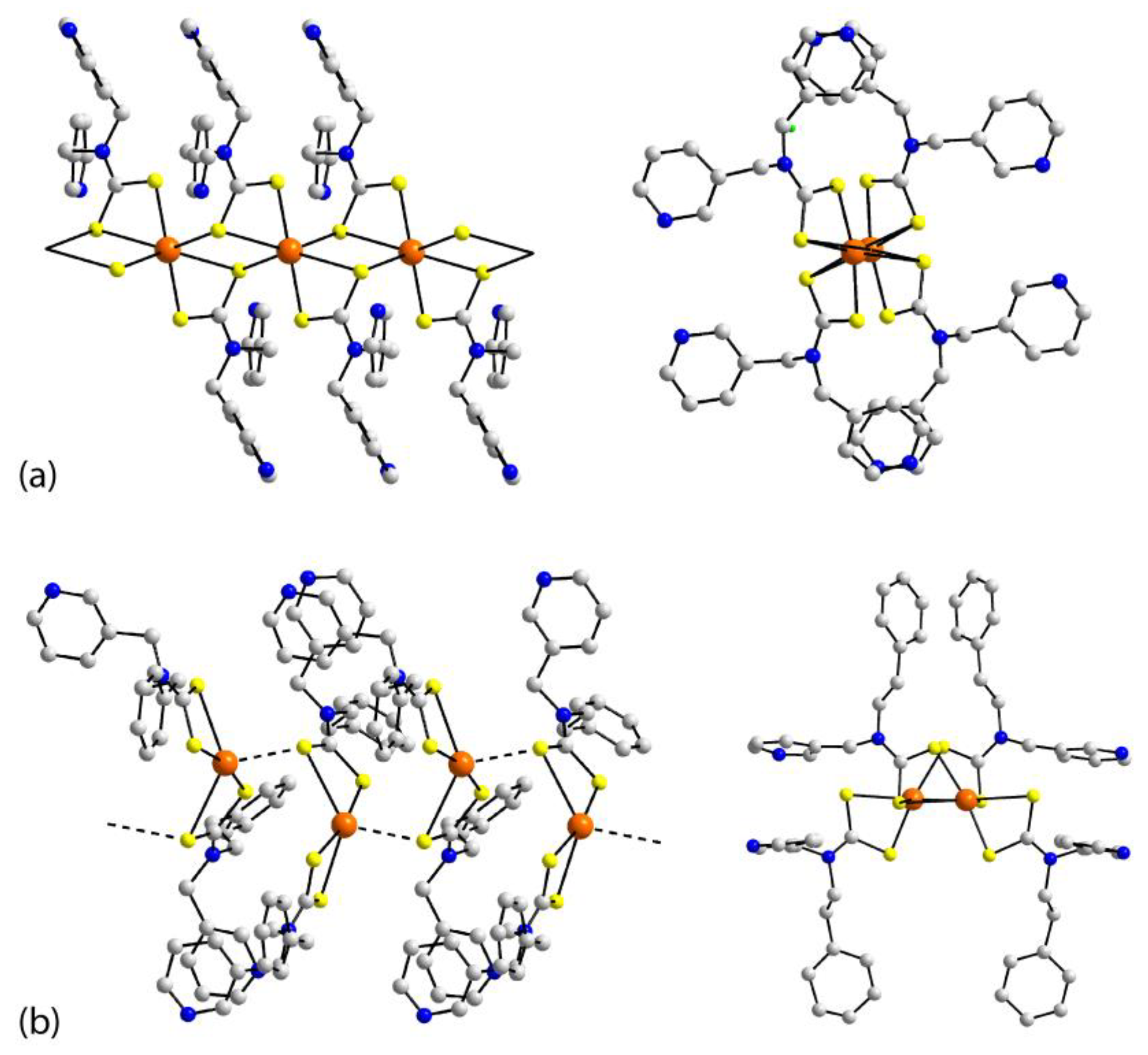
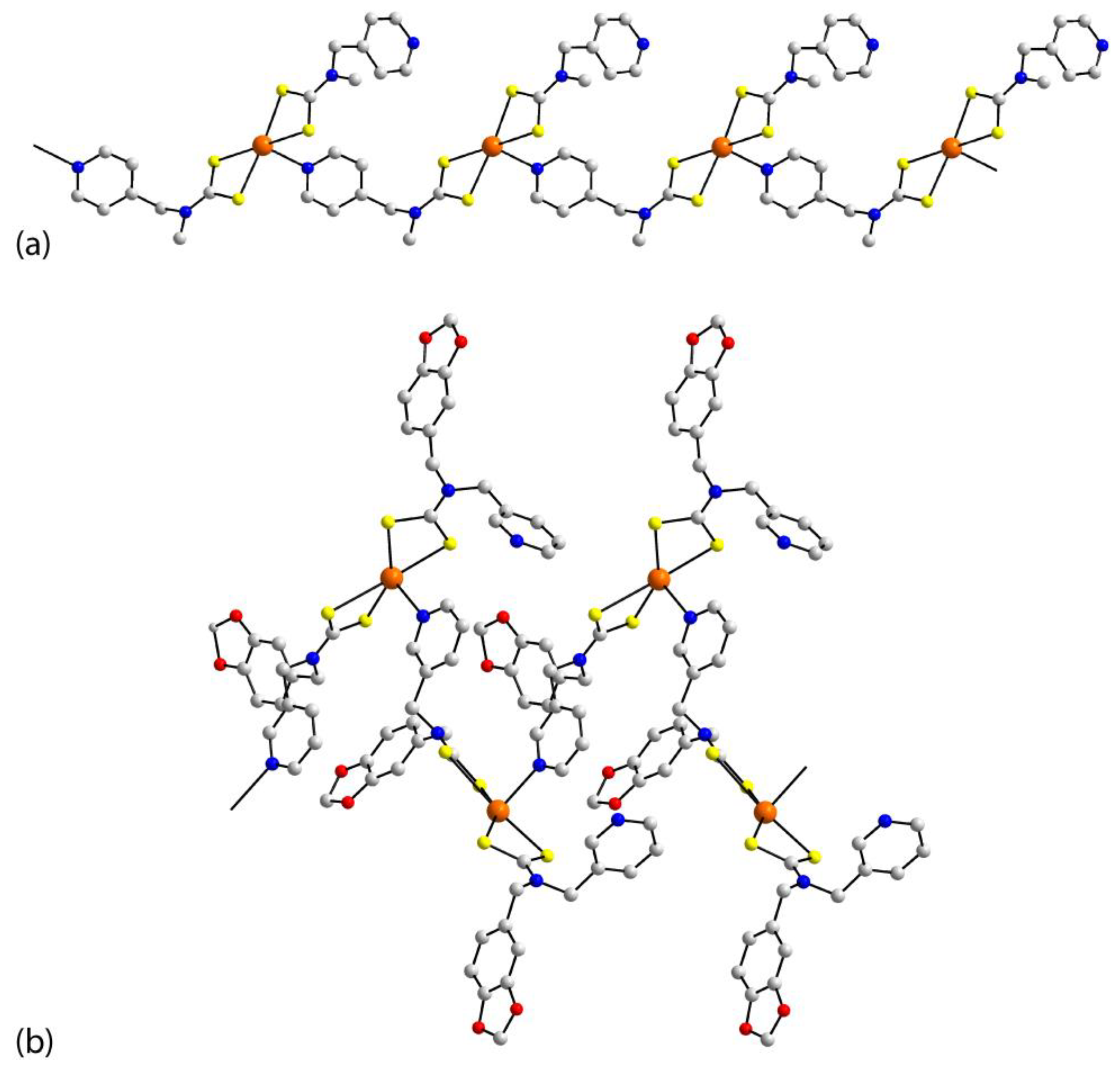

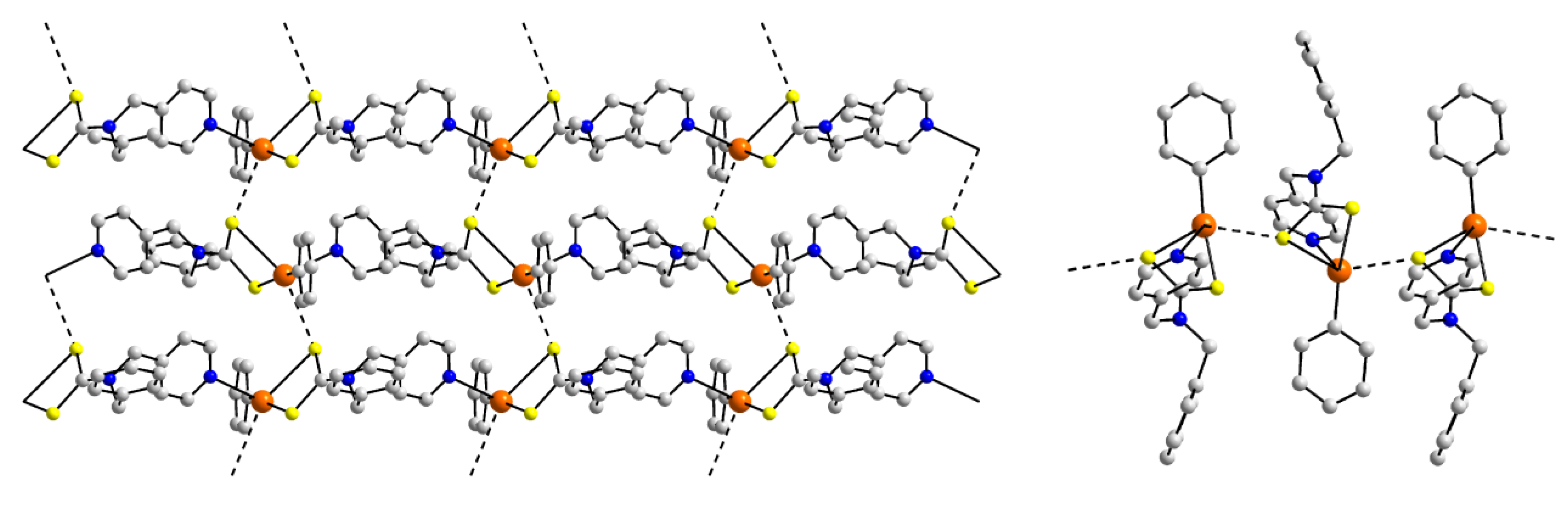



| Crystal | Formulation | Motif | CSD REFCODE | Ref. |
|---|---|---|---|---|
| 1 | Ni(L1)2 | monomer | DEXFIX | [18] |
| 2 | Ni(L2)2 | monomer | DIFHIL | [18] |
| 3 | Ni(L3)2 | monomer | HUTNOB | [19] |
| 4 | Ni(L4)2 | monomer | LUKTIW | [20] |
| 5a | (NN1)Ni(L5) | monomer | VOTDEP | [21] |
| 6 | Pd(L1)2 | monomer | HOMXEO | [22] |
| 7 | [(4-tol)3P]Pd(L6)Cl | monomer | ROHKAD | [23] |
| 8 | Pt(L1)2 | monomer | LASPIH | [24] |
| 9 | Pt(L7)2 | monomer | UHOGIJ | [25] |
| 10 | [(4-tol)3P]Pt(L6)Cl | monomer | RIZNEW | [26] |
| 11 | Cu(L1)2 | monomer | MISLUX | [27] |
| 12 | Cu(L7)2 | monomer | ZUWLUY | [28] |
| 13 | (Ph3P)2Cu(L8).2H2O | monomer | LEBDIH | [29] |
| 14 | (Ph3P)2Cu(L9) | monomer | NOJVIT | [30] |
| 15 | (Ph3P)2Cu(L10).0.5(CH2Cl2).0.5(H2O) | monomer | MORNIT | [31] |
| 16 | [(Ph3P)2Ag(L1)]2 | dimer | GONMED | [32] |
| 17 | [(Ph3P)2Ag(L11)]2 | dimer | GONMIH | [32] |
| 18 | [Ag(L1)]n | 1-D | GONLUS | [32] |
| 19 | [Ag(L11)]n | 1-D | GONMAZ | [32] |
| 20 | [Au(L7)]2 | monomer | XIRYII | [33] |
| 21 | [Au(L12)]2.isophthalic acid | monomer | EYURAS | [34] |
| 22 | [Zn(L1)2]2.EtOH | dimer | FODSIC | [35] |
| 23 | [Zn(L13)2]2 | dimer | UTEJOU | [36] |
| 24 | [Zn(L14)2]n.0.5(4-methylpyridine) | 1-D | FECRIR | [37] |
| 25 | [Zn(L5)2]2.2(DMF) | 2-D | UTEJUA | [36] |
| 26 | Cd(L13)2(2,2′-bipyridyl) | monomer | GIVGUQ | [38] |
| 27 | Cd(L13)2(1,10-phenanthroline) | monomer | GIVGOK | [38] |
| 28 | Cd(L14)2(1,10-phenanthroline) | monomer | UTEKIP | [36] |
| 29 | [Cd(L15)]2.2(DMF) | dimer | ZODSUI | [39] |
| 30 | [Cd(L14)]n.3-methylpyridine | 2-D | GAMVAU | [40] |
| 31 | [Cd(L1)]n | 2-D | ZODSAO | [39] |
| 32 | [Cd(L11)]n | 2-D | ZODSES | [39] |
| 33 | [Cd(L16)]n | 2-D | ZODSOC | [39] |
| 34 | [Cd(L13)]n.acetonitrile | 2-D | UTEKAH | [36] |
| 35 | [Cd(L2)]n | 2-D | ZODSIW | [39] |
| 36 | Hg(L1)2 | monomer | FODSAU | [35] |
| 37 | Hg(L8)2 | monomer | EBUTAY | [41] |
| 38 | Hg(L17)2 | monomer | XOBCEY | [42] |
| 39 | Hg(L18)2 | monomer | YOMYOQ | [43] |
| 40 | [Hg(L13)2]2 | dimer | UTEKEL | [36] |
| 41 | [Hg(L19)2]2·MeOH | dimer | XOBCAU | [42] |
| 42 | PhHg(L5) | dimer | EKIYON | [44] |
| 43 | PhHg(L17) | dimer | XOBBOH | [42] |
| 44 | PhHg(L20)·2(MeOH) | dimer | FODRUN | [35] |
| 45 | [PhHg(L18)]2 | dimer | YOMYAC | [43] |
| 46 | [PhHg(L21)]2 | dimer | EBUSOL | [41] |
| 47 | [Hg(L10)2]n | 1-D | YOMYIK | [43] |
| 48 | [Hg(L22)2]n | 1-D | FODROH | [35] |
| 49 | [Hg(L21)2]n | 1-D | FODREX | [35] |
| 50 | [Hg(L23)2]n | 1-D | FODRAT | [35] |
| 51 | [Hg(L9)2]n | 2-D | FODRIB | [35] |
| 52 | [PhHg(L8)]n | 2-D | EBUSIF | [41] |
| 53 | [Tl(L24)]n | 1-D | FEGHEH | [45] |
| 54 | [Tl(L1)]n | 2-D | GOQHUR | [46] |
| 55 | [Tl(L11)]n | 2-D | GOQJED | [46] |
| 56 | [Tl(L13)]n | 2-D | GOQKAA | [46] |
| 57 | [Tl(L23)]n | 2-D | GOGJON | [46] |
| 58 | [Tl(L16)]n | 2-D | FEGHOR | [45] |
| 59 | [Tl(L25)]n | 2-D | FEGHUX | [45] |
| 60 | [Tl(L26)]n | 2-D | FEGJAF | [45] |
| 61 | (nBu)2Sn(L8)2 | monomer | UGEFAP | [47] |
| 62 | (nBu)2Sn(L14)2 | monomer | CEHKUX | [48] |
| 63 | Ph2Sn(L1)2 | monomer | UGEFET | [47] |
| 64 | Ph2Sn(L14)2 | monomer | CEHLAE | [48] |
| 65 | Ph3Sn(L27) | monomer | TOHBOJ | [49] |
| 66 | [Ph3Sn]2(L28) | dimer | TOHGEE | [49] |
| 67 | [(PhCH2)2Sn)(L28)]2·CH2Cl2 | dimer | TOHBUP | [49] |
| 68 | [Me2Sn(L14)2]2 | dimer | CEHKOR | [48] |
| 69 | [Ph3Sn(L11)]n | 1-D | UGEFIX | [47] |
| 70 | [Bi(L6)3]2 | dimer | JURXAX | [50] |
| 71 | [Bi(L25)3]2 | dimer | NOTTIC | [51] |
| 72 | [Bi(L29)3]2 | dimer | NOTTOI | [51] |
| 73 | [Bi(L30)3]n·0.5(HCl) | 1-D | NOTTUO | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiekink, E.R.T. On the Coordination Role of Pyridyl-Nitrogen in the Structural Chemistry of Pyridyl-Substituted Dithiocarbamate Ligands. Crystals 2021, 11, 286. https://doi.org/10.3390/cryst11030286
Tiekink ERT. On the Coordination Role of Pyridyl-Nitrogen in the Structural Chemistry of Pyridyl-Substituted Dithiocarbamate Ligands. Crystals. 2021; 11(3):286. https://doi.org/10.3390/cryst11030286
Chicago/Turabian StyleTiekink, Edward R.T. 2021. "On the Coordination Role of Pyridyl-Nitrogen in the Structural Chemistry of Pyridyl-Substituted Dithiocarbamate Ligands" Crystals 11, no. 3: 286. https://doi.org/10.3390/cryst11030286
APA StyleTiekink, E. R. T. (2021). On the Coordination Role of Pyridyl-Nitrogen in the Structural Chemistry of Pyridyl-Substituted Dithiocarbamate Ligands. Crystals, 11(3), 286. https://doi.org/10.3390/cryst11030286